Identification of ‘Calanda’-Type Peach Genotypes Tolerant to Monilinia laxa (Aderh. & Ruhland) Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Spores and Evaluation of Tolerance to Brown Rot
2.3. Determination of the Physicochemical Fruit Quality Traits
2.4. Determination of Antioxidant Compounds
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Basic Fruit Traits on the Population and Effect of Storage and Inoculation
3.2. Evaluation of Tolerance/Susceptibility to M. laxa in the Years 2014–2015
3.3. Biochemical Composition in Flesh
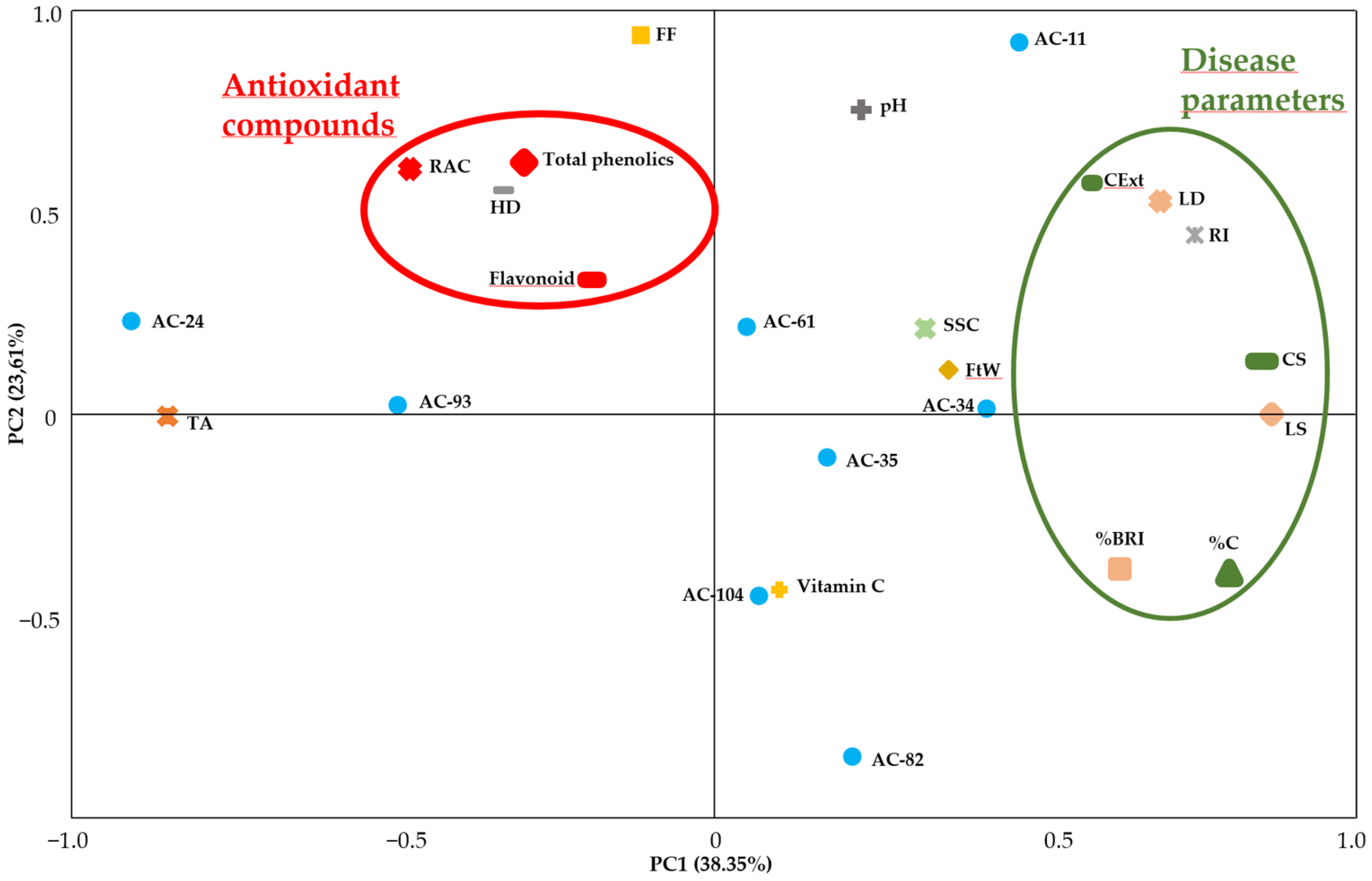
3.4. Selection of Genotypes with Better Quality Characteristics and Higher Tolerance to M. laxa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agricultural Organization of the United Nations. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 4 August 2022).
