Ferrate-Modified Biochar for Greenhouse Gas Mitigation: First-Principles Calculation and Paddy Field Trails
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Theoretical Calculation
2.2. Field Experiment Location
2.3. Experimental Design and Management
2.4. Methods of Sample Collection and Analysis
2.4.1. The Soil Chemical Properties Preparation and Quantification
2.4.2. Greenhouse Gas Acquisition and Measurement in Paddy Fields
2.4.3. Soil Acquisition and Soil Enzyme Activities Measurement
2.4.4. Soil Acquisition and Soil Organic Carbon Measurement
2.4.5. Yield Determination
2.5. Statistical Analyses
3. Results
3.1. Theoretical Prediction
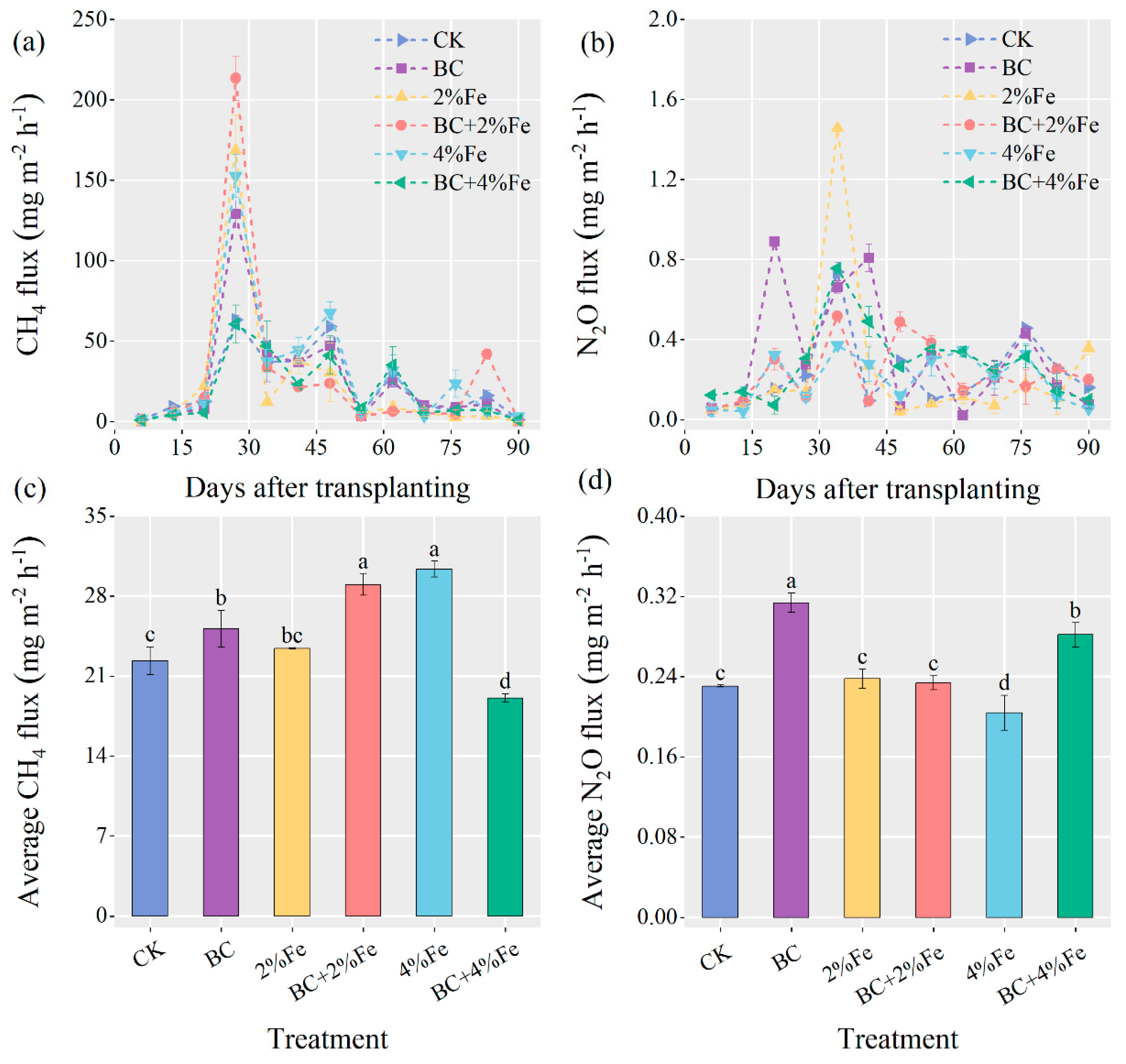
3.2. CH4 and N2O Emissions
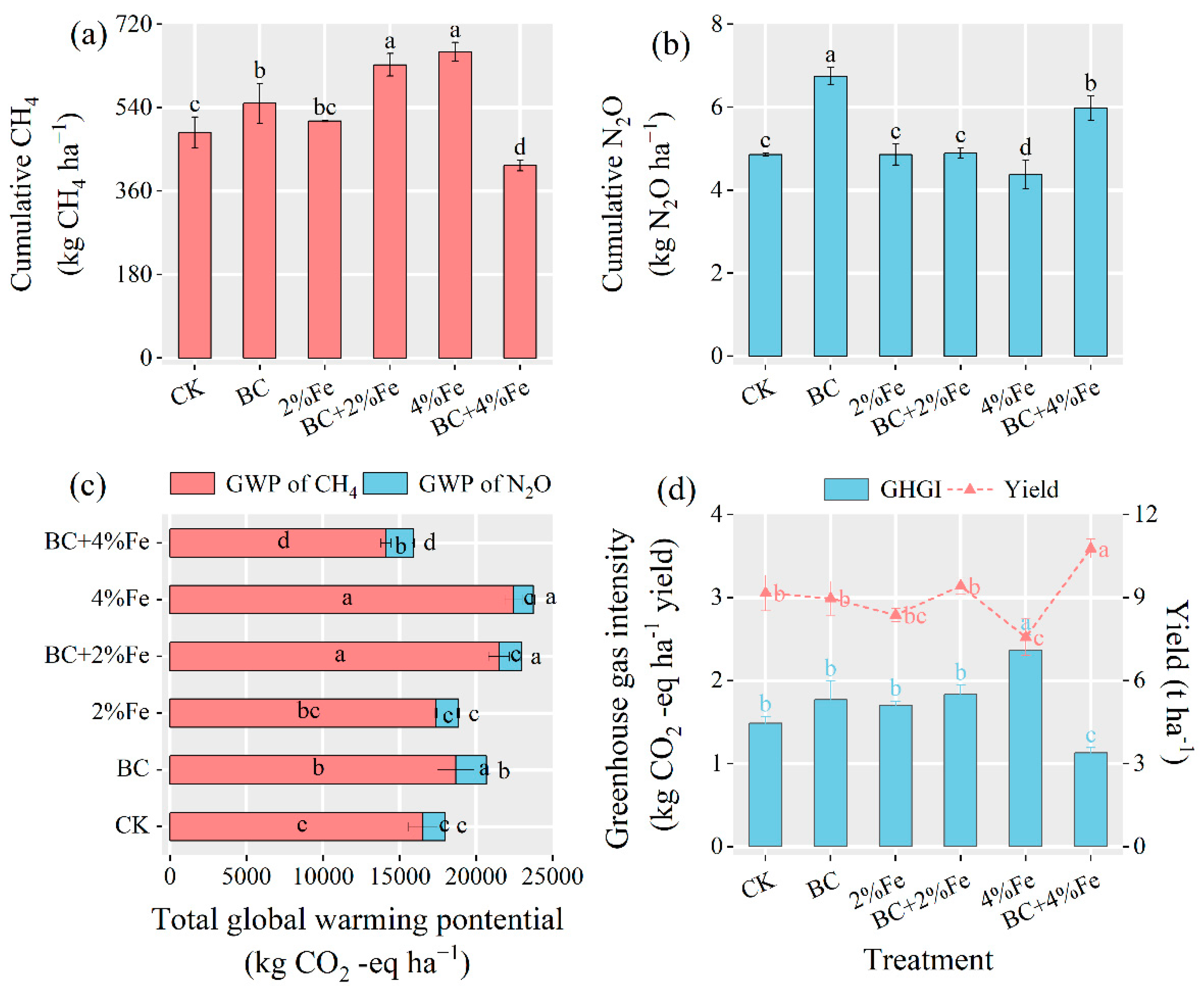
3.3. Greenhouse Effect and Yield
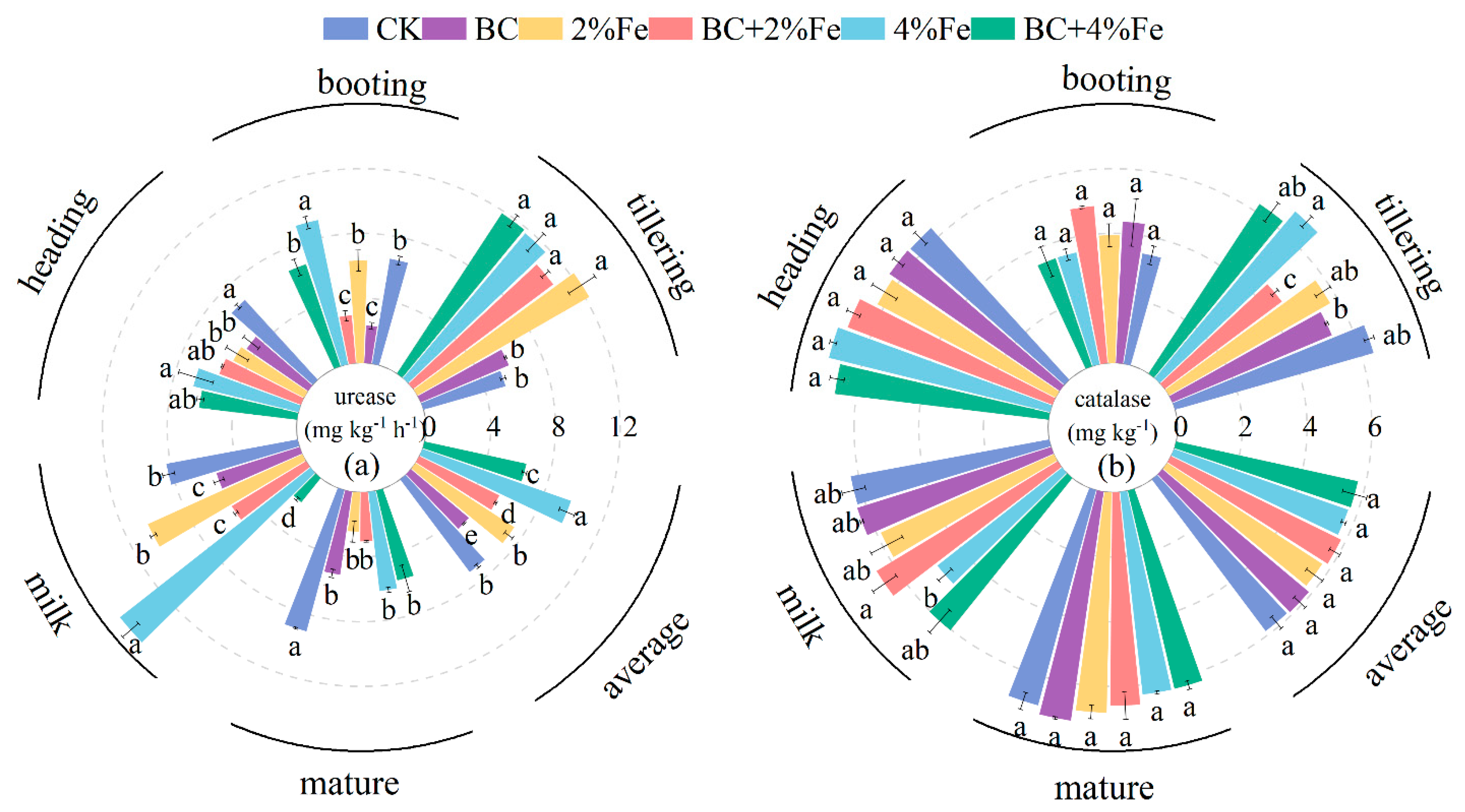
3.4. Soil Urease Activities
3.5. Soil Catalase Activities
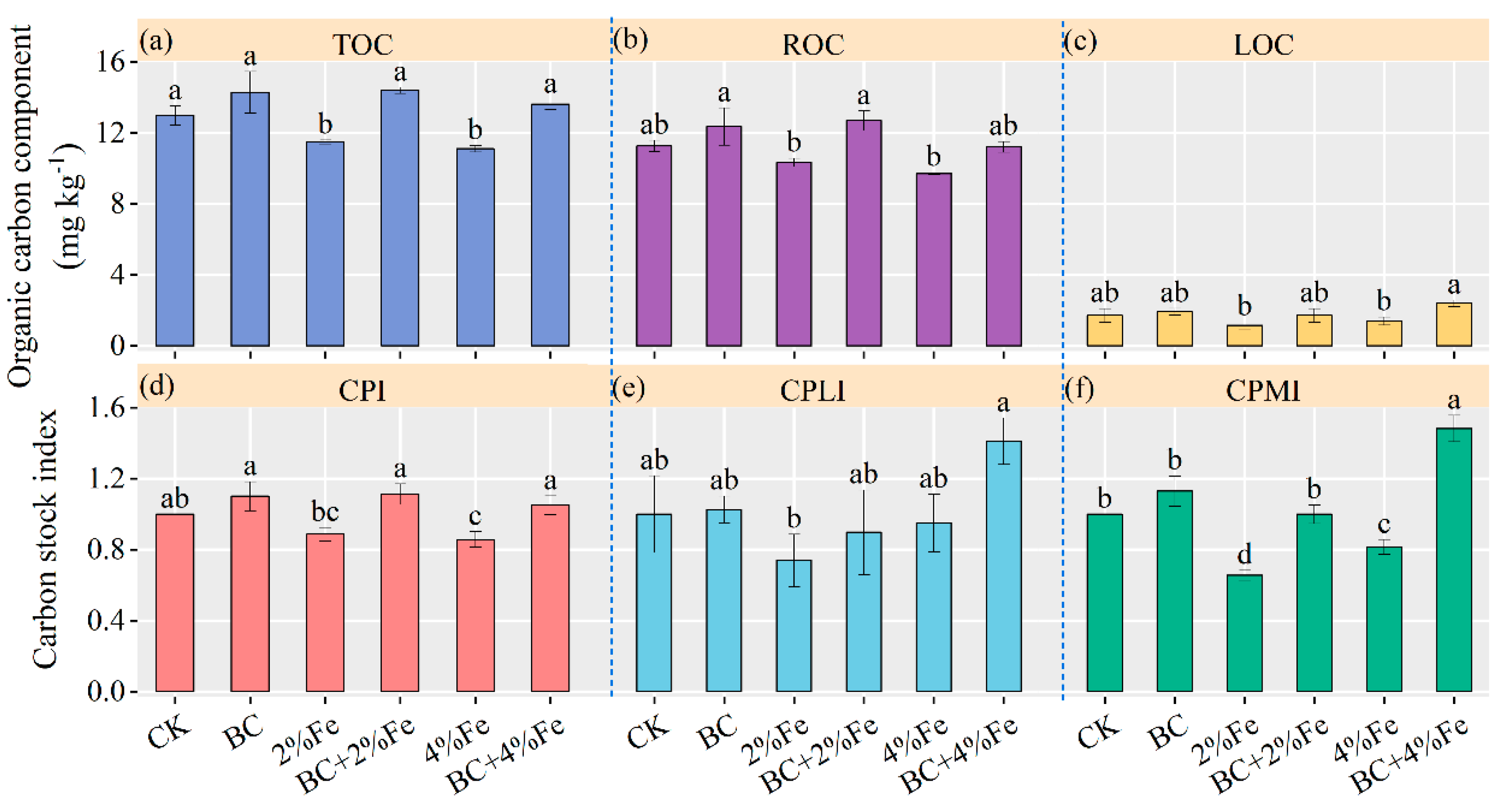
3.6. Soil Organic Carbon
3.7. Relationship Analysis of Greenhouse Gas Emissions, Yields, Organic Carbon Characteristics, and Soil Enzymes Activities
4. Discussion
4.1. Soil Organic Carbon
4.2. Soil Enzymes
4.3. Trends of CH4 Emission Fluxes
4.4. Differences in Cumulative CH4 Emissions between Treatments
4.5. Differences in N2O Emissions and Greenhouse Effect between Treatments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Yan, S.; Zhang, J.; Jiang, Y.; Deng, A. Win-win strategy for national food security and agricultural double-carbon goals. Sci. Agric. Sin. 2021, 54, 3892–3902. [Google Scholar]
