Differences in the Mode of Action of Florpyrauxifen-Benzyl between Barnyardgrass and Yerbadetajo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Plant Material
2.3. Whole-Plant Bioassay to Determine Herbicide Sensitivity
2.4. Liquid Chromatography–Mass Spectrometry Method to Detect 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Content
2.5. Liquid Chromatography–Mass Spectrometry Method to Detect ABA Content
2.6. High-Throughput Sequencing to Screen for Differentially Expressed Genes (DEGs)
2.7. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) to Verify DEGs
3. Results
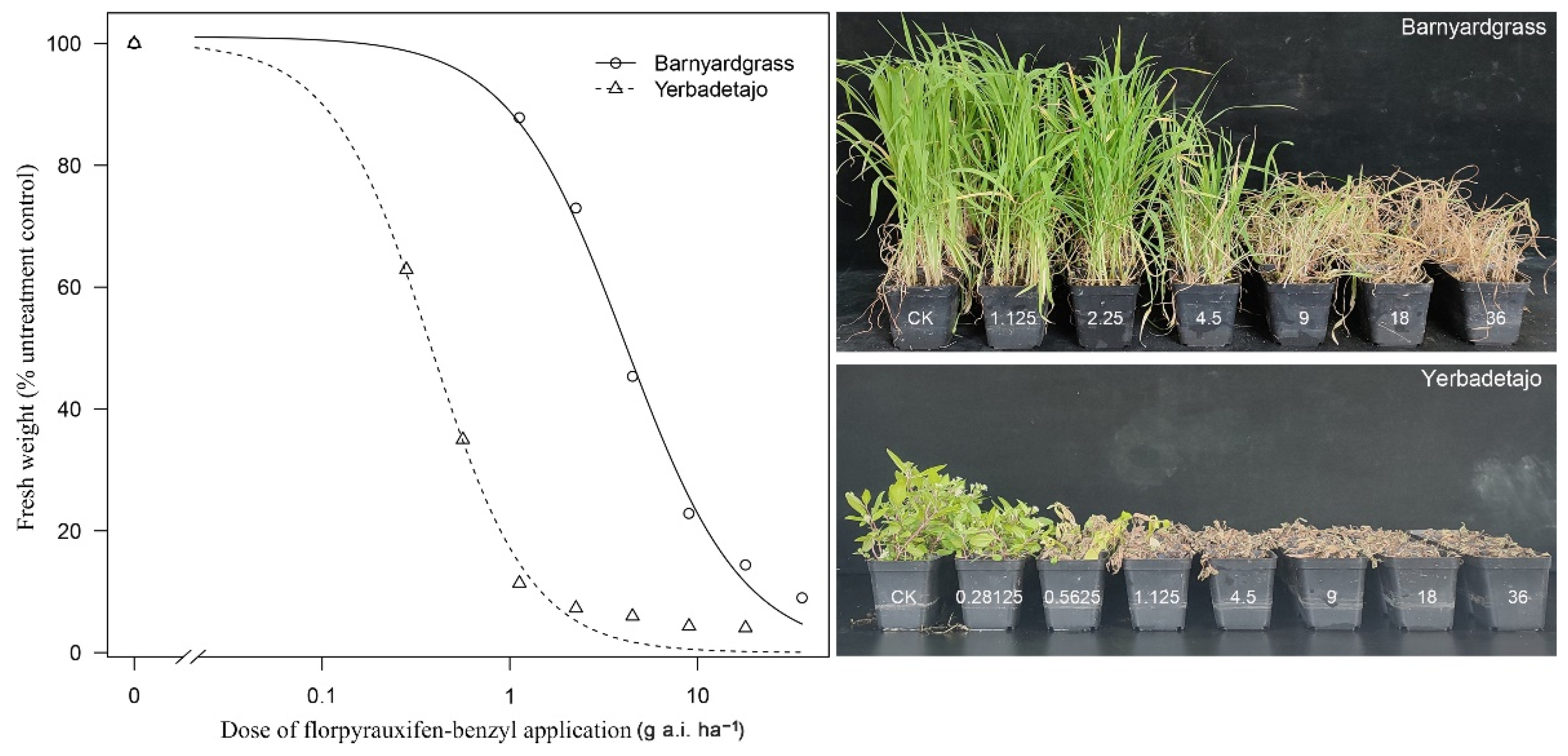
3.1. Sensitivity to Florpyrauxifen-Benzyl
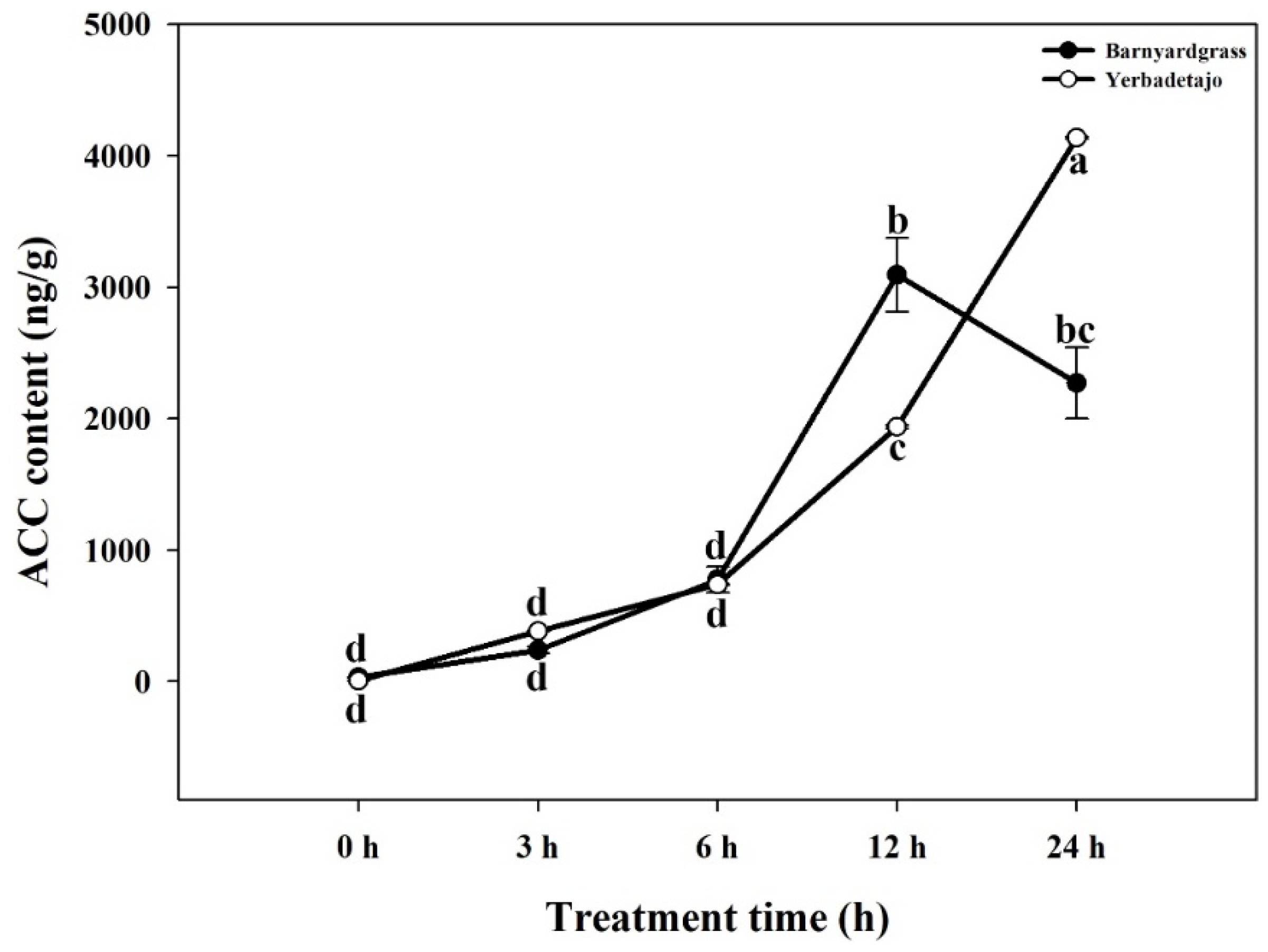
3.2. ACC Production
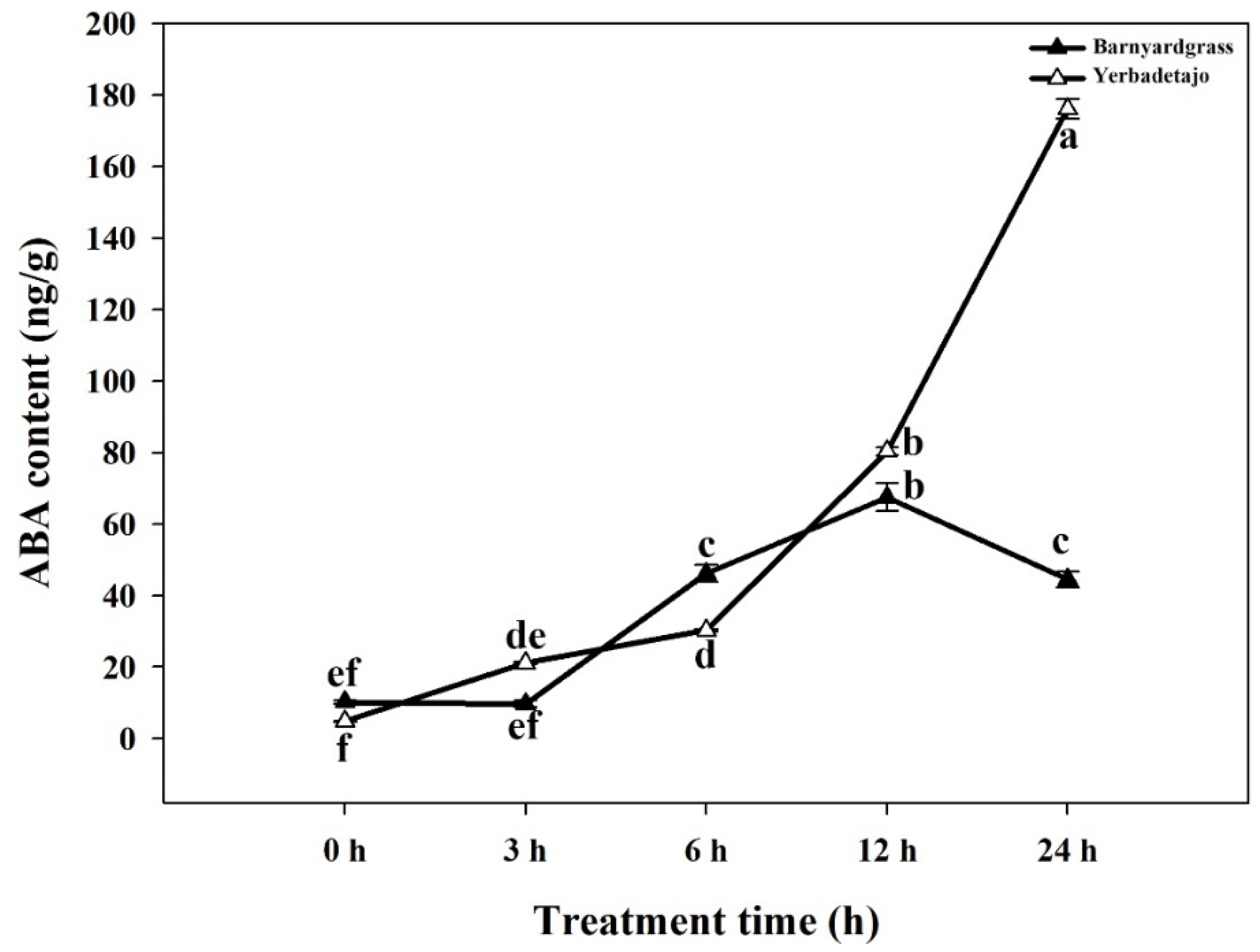
3.3. ABA Production
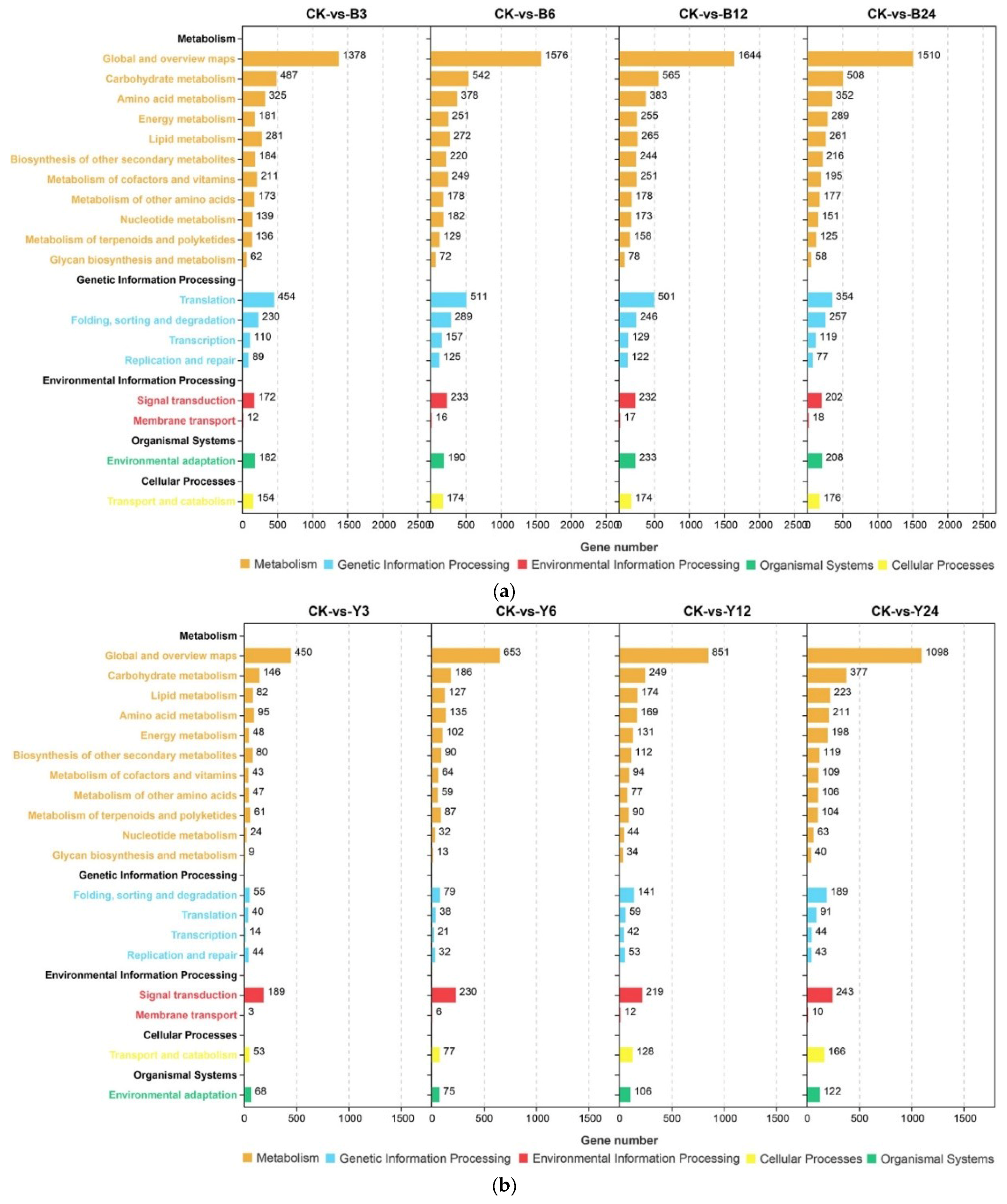
3.4. DEGs Related to ACC and ABA Biosyntheses
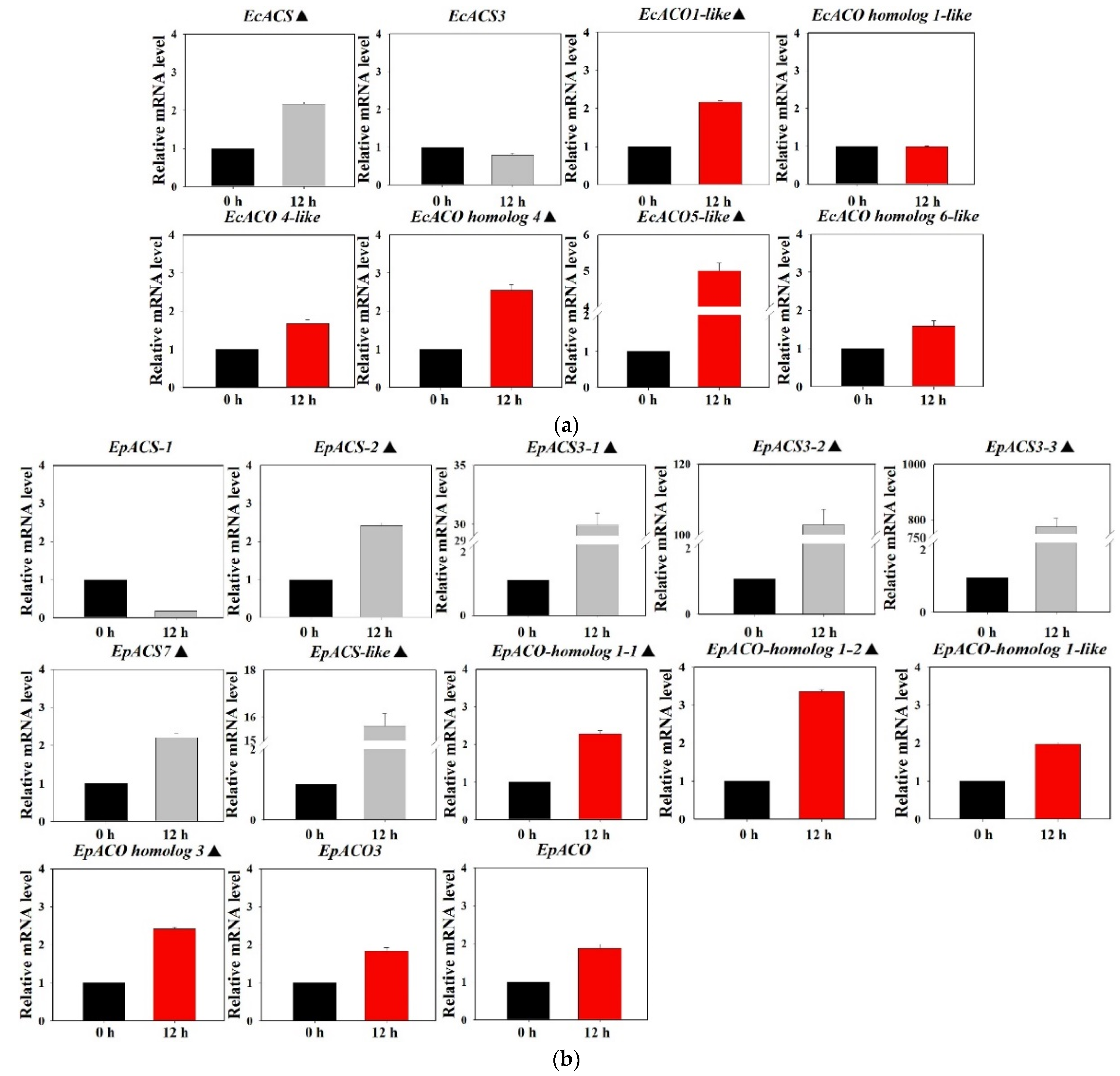
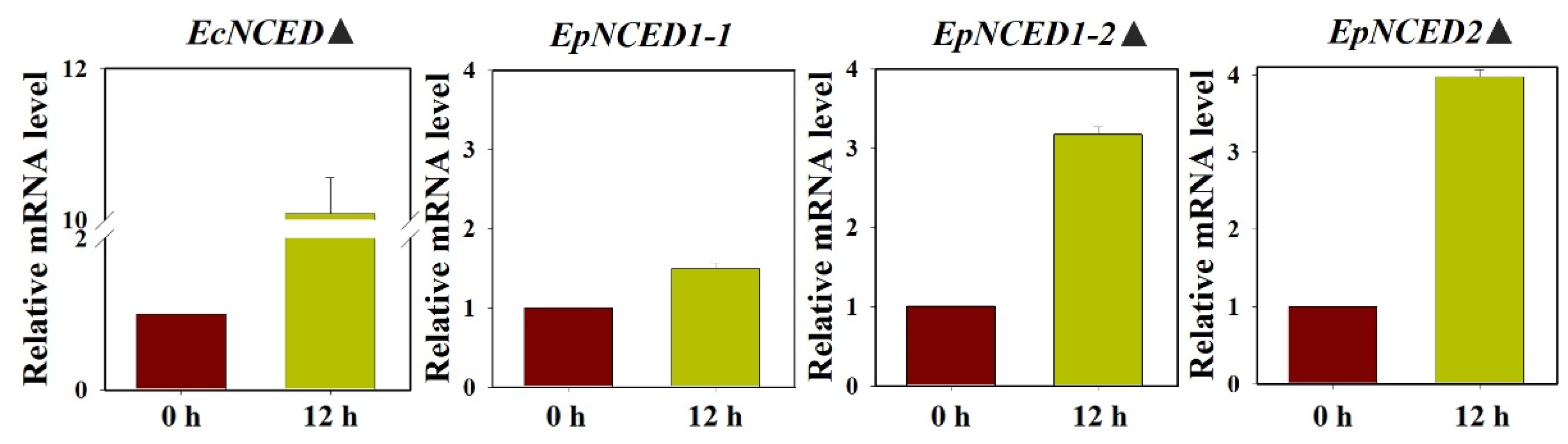
3.5. The Expression Profiles of ACS, ACO, and NCED
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, K.R. Introduction: Rice in historical and social perspectives. Rice Qual. Woodhead Publ. Ser. Food Sci. Technol. Nutr. Elsevier Sci. 2013, 1–25. [Google Scholar] [CrossRef]
- Philip, D.; Jayeoba, O.; Ndripaya, Y.; Fatunbi, A. Innovation Opportunities in the Rice Value Chain in Nigeria. FARA Res. Rep. 2018, 2, 1–48. [Google Scholar]
- Alibu, S.; Mamadou, F. How Does Water Stress and Nitrogen Fertilizer Affect the Growth and Yield of Upland Rice (Oryza sativa L.). J. Food Nutr. Res. 2021, 9, 215–222. [Google Scholar] [CrossRef]
- Zhang, Z.P. Development of chemical weed control and integrated weed management in China. Weed Biol. Manag. 2003, 3, 197–203. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Jabran, K.; Shahid, M.; Ali, H.H.; Chauhan, B.; Ehsanullah. Eco-biology and management of Echinochloa crus-galli. Crop Prot. 2015, 75, 151–162. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 16 May 2022).