- Charrier, G.; Ngao, J.; Saudreau, M.; Méglio, T. Effects of environmental factors and management practices on microclimate, winter physiology, and frost resistance in trees. Front. Plant Sci. 2015, 6, 259. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.; Holtz, B.A.; Morgan, D.P.; Michailides, T.J. Significance of thinned fruit as a source of the secondary inoculum of Monilinia fructicola in California nectarine orchards. Plant Dis. 1997, 81, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Peach brown rot: Still in search of an ideal management option. Agriculture 2018, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Rungjindamai, N.; Jeffries, P.; Xu, X.M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Villarino, M.; Egüen, B.; Melgarejo, P.; Lamarca, N.; Segarra, J.; Usall, J.; Melgarejo, P.; de Cal, A. Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- De Cal, A.; Egüen, B.; Melgarejo, P. Vegetative compatibility groups and sexual reproduction among Spanish Monilinia fructicola isolates obtained from peach and nectarine orchards, but not Monilinia laxa. Fungal Biol. 2014, 118, 484–494. [Google Scholar] [CrossRef]
- Villarino, M.; Melgarejo, P.; De Cal, A. Growth and aggressiveness factors affecting Monilinia spp. survival peaches. Int. J. Food Microbiol. 2016, 224, 22–27. [Google Scholar] [CrossRef]
- Mari, M.; Casalini, L.; Baraldi, E.; Bertolini, P. Susceptibility of apricot and peach fruit to Monilinia laxa during phenological stages. Postharvest Biol. Technol. 2003, 30, 105–109. [Google Scholar] [CrossRef]
- Papavasileiou, A.; Testempasis, S.; Michailides, T.J.; Karaoglanidis, G.S. Frequency of brown rot fungi on blossoms and fruit in stone fruit orchards in Greece. Plant Pathol. 2015, 64, 416–424. [Google Scholar] [CrossRef]
- Bernat, M.; Segarra, J.; Casal, C.; Torres, R.; Teixidó, N.; Usall, J. Identification of fungal population in the environment and on surfaces of stone fruit packinghouses. Eur. J. Plant Pathol. 2017, 148, 723–731. [Google Scholar] [CrossRef]
- Egüen, B.; Melgarejo, P.; De Cal, A. Sensitivity of Monilinia fructicola from Spanish peach orchards to thiophanate-methyl, iprodione, and cyproconazole: Fitness analysis and competitiveness. Eur. J. Plant Pathol. 2015, 141, 789–801. [Google Scholar] [CrossRef]
- Casals, C.; Torres, R.; Teixidó, N.; De Cal, A.; Segarra, J.; Usall, J. Brown rot on stone fruit: From epidemiology studies to the development of effective control strategies. Sci. Hortic. 2022, 301, 111096. [Google Scholar] [CrossRef]
- Holb, I.J.; Schnabel, G. Differential effect of triazoles on mycelial growth and disease measurements of Monilinia fructicola isolates with reduced sensitivity to DMI fungicides. Crop Prot. 2007, 26, 753–759. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Egüen, B.; Melgarejo, P.; De Cal, A. The effect of fungicide resistance on the structure of Monilinia laxa populations in Spanish peach and nectarine orchards. Eur. J. Plant Pathol. 2016, 145, 815–827. [Google Scholar] [CrossRef]
- Mari, M.; Di Francesco, A.; Bertolini, P. Control of fruit postharvest diseases: Old issues and innovative approaches. Stewart Postharvest Rev. 2014, 10, 1–4. [Google Scholar] [CrossRef]
- Casals, C.; Guijarro, B.; De Cal, A.; Torres, R.; Usall, J.; Perdrix, V.; Hilscher, U.; Ladurner, E.; Smetsf, T.; Teixidó, N. Field validation of biocontrol strategies to control brown rot on stone fruit in several European countries. Pest Manag. Sci. 2021, 77, 2502–2511. [Google Scholar] [CrossRef]
- Kreidl, S.; Edwards, J.; Villalta, O.N. Assessment of pathogenicity and infection requirements of Monilinia species causing brown rot of stone fruit in Australian orchards. Australas. Plant Pathol. 2015, 44, 419–430. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Rubos, A.; Thomidis, T.; Tsipouridis, C.; Navrozidis, E.; Michailidou, O. Susceptibility of peach—Nectarine cultivars on brown rot infections. Ann. Univ. Oradea Fascicle Environ. Protect. 2008, 13, 214–217. [Google Scholar]