- Hussain, S.; Peng, S.; Fahad, S.; Khaliq, A.; Huang, J.; Cui, K.; Nie, L. Rice management interventions to mitigate greenhouse gas emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 3342–3360. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tan, D.K.Y.; Munsif, F.; Afridi, M.Z.; Shah, F.; Wei, F.; Fahad, S.; Zhou, R. Nitrogen nutrition in cotton and control strategies for greenhouse gas emissions: A review. Environ. Sci. Pollut. Res. 2017, 24, 23471–23487. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Niu, H.-D.; Zhou, P.; Jia, R.-J.; Gao, M. Effects of biochar on the net greenhouse gas emissions under continuous flooding and water-saving irrigation conditions in paddy soils. Sustainability 2018, 10, 1403. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report: Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Mallapaty, S. How China could be carbon neutral by mid-century. Nature 2020, 586, 482–484. [Google Scholar] [CrossRef]
- Cui, J.; Glatzel, S.; Bruckman, V.J.; Wang, B.; Lai, D.Y.F. Long-term effects of biochar application on greenhouse gas production and microbial community in temperate forest soils under increasing temperature. Sci. Total Environ. 2021, 767, 145021. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Shahzad, S.M.; Chatterjee, N.; Arif, M.S.; Farooq, T.H.; Altaf, M.M.; Tufail, M.A.; Dar, A.A.; Mehmood, T. Nitrous oxide emission from agricultural soils: Application of animal manure or biochar? A global meta-analysis. J. Environ. Manag. 2021, 285, 112170. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergstrom, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Dong, Y.; Li, B.; Xiong, Z. Biochar amendment reduced greenhouse gas intensities in the rice-wheat rotation system: Six-year field observation and meta-analysis. Agric. For. Meteorol. 2019, 278, 107625. [Google Scholar] [CrossRef]