- Gealy, D.R.; Fischer, A.J. 13C Discrimination: A Stable Isotope Method to Quantify Root Interactions between C3 Rice (Oryza sativa) and C4 Barnyardgrass (Echinochloa crus-galli) in Flooded Fields. Weed Sci. 2010, 58, 359–368. [Google Scholar] [CrossRef]
- Marambe, B.; Amarasinghe, L. Propanil-resistant barnyardgrass [Echinochloa crus-galli (L.) Beauv.] in Sri Lanka: Seedling growth under different temperatures and control. Weed Biol. Manag. 2002, 2, 194–199. [Google Scholar] [CrossRef]
- Juliano, L.M.; Casimero, M.C.; Llewellyn, R. Multiple herbicide resistance in barnyardgrass (Echinochloa crus-galli) in direct-seeded rice in the Philippines. Int. J. Pest Manag. 2010, 56, 299–307. [Google Scholar] [CrossRef]
- Gibson, K.; Fischer, A.; Foin, T.; Hill, J. Implications of delayed Echinochloa spp. germination and duration of competition for integrated weed management in water-seeded rice. Weed Res. 2002, 42, 351–358. [Google Scholar] [CrossRef]
- Clay, S.A.; Kleinjan, J.; Clay, D.E.; Forcella, F.; Batchelor, W. Growth and Fecundity of Several Weed Species in Corn and Soybean. Agron. J. 2005, 97, 294–302. [Google Scholar] [CrossRef]
- Ottis, B.V.; Talbert, R.E. Barnyardgrass (Echinochloa crus-galli) Control and Rice Density Effects on Rice Yield Components. Weed Technol. 2007, 21, 110–118. [Google Scholar] [CrossRef]
- Wilson, M.J.; Norsworthy, J.K.; Scott, R.C.; Gbur, E.E. Program Approaches to Control Herbicide-Resistant Barnyardgrass (Echinochloa crus-galli) in Midsouthern United States Rice. Weed Technol. 2014, 28, 39–46. [Google Scholar] [CrossRef]
- Xu, J.; Lv, B.; Wang, Q.; Li, J.; Dong, L. A resistance mechanism dependent upon the inhibition of ethylene biosynthesis. Pest Manag. Sci. 2013, 69, 1407–1414. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Q.; Yao, Z.; Zhu, L.; Dong, L. Penoxsulam-resistant barnyardgrass (Echinochloa crus-galli) in rice fields in China. Weed Biol. Manag. 2015, 16, 16–23. [Google Scholar] [CrossRef]
- Liu, H.Y.; Han, H.T.; Zuo, H.L.; Gao, L.; Zhong, G.H. Effect of pyrazosulfuron against Echinochloa crus-galli in rice fields in South China. Guangdong Agric. Sci. 2011, 38, 3. (In Chinese) [Google Scholar]
- Liu, J.; Fang, J.; He, Z.; Li, J.; Dong, L. Target site–based resistance to penoxsulam in late watergrass (Echinochloa phyllopogon) from China. Weed Sci. 2019, 67, 380–388. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Yu, H.; Cui, H. Detection of resistance of Eclipta prostrata L. to pyrazosulfuron-ethyl in some provinces of China. Plant Prot. 2017, 196–201. (In Chinese) [Google Scholar]
- Zhao, H.Y.; Lu, Z.C.; Qiao, L.; Yang, L.M. Comparative Test of Weed Control Efficacy of Five Herbicides in Dry Direct-seeding Rice Field. Weed Sci. 2012, 48–50. (In Chinese) [Google Scholar]
- Li, R.; Luo, X.; Dong, L.; Zhao, G.; Fu, Y. Sensitivity of Eclipta prostrata populations to herbicides. Jiangsu J. Agric. Sci. 2013, 1514–1516. (In Chinese) [Google Scholar]
- Li, Y.H. Weeds of China; China Agroculture Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Zhou, X.G.; Zhang, H. Primary Color Map of Common Weeds in Sichuan Farmland; Sichuan Publishing House of Science & Technology: Chengdu, China, 2006. (In Chinese) [Google Scholar]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds; Distribution and biology, University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Li, S.S.; Zhang, L.J.; Qiang, S. Weed Community Characteristics and Comprehensive Evaluation on Weeds in Paddy Fields under Labour-Saving Cultivations in Middle Jiangsu Province, China. Chin. J. Rice Sci. 2009, 23, 207–214. (In Chinese) [Google Scholar]
- Huang, B.Q.; Lin, S.X. Study on the resistance of the barnyardgrass in the paddy fields to the butachlor in China. J. South China Agric. Univ. 1993, 14, 103–108. (In Chinese) [Google Scholar]
- Fang, J.; Liu, T.; Zhang, Y.; Li, J.; Dong, L. Target site–based penoxsulam resistance in barnyardgrass (Echinochloa crus-galli) from China. Weed Sci. 2019, 67, 281–287. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Gu, T.; Dong, M.; Peng, Q.; Bai, L.; Li, Y. Quantitative proteomics reveals ecological fitness cost of multi-herbicide resistant barnyardgrass (Echinochloa crus-galli L.). J. Proteom. 2017, 150, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Ji, M.; Zang, X.; Chen, S.; Du, Y. The resistance level and acetyl co-enzyme A carboxylase activity of Echinochloa phyllopogon populations to metamifop in paddies. J. Plant Prot. 2017, 44, 1040–1045. (In Chinese) [Google Scholar]
- Wang, H.; Sun, X.; Yu, J.; Li, J.; Dong, L. The phytotoxicity mechanism of florpyrauxifen-benzyl to Echinochloa crus-galli (L.) P. Beauv and weed control effect. Pestic. Biochem. Physiol. 2021, 179, 104978. [Google Scholar] [CrossRef]
- China Pesticide Information Network. 2020. Available online: https://www.chinapesticide.org.cn/ (accessed on 16 May 2022).