- Gradziel, T.M.; Wang, D. Evaluation of brown rot resistance and its relation to enzymatic browning in clingstone peach germplasm. J. Am. Soc. Hortic. Sci. 1993, 118, 675–679. [Google Scholar] [CrossRef]
- Quilot-Turion, B.; Ould-Sidi, M.-M.; Kadrani, A.; Hilgert, N.; Génard, M.; Lescourret, F. Optimization of parameters of the ‘Virtual Fruit’ model to design peach genotype for sustainable production systems. Eur. J. Agron. 2012, 42, 34–48. [Google Scholar] [CrossRef]
- Martínez-García, P.J.; Parfitt, D.E.; Bostock, R.M.; Fresnedo-Ramírez, J.; Vazquez-Lobo, A.; Ogundiwin, E.A.; Gradziel, T.M.; Crisosto, C.H. Application of genomic and quantitative genetic tools to identify candidate resistance genes for brown rot resistance in peach. PLoS ONE 2013, 8, e78634. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Torres, R.; Casals, C.; Teixidó, N.; Segarra, J.; Usall, J. Developing a methodology for identifying brown rot resistance in stone fruit. Eur. J. Plant Pathol. 2019, 154, 287–303. [Google Scholar] [CrossRef]
- Obi, V.; Barriuso, J.J.; Moreno, M.Á.; Giménez, R.; Gogorcena, Y. Optimizing protocols to evaluate brown rot (Monilinia laxa) susceptibility in peach and nectarine fruits. Australas. Plant Pathol. 2017, 46, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Villarino, M.; Sandín-España, P.; Melgarejo, P.; De Cal, A. High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agric. Food Chem. 2011, 59, 3205–3213. [Google Scholar] [CrossRef]
- Oliveira-Lino, L.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown rot strikes Prunus fruit: An ancient fight almost always lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef] [Green Version]
- Obi, V.; Barriuso, J.J.; Usall, J.; Gogorcena, Y. Breeding strategies for identifying superior peach genotypes resistant to brown rot. Sci. Hortic. 2019, 246, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Obi, V.; Montenegro, J.; Barriuso, J.J.; Saidani, F.; Aubert, C.; Gogorcena, Y. Is the tolerance of commercial peach cultivars to brown rot caused by Monilinia laxa modulated by its antioxidant content? Plants 2020, 9, 589. [Google Scholar] [CrossRef]
- Dos Santos, J.; Raseira, M.C.B.; Zanandrea, I. Resistance to brown rot in peach plants. Bragantia 2012, 71, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Gradziel, T.M. Changes in susceptibility to brown rot with ripening in three clingstone peach genotypes. J. Am. Soc. Hortic. Sci. 1994, 119, 101–105. [Google Scholar] [CrossRef]
- Fu, W.; da Silva Linge, C.; Gasic, K. Genome-wide association study of brown rot (Monilinia spp.) tolerance in peach. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Eduardo, I.; Usall, J.; Casals, C.; Arús, P.; Teixidó, N.; Torres, R. Exploring sources of resistance to brown rot in an interspecific almond × peach population. J. Sci. Food Agric. 2019, 99, 4105–4113. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, A.; Feliciano, A.J.; Ogawa, J.M. Monilinia fructicola resistance in the peach cultivar Bolinha. Phytopathology 1987, 77, 776–780. [Google Scholar] [CrossRef]
- Pacheco, I.; Bassi, D.; Eduardo, I.; Ciacciulli, A.; Pirona, R.; Rossini, L.; Vecchietti, A. QTL mapping for brown rot (Monilinia fructigena) resistance in an intraspecific peach (Prunus persica L. Batsch) F1 progeny. Tree Genet. Genomes 2014, 10, 1223–1242. [Google Scholar] [CrossRef]
- Dini, M.; Raseira, M.C.B.; Scariotto, S.; Ueno, B. Breeding peaches for brown rot resistance in Embrapa. Agronomy 2022, 12, 2306. [Google Scholar] [CrossRef]
- Monteagudo, A.; Font i Forcada, C.; Estopañán, G.; Dodd, R.S.; Alonso, J.M.; Rubio-Cabetas, M.J.; Fernandez i Marti, Á. Biochemical analyses and expression of cold transcription factors of the late PDO ‘Calanda’ peach under different post-harvest conditions. Sci. Hortic. 2018, 238, 116–125. [Google Scholar] [CrossRef]
- B. O. A. (Boletín Oficial de Aragón). Núm. 288. Available online: http://www.melocotondecalanda.com (accessed on 4 September 2022).