- Scheer, C.; Grace, P.R.; Rowlings, D.W.; Kimber, S.; Van Zwieten, L. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil 2011, 345, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shen, J.; Li, Y.; Su, Y.; Ge, T.; Jones, D.L.; Wu, J. Effects of biochar amendment on the net greenhouse gas emission and greenhouse gas intensity in a Chinese double rice cropping system. Eur. J. Soil Biol. 2014, 65, 30–39. [Google Scholar] [CrossRef]
- Xu, W.; Whitman, W.B.; Gundale, M.J.; Chien, C.C.; Chiu, C.Y. Functional response of the soil microbial community to biochar applications. GCB Bioenergy 2021, 13, 269–281. [Google Scholar] [CrossRef]
- Kubaczyński, A.; Walkiewicz, A.; Pytlak, A.; Grządziel, J.; Gałązka, A.; Brzezińska, M. Biochar dose determines methane uptake and methanotroph abundance in Haplic Luvisol. Sci. Total Environ. 2022, 806, 151259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yang, S.; Pang, Q.; Xu, Y.; Chen, X.; Sun, X.; Qi, S.; Yu, W. Biochar improved soil health and mitigated greenhouse gas emission from controlled irrigation paddy field: Insights into microbial diversity. J. Clean. Prod. 2021, 318, 128595. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Maroušek, J.; Rowland, Z.; Valášková, K.; Král, P. Techno-economic assessment of potato waste management in developing economies. Clean Technol. Environ. Policy 2020, 22, 937–944. [Google Scholar]
- Stavkova, J.; Maroušek, J. Novel sorbent shows promising financial results on P recovery from sludge water. Chemosphere 2021, 276, 130097. [Google Scholar] [CrossRef]
- Ayub, M.A.; Usman, M.; Faiz, T.; Umair, M.; Rizwan, M.; Ali, S.; Zia ur Rehman, M. Restoration of degraded soil for sustainable agriculture. In Soil Health Restoration and Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 31–81. [Google Scholar]