- Epp, J.B.; Alexander, A.L.; Balko, T.W.; Buysse, A.M.; Brewster, W.K.; Bryan, K.; Daeuble, J.F.; Fields, S.C.; Gast, R.E.; Green, R.A.; et al. The discovery of Arylex™ active and Rinskor™ active: Two novel auxin herbicides. Bioorg. Med. Chem. 2016, 24, 362–371. [Google Scholar] [CrossRef]
- Miller, M.R.; Norsworthy, J.K.; Scott, R.C. Evaluation of Florpyrauxifen-benzyl on Herbicide-Resistant and Herbicide-Susceptible Barnyardgrass Accessions. Weed Technol. 2017, 32, 126–134. [Google Scholar] [CrossRef]
- Travlos, I.; Kanatas, P.; Tsekoura, A.; Gazoulis, I.; Papastylianou, P.; Kakabouki, I.; Antonopoulos, N. Efficacy of Different Herbicides on Echinochloa colona (L.) Link Control and the First Case of Its Glyphosate Resistance in Greece. Agronomy 2020, 10, 1056. [Google Scholar] [CrossRef]
- Lim, S.-H.; Kim, H.; Noh, T.-K.; Lim, J.-S.; Yook, M.-J.; Kim, J.-W.; Yi, J.-H.; Kim, D.-S. Baseline Sensitivity of Echinochloa crus-gall and E. oryzicola to Florpyrauxifen-Benzyl, a New Synthetic Auxin Herbicide, in Korea. Front. Plant Sci. 2021, 12, 1065. [Google Scholar] [CrossRef]
- Hwang, J.I.; Norsworthy, J.K.; González-Torralva, F.; Priess, G.L.; Barber, L.T.; Butts, T.R. Non-target-site resistance mechanism of barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] to florpyrauxifen-benzyl. Pest Manag. Sci. 2022, 78, 287–295. [Google Scholar] [CrossRef]
- Velásquez, J.C.; Bundt, A.D.C.; Camargo, E.R.; Andres, A.; Viana, V.E.; Hoyos, V.; Plaza, G.; de Avila, L.A. Florpyrauxifen-Benzyl Selectivity to Rice. Agriculture 2021, 11, 1270. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. Former. Pestic. Sci. 2009, 66, 113–120. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Pan, X.; Liu, D.; Napier, R.; Dong, L. Quinclorac resistance induced by the suppression of the expression of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase genes in Echinochloa crus-galli var. zelayensis. Pestic. Biochem. Physiol. 2018, 146, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuor, H.; Milan, M.; Eckert, J.W.; Fischer, A.J. Quinclorac resistance: A concerted hormonal and enzymatic effort in Echinochloa phyllopogon. Pest Manag. Sci. 2011, 68, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K.; Scheltrup, F. Selective Induction of 1-Aminocyclopropane-1-carboxylic Acid (ACC) Synthase Activity Is Involved in the Selectivity of the Auxin Herbicide Quinclorac between Barnyard Grass and Rice. Pestic. Biochem. Physiol. 1997, 58, 145–153. [Google Scholar] [CrossRef]
- Grossmann, K.; Kwiatkowski, J. Evidence for a Causative Role of Cyanide, Derived from Ethylene Biosynthesis, in the Herbicidal Mode of Action of Quinclorac in Barnyard Grass. Pestic. Biochem. Physiol. 1995, 51, 150–160. [Google Scholar] [CrossRef]
- Abdallah, I.; Fischer, A.J.; Elmore, C.L.; Saltveit, M.E.; Zaki, M. Mechanism of resistance to quinclorac in smooth crabgrass (Digitaria ischaemum). Pestic. Biochem. Physiol. 2006, 84, 38–48. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, L.; Sun, Y.; Zhang, T.; Dong, L.; Li, J. Resistance to quinclorac caused by the enhanced ability to detoxify cyanide and its molecular mechanism in Echinochloa crus-galli var. zelayensis. Pestic. Biochem. Physiol. 2017, 143, 231–238. [Google Scholar] [CrossRef]
- Kraft, M.; Kuglitsch, R.; Kwiatkowski, J.; Frank, M.; Grossmann, K. Indole-3-acetic acid and auxin herbicides up-regulate 9-cis-epoxycarotenoid dioxygenase gene expression and abscisic acid accumulation in cleavers (Galium aparine): Interaction with ethylene. J. Exp. Bot. 2007, 58, 1497–1503. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xu, J.; Qi, Y.; He, S.; Li, J.; Dong, L. Differences in ABA synthesis and oxidase stress between quinclorac-resistant and-sensitive Echinochloa crus-galli var. zelayensis. Int. J. Pest Manag. 2021, 1–13. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, X.; Sun, X.; Li, J.; Dong, L. Is the protection of photosynthesis related to the mechanism of quinclorac resistance in Echinochloa crus-galli var. zelayensis? Gene 2019, 683, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org (accessed on 3 January 2022).