- Crisosto, C.H. Stone fruit maturity indices: A descriptive review. Postharvest News Inf. 1994, 5, 68. [Google Scholar]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Okamura, M. An improved method for determination of l-ascorbic acid and l-dehydroascorbic acid in blood plasma. Clin. Chim. Acta 1980, 103, 259–268. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-William, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Tecnol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Llácer, G.; Badenes, M.L. Peach breeding in Spain. Acta Hortic. 2012, 63–68. [Google Scholar] [CrossRef]
- Anthony, B.R.; Phillips, D.J.; Badr, S.; Aharoni, Y. Decay control and quality maintenance after moist air heat treatment of individually plastic-wrapped nectarines. J. Am. Soc. Hortic. Sci. 1989, 114, 946–949. [Google Scholar] [CrossRef]
- Casals, C.; Teixidó, N.; Viñas, I.; Llauradó, S.; Usall, J. Control of Monilinia spp. on stone fruit by curing treatments. Part I. The effect of temperature, exposure time and relative humidity on curing efficacy. Postharvest Biol. Technol. 2010, 56, 19–25. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, S.; Sun, D.W.; Wang, Z. Effects of heat treatment on postharvest quality of peaches. J. Food Eng. 2002, 54, 17–22. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Bostock, R.M.; Adaskaveg, J.E. Resistance to brown rot disease in peach is determined by multiple structural and biochemical components. Acta Hortic. 2003, 622, 347–352. [Google Scholar] [CrossRef]
- Oliveira-Lino, L.; Quilot-Turion, B.; Dufour, C.; Corre, M.-N.; Lessire, R.; Génard, M.; Poësel, J.-L. Cuticular waxes of nectarines (Prunus persica L. Batsch) during fruit development in relation to surface conductance and susceptibility to Monilinia laxa. J. Exp. Bot. 2020, 71, 5521–5537. [Google Scholar] [CrossRef]
- Mustafa, M.H.; Bassi, D.; Corre, M.N.; Oliveira-Lino, L.; Signoret, V.; Quilot-Turion, B.; Cirilli, M. Phenotyping brown rot susceptibility in stone fruit: A literature review with emphasis on peach. Horticulturae 2021, 7, 115. [Google Scholar] [CrossRef]
- Kappel, F.; Sholberg, P.L. Screening sweet cherry cultivars from the Pacific Agri-Food Research Centre Summerland breeding program for resistance to brown rot (Monilinia fructicola). Can. J. Plant Sci. 2008, 88, 747–752. [Google Scholar] [CrossRef]
- Xu, X.M.; Guerin, L.; Robinson, J.D. Effects of temperature and relative humidity on conidial germination and viability, colonization and sporulation of Monilinia fructigena. Plant Pathol. 2001, 50, 561–568. [Google Scholar] [CrossRef]
- Burnett, A.L.; Lalancette, N.; McFarland, K.A. Effect of QoI fungicides on colonization and sporulation of Monilinia fructicola on peach fruit and blossom blight cankers. Plant Dis. 2010, 94, 1000–1008. [Google Scholar] [CrossRef]
- Obi, V.; Barriuso, J.; Gogorcena, Y. Effects of pH and titratable acidity on the growth and development of Monilinia laxa (Aderh. & Ruhl.) in vitro and in vivo. Eur. J. Plant Pathol. 2018, 145, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Lopresti, J.; Goodwin, I.; McGlasson, B.; Holford, P.; Golding, J. Variability in size and soluble solids concentration in peaches and nectarines. Hortic. Rev. 2014, 42, 253–311. [Google Scholar] [CrossRef]