- Alghamdi, A.G. Biochar as a potential soil additive for improving soil physical properties—A review. Arab. J. Geosci. 2018, 11, 766. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Jun, Z.; Qingling, F.; Houben, D.; Hongqing, H. Efficiency of KOH-modified rice straw-derived biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil. Pedosphere 2020, 30, 874–882. [Google Scholar] [CrossRef]
- Herawati, A.; Syamsiyah, J.; Baldan, S.; Arifin, I. Application of soil amendments as a strategy for water holding capacity in sandy soils. IOP Conf. Ser. Earth Environ. Sci. 2021, 724, 012014. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Dong, C.-D.; Su, J.-F.; Wang, P.-Y.; Chen, C.-W.; Chang, J.-S.; Kim, H.; Huang, C.-P.; Hung, C.-M. The role of biochar in regulating the carbon, phosphorus, and nitrogen cycles exemplified by soil systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Li, W.; Xiao, Q.; Hu, C.; Liu, B.; Sun, R. A comparison of the efficiency of different urease inhibitors and their effects on soil prokaryotic community in a short-term incubation experiment. Geoderma 2019, 354, 113877. [Google Scholar] [CrossRef]
- Pitts, G.; Allam, A.I.; Hollis, J.P. Beggiatoa: Occurrence in the rice rhizosphere. Science 1972, 178, 990–992. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Wei, X.; Kuzyakov, Y.; Li, B.; Kim, P.J.; Wu, J.; Liu, S.; Ge, T. Sources and intensity of CH4 production in paddy soils depend on iron oxides and microbial biomass. Biol. Fertil. Soils 2022, 58, 181–191. [Google Scholar] [CrossRef]
- Luo, D.; Meng, X.; Zheng, N.; Li, Y.; Yao, H.; Chapman, S.J. The anaerobic oxidation of methane in paddy soil by ferric iron and nitrate, and the microbial communities involved. Sci. Total Environ. 2021, 788, 147773. [Google Scholar] [CrossRef]
- Conrad, R. Microbial ecology of methanogens and methanotrophs. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 1–63. [Google Scholar]