- Li, G.; Wu, S.; Cai, L.; Wang, Q.; Zhao, X.; Wu, C. Identification and mRNA expression profile of glutamate receptor-like gene in quinclorac-resistant and susceptible Echinochloa crus-galli. Gene 2013, 531, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Z.; Cai, J.; Gao, H.; Zhao, H.; Dong, L. High-throughput sequencing reveals differential regulation of miRNAs in fenoxaprop-P-ethyl-resistant Beckmannia syzigachne. Sci. Rep. 2016, 6, 28725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón Villalobos, L.I.A.; Lee, S.; De Oliveira, C.; Ivetac, A.; Brandt, W.; Armitage, L.; Sheard, L.B.; Tan, X.; Parry, G.; Mao, H. A combinatorial TIR1/AFB–Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 2012, 8, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeClere, S.; Wu, C.; Westra, P.; Sammons, R.D. Cross-resistance to dicamba, 2, 4-D, and fluroxypyr in Kochia scoparia is endowed by a mutation in an AUX/IAA gene. Proc. Natl. Acad. Sci. USA 2018, 115, E2911–E2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, K.; Kwiatkowski, J. The Mechanism of Quinclorac Selectivity in Grasses. Pestic. Biochem. Physiol. 2000, 66, 83–91. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Napier, R.; Dong, L.; Li, J. Mode of Action of a Novel Synthetic Auxin Herbicide Halauxifen-Methyl. Agronomy 2022, 12, 1659. [Google Scholar] [CrossRef]
- Chutichude, P.; Chutichudet, B.; Boontiang, K. Influence of 1-MCP Fumigation on Flowering Weight Loss, Water Uptake, Longevity, Anthocyanin Content and Colour of Patumma (Curcuma alismatifolia) cv. Chiang Mai Pink. Int. J. Agric. Res. 2010, 6, 29–39. [Google Scholar] [CrossRef]
- Brown, K.M. Ethylene and abscission. Physiol. Plantarum. 1997, 100, 567–576. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, Y.T.; Kao, C.H. Ammonium ion, ethylene, and NaCl-induced senescence of detached rice leaves. Plant Growth Regul. 2002, 37, 85–92. [Google Scholar] [CrossRef]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plantarum. 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Woltering, E.J.; Van Doorn, W.G. Role of ethylene in senescence of petals—Morphological and taxonomical relationships. J. Exp. Bot. 1988, 39, 1605–1616. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Ikegaya, H.; Iwase, H.; Hatanaka, K.; Sakurada, K.; Yoshida, K.-I.; Takatori, T. Diagnosis of cyanide intoxication by measurement of cytochrome c oxidase activity. Toxicol. Lett. 2001, 119, 117–123. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van Montagu, M.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Fipke, M.; Vidal, R. Integrative Theory of the Mode of Action of Quinclorac: Literature Review1. Planta Daninha 2016, 34, 393–402. [Google Scholar] [CrossRef]
- Iwakami, S.; Uchino, A.; Kataoka, Y.; Shibaike, H.; Watanabe, H.; Inamura, T. Cytochrome P450 genes induced by bispyribac-sodium treatment in a multiple-herbicide-resistant biotype of Echinochloa phyllopogon. Pest Manag. Sci. 2013, 70, 549–558. [Google Scholar] [CrossRef]
- Yuan, J.S.; Tranel, P.J.; Stewart, C.N., Jr. Non-target-site herbicide resistance: A family business. Trends Plant Sci. 2007, 12, 6–13. [Google Scholar] [CrossRef]
- Barry, C.S.; Blume, B.; Bouzayen, M.; Cooper, W.; Hamilton, A.J.; Grierson, D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996, 9, 525–535. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botella, J.R.; Schlagnhaufer, C.D.; Arteca, J.M.; Arteca, R.N.; Phillips, A.T. Identification of two new members of the l-aminocyclopropane-l-carboxylate synthase-encoding multigene family in mung bean. Gene 1993, 123, 249–253. [Google Scholar] [CrossRef]
- Kende, H.; Zeevaart, J. The Five "Classical" Plant Hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Abel, S.; A Keller, J.; Shen, N.F.; Theologis, A. The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1992, 89, 11046–11050. [Google Scholar] [CrossRef] [Green Version]
- Yip, W.K.; Moore, T.; Yang, S.F. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc. Natl. Acad. Sci. USA 1992, 89, 2475–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottmann, W.H.; Peter, G.F.; Oeller, P.W.; Keller, J.A.; Shen, N.F.; Nagy, B.P.; Taylor, L.P.; Campbell, A.D.; Theologis, A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J. Mol. Biol. 1991, 222, 937–961. [Google Scholar] [CrossRef]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical Diversity among the 1-Amino-cyclopropane-1-Carboxylate Synthase Isozymes Encoded by the Arabidopsis Gene Family. J. Biol. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A. Elucidation of the Indirect Pathway of Abscisic Acid Biosynthesis by Mutants, Genes, and Enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef]