- Liu, Y.; Luo, H.; Tie, B.; Li, D.; Liu, S.; Lei, M.; Du, H. The long-term effectiveness of ferromanganese biochar in soil Cd stabilization and reduction of Cd bioaccumulation in rice. Biochar 2021, 3, 499–509. [Google Scholar] [CrossRef]
- Ciais, P.; Wattenbach, M.; Vuichard, N.; Smith, P.; Piao, S.; Don, A.; Luyssaert, S.; Janssens, I.; Bondeau, A.; Dechow, R. The European carbon balance. Part 2: Croplands. Glob. Chang. Biol. 2010, 16, 1409–1428. [Google Scholar] [CrossRef]
- Fan, L.; Dippold, M.A.; Ge, T.; Wu, J.; Thiel, V.; Kuzyakov, Y.; Dorodnikov, M. Anaerobic oxidation of methane in paddy soil: Role of electron acceptors and fertilization in mitigating CH4 fluxes. Soil Biol. Biochem. 2020, 141, 107685. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Sun, Q. Magnetism of phthalocyanine-based organometallic single porous sheet. J. Am. Chem. Soc. 2011, 133, 15113–15119. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhou, W.; Long, W.; Wang, H.; Long, P.; Xu, Y.; Fu, Z. Matter Production Characteristics and Nitrogen Use Efficiency under Different Nitrogen Application Patterns in Chinese Double-Cropping Rice Systems. Agronomy 2022, 12, 1165. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Jiang, J.; Zheng, X.; Sass, R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, G.; Xu, Y.; Wang, L.; Lin, D.; Liang, X.; Shi, X. In situ stabilization remediation of cadmium contaminated soils of wastewater irrigation region using sepiolite. J. Environ. Sci. 2012, 24, 1799–1805. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Li, Y.-M.; Duan, Y.; Wang, G.-L.; Wang, A.-Q.; Shao, G.-Z.; Meng, X.-H.; Hu, H.-Y.; Zhang, D.-M. Straw alters the soil organic carbon composition and microbial community under different tillage practices in a meadow soil in Northeast China. Soil Tillage Res. 2021, 208, 104879. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, S.; Wang, T.; Yang, S.-Z.; Johannessen, B.; Chen, H.; Liu, C.; Ye, Y.; Wu, Y.; Peng, Y. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li–S batteries. Nano Lett. 2019, 20, 1252–1261. [Google Scholar] [CrossRef]
- Song, B.; Almatrafi, E.; Tan, X.; Luo, S.; Xiong, W.; Zhou, C.; Qin, M.; Liu, Y.; Cheng, M.; Zeng, G. Biochar-based agricultural soil management: An application-dependent strategy for contributing to carbon neutrality. Renew. Sustain. Energy Rev. 2022, 164, 112529. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar] [CrossRef]
- Khan, M.N.; Li, D.; Shah, A.; Huang, J.; Zhang, L.; Núñez-Delgado, A.; Han, T.; Du, J.; Ali, S.; Sial, T.A. The impact of pristine and modified rice straw biochar on the emission of greenhouse gases from a red acidic soil. Environ. Res. 2022, 208, 112676. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Keiluweit, M.; Yang, Y.; Yang, Y.; Jin, J.; Sun, H.; Wu, F.; Xing, B. Mobilization of ferrihydrite-associated organic carbon during Fe reduction: Adsorption versus coprecipitation. Chem. Geol. 2019, 503, 61–68. [Google Scholar] [CrossRef]
- Yao, J.; Qin, S.; Liu, T.; Clough, T.J.; Wrage-Mönnig, N.; Luo, J.; Hu, C.; Ge, T.; Zhou, S. Rice root Fe plaque enhances oxidation of microbially available organic carbon via Fe (III) reduction-coupled microbial respiration. Soil Biol. Biochem. 2022, 167, 108568. [Google Scholar] [CrossRef]