- Tan, B.-C.; Joseph, L.M.; Deng, W.-T.; Liu, L.; Li, Q.-B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
| Substance | Polarity | Parent Ion (m/z) | Daughter Ion (m/z) | De Clustering Voltage (V) | Collision Energy (V) |
|---|---|---|---|---|---|
| ACC | + | 102.0 | 55.9 */84.0 | 40 | 19/42 |
| Time (min) | Flow Velocity (mL/min) | A% |
|---|---|---|
| 0–1 | 0.3 | 20 |
| 1–3 | 0.3 | Increase from 20 to 50 |
| 3–9 | 0.3 | Increase from 50 to 80 |
| 9–10.5 | 0.3 | 80 |
| 10.5–10.6 | 0.3 | Decrease from 80 to 20 |
| 10.6–13.5 | 0.3 | 20 |
| Substance | Polarity | Parent Ion (m/z) | Daughter Ion (m/z) | De Clustering Voltage (V) | Collision Energy (V) |
|---|---|---|---|---|---|
| ABA | - | 263.1 | 153.1 */204.2 | −60 | −14/−27 |
| Weed Species | Gene Expression Trend | Time after Florpyrauxifen-Benzyl Treatment | |||
|---|---|---|---|---|---|
| 3 h | 6 h | 12 h | 24 h | ||
| Barnyardgrass | Up-regulated genes | 9162 | 11,545 | 12,150 | 9440 |
| Down-regulated genes | 4148 | 4047 | 4189 | 3289 | |
| All Differentially expressed genes | 13,310 | 15,592 | 16,339 | 12,729 | |
| Yerbadetajo | Up-regulated genes | 2172 | 3589 | 3860 | 4567 |
| Down-regulated genes | 3925 | 3427 | 5718 | 6637 | |
| All Differentially expressed genes | 6097 | 7016 | 9578 | 11,204 | |
| Genes | Best BLASTX Match | |
|---|---|---|
| Name and Accession Number | Ident (%) | |
| EcACS | ACS (Echinochloa crus-galli) (KT245168.1) | 96.95 |
| EcACS3 | ACS 3 (E. crus-galli var. zelayensis) (KY963551) | 100.00 |
| EcACO1-like | ACO 1-like (E. crus-galli var. zelayensis) (KY963553.1) | 96.64 |
| EcACO homolog 1 | EcACO homolog 1 (Setaria italica) (XM_004975083.3) | 89.89 |
| EcACO 4-like | ACO 4-like (E. crus-galli var. zelayensis) (KY963555) | 100.00 |
| EcACO homolog 4 | ACO 4-like (E. crus-galli var. zelayensis) (KY963556) | 100.00 |
| EcACO5-like | ACO 5-like (E. crus-galli var. zelayensis) (KY963548) | 100.00 |
| EcACO homolog 6-like | ACO 6-like (E. crus-galli var. zelayensis) (KY963557) | 100.00 |
| EcNCED | NCED (E. crus-galli var. zelayensis) [45] | 100.00 |
| EpACS-1 | ACS (Helianthus annuus) (XM_022113477.2) | 85.06 |
| EpACS-2 | ACS (Helianthus annuus) (XM_022144560.2) | 88.86 |
| EpACS3-1 | ACS 3 (Helianthus annuus) (XM_022154426.2) | 88.42 |
| EpACS3-2 | ACS 3 (Helianthus annuus) (XM_022142807.2) | 88.39 |
| EpACS3-3 | ACS 3 (Helianthus annuus) (XM_022179292.2) | 86.04 |
| EpACS7 | ACS 7 (Helianthus annuus) (XM_022131322.1) | 91.77 |
| EpACS-like | ACS-like (Helianthus annuus) (XM_035986598.1) | 86.21 |
| EpACO-homolog 1-1 | ACO homolog 1 (Helianthus annuus) (XM_035987786.1) | 85.21 |
| EpACO-homolog 1-2 | ACO homolog 1 (Helianthus annuus) (XM_022146678.2) | 81.67 |
| EpACO-homolog 1-like | ACO homolog 1-like (Helianthus annuus) (XM_022174330.2) | 85.69 |
| EpACO homolog 3 | ACO homolog 3 (Helianthus annuus) (XM_022170011.2) | 88.71 |
| EpACO3 | ACO 3 (Helianthus annuus) (XM_022155132.2) | 88.06 |
| EpACO | ACO (Helianthus annuus) (XM_022155410.2) | 84.07 |
| EpNCED1-1 | NCED1 (Helianthus annuus) (XM_022157417.2) | 85.73 |
| EpNCED1-2 | NCED1 (Helianthus annuus) (XM_022133305.2) | 85.99 |
| EpNCED2 | NCED2 (Helianthus annuus) (XM_022179446.2) | 83.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, J.; Shen, G.; Tian, Z. Differences in the Mode of Action of Florpyrauxifen-Benzyl between Barnyardgrass and Yerbadetajo. Agronomy 2022, 12, 2656. https://doi.org/10.3390/agronomy12112656
Gao Y, Li J, Shen G, Tian Z. Differences in the Mode of Action of Florpyrauxifen-Benzyl between Barnyardgrass and Yerbadetajo. Agronomy. 2022; 12(11):2656. https://doi.org/10.3390/agronomy12112656
Chicago/Turabian StyleGao, Yuan, Jun Li, Guohui Shen, and Zhihui Tian. 2022. "Differences in the Mode of Action of Florpyrauxifen-Benzyl between Barnyardgrass and Yerbadetajo" Agronomy 12, no. 11: 2656. https://doi.org/10.3390/agronomy12112656
APA StyleGao, Y., Li, J., Shen, G., & Tian, Z. (2022). Differences in the Mode of Action of Florpyrauxifen-Benzyl between Barnyardgrass and Yerbadetajo. Agronomy, 12(11), 2656. https://doi.org/10.3390/agronomy12112656