- Primožič, M.; Podrepšek, G.H.; Pavlovič, I.; Škerget, M.; Knez, Ž.; Leitgeb, M. Enzyme immobilization onto biochar produced by the hydrothermal carbonization of biomass. Acta Chim. Slov. 2019, 66, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Ergang, W.; Xing, Y.; Hanbo, C.; Sabry, M.S.; Binoy, S.; Song, X.; Hocheol, S.; Yong, L.; Rinklebe, J.; Deyi, H.; et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J. Hazard. Mater. 2021, 407, 124344. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Dash, P.K.; Swain, C.K.; Padhy, S.R.; Roy, K.S.; Neogi, S.; Berliner, J.; Adak, T.; Pokhare, S.S.; Baig, M.J.; et al. Mechanism of plant mediated methane emission in tropical lowland rice. Sci. Total Environ. 2019, 651, 84–92. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- He, Y.; Yao, Y.; Jia, Z.; Chen, X.; Zhou, L.; Shao, J.; Liu, R.; Zhou, G.; Fu, Y.; Sun, X.; et al. Antagonistic interaction between biochar and nitrogen addition on soil greenhouse gas fluxes: A global synthesis. Glob. Chang. Biol. Bioenergy 2021, 13, 1636–1648. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Q.; Fu, L.; Hu, Y.; Zhong, L.; Zhang, S.; Liu, Q.; Xie, Q. The effect of modified biochar on methane emission and succession of methanogenic archaeal community in paddy soil. Chemosphere 2022, 304, 135288. [Google Scholar] [CrossRef]
- Khan, M.N.; Huang, J.; Shah, A.; Li, D.; Daba, N.A.; Han, T.; Du, J.; Qaswar, M.; Anthonio, C.K.; Sial, T.A.; et al. Mitigation of greenhouse gas emissions from a red acidic soil by using magnesium-modified wheat straw biochar. Environ. Res. 2022, 203, 111879. [Google Scholar] [CrossRef]
- Gupta, K.; Kumar, R.; Baruah, K.K.; Hazarika, S.; Karmakar, S.; Bordoloi, N. Greenhouse gas emission from rice fields: A review from Indian context. Environ. Sci. Pollut. Res. 2021, 28, 30551–30572. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, J.; Ren, X.; Schaeffer, S.M. Effects of phosphorus modified nZVI-biochar composite on emission of greenhouse gases and changes of microbial community in soil. Environ. Pollut. 2021, 274, 116483. [Google Scholar] [CrossRef]
- Wu, F.; Jia, Z.; Wang, S.; Chang, S.X.; Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fertil. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
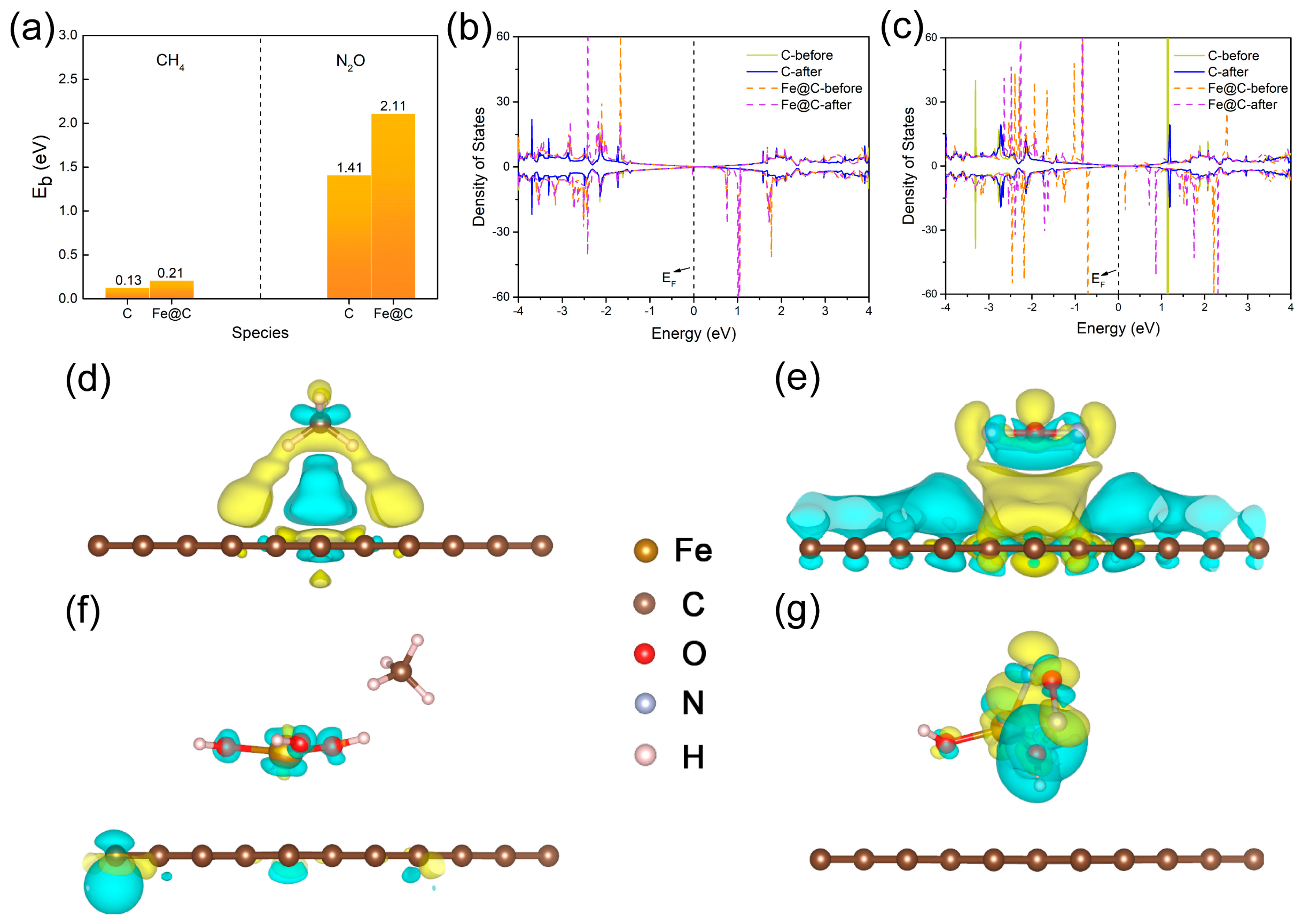

| Material | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AN (mg kg−1) | RAP (mg kg−1) | RAK (g kg−1) |
|---|---|---|---|---|---|---|
| Soil | 0.68 | 0.74 | 6.45 | 77.30 | 15.77 | 13.50 |
| Biochar | 0.26 | 5.77 | 47.20 | 176.00 | 242.00 | 22.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Zhang, Y.; Zhong, K.; Xiong, R.; Long, P.; Xu, Y.; Ma, X.; Wu, Q.; Wang, H.; Fu, Z. Ferrate-Modified Biochar for Greenhouse Gas Mitigation: First-Principles Calculation and Paddy Field Trails. Agronomy 2022, 12, 2661. https://doi.org/10.3390/agronomy12112661
Zhou W, Zhang Y, Zhong K, Xiong R, Long P, Xu Y, Ma X, Wu Q, Wang H, Fu Z. Ferrate-Modified Biochar for Greenhouse Gas Mitigation: First-Principles Calculation and Paddy Field Trails. Agronomy. 2022; 12(11):2661. https://doi.org/10.3390/agronomy12112661
Chicago/Turabian StyleZhou, Wentao, Yalan Zhang, Kangyu Zhong, Rui Xiong, Pan Long, Ying Xu, Xin Ma, Qing Wu, Hongrui Wang, and Zhiqiang Fu. 2022. "Ferrate-Modified Biochar for Greenhouse Gas Mitigation: First-Principles Calculation and Paddy Field Trails" Agronomy 12, no. 11: 2661. https://doi.org/10.3390/agronomy12112661
APA StyleZhou, W., Zhang, Y., Zhong, K., Xiong, R., Long, P., Xu, Y., Ma, X., Wu, Q., Wang, H., & Fu, Z. (2022). Ferrate-Modified Biochar for Greenhouse Gas Mitigation: First-Principles Calculation and Paddy Field Trails. Agronomy, 12(11), 2661. https://doi.org/10.3390/agronomy12112661