- Cantín, C.M.; Moreno, M.Á.; Gogorcena, Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) Batsch] breeding progenies. J. Agric. Food Chem. 2009, 57, 4586–4592. [Google Scholar] [CrossRef]
- Abidi, W.; Cantín, C.M.; Jiménez, S.; Giménez, R.; Moreno, M.Á.; Gogorcena, Y. Influence of antioxidant compounds, total sugars and genetic background on the chilling injury susceptibility of a non-melting peach (Prunus persica (L.) Batsch) progeny. J. Sci. Food Agric. 2015, 95, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Milatović, D.; Nikolić, D.; Durović, D. Variability, heritability and correlations of some factors affecting productivity in peach. Hortic. Sci. 2010, 37, 79–87. [Google Scholar] [CrossRef]

| Progeny | ‘Andross’ | ‘Calante’ | ||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014–2015 | 2014–2015 | |||||
| Parameters * | N (2014–2015) | Minimum | Maximum | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Harvest date (HD) | 42-8 | 222 | 266 | 248 ± 2.38 | 254 ± 4.70 | 249 ± 2.15 | 241 ± 3.50 | 278 ± 0.50 |
| Fruit weight (FtW) | 41-8 | 158.82 | 364.00 | 210.80 ± 5.55 | 249.97 ± 7.62 | 217.19 ± 5.22 | 166.55 ± 4.55 | 295.05 ± 9.40 |
| Fruit firmness (FF) | 274-60 | 14.21 | 66.15 | 38.72 ± 0.62 | 33.89 ± 1.13 | 37.86 ± 0.58 | 28.12 ± 2.31 | 45.26 ± 1.51 |
| SSC | 42-8 | 8.40 | 13.00 | 11.00 ± 0.18 | 11.26 ± 0.53 | 11.04 ± 0.17 | 14.15 ± 0.38 | 14.55 ± 0.32 |
| pH | 42-8 | 3.80 | 4.04 | 4.38 ± 0.06 | 3.90 ± 0.03 | 4.30 ± 0.05 | 4.31 ± 0.03 | 3.65 ± 0.03 |
| Titratable acidity (TA) | 42-8 | 0.24 | 0.86 | 0.41 ± 0.01 | 0.63 ± 0.04 | 0.45 ± 0.02 | 0.35 ± 0.01 | 0.72 ± 0.02 |
| Ripening index (RI) | 42-8 | 12.80 | 40.96 | 27.60 ± 0.89 | 18.25 ± 1.26 | 26.11 ± 0.91 | 40.69 ± 0.83 | 20.09 ± 0.81 |
| Genotype | HD | FtW | FF Harvest | FF Incubated | FF Inoculated | SSC Harvest | SSC Incubated | SSC Inoculated | pH | TA | RI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC-11 | 258 b | 235.25 | 48.76 c | 36.20 c | 31.56 f | 10.80 | 10.19 abcd | 10.09 cd | 4.78 | 0.46 | 28.75 |
| AC-24 | 262 b | 216.46 | 39.99 b | 28.13 a | 26.36 cd | 10.75 | 9.28 ab | 10.66 d | 4.31 | 0.65 | 18.29 |
| AC-34 | 258 b | 251.52 | 34.70 ab | 30.33 ab | 29.00 e | 12.40 | 12.00 e | 10.86 d | 4.11 | 0.51 | 25.81 |
| AC-35 | 235 a | 225.57 | 32.60 ab | 30.04 ab | 24.20 bc | 11.05 | 11.39 cde | 9.53 bc | 4.03 | 0.49 | 22.74 |
| AC-61 | 258 b | 222.56 | 37.40 b | 35.40 c | 28.05 de | 11.45 | 11.78 de | 10.45 d | 4.23 | 0.49 | 23.28 |
| AC-82 | 226 a | 237.96 | 27.76 a | 26.12 a | 22.97 b | 10.90 | 10.90 bcde | 10.21 cd | 4.00 | 0.50 | 22.39 |
| AC-93 | 262 b | 236.86 | 40.34 b | 33.42 bc | 32.24 f | 10.00 | 9.90 abc | 9.26 b | 3.85 | 0.61 | 16.65 |
| AC-104 | 262 b | 204.27 | 32.83 ab | 26.36 a | 18.72 a | 9.25 | 9.07 a | 8.27 a | 4.15 | 0.48 | 19.20 |
| Progeny | ‘Andross’ | ‘Calante’ | ||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014–2015 | 2014–2015 | |||||
| Parameters # | N (2014–2015) | Minimum | Maximum | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| % Brown rot incidence | 42-8 | 45.00 | 100.00 | 90.26 ± 1.83 | 93.75 ± 3.10 | 90.82 ± 1.62 | 70.00 ± 30.00 | 87.50 ± 12.50 |
| Lesion diameter | 690-150 | 7.19 | 79.89 | 52.51 ± 0.68 | 45.84 ± 0.65 | 51.32 ± 0.57 | 33.28 ± 2.60 | 53.55 ± 3.48 |
| Lesion severity | 690-150 | 4.67 | 76.63 | 48.69 ± 0.68 | 43.47 ± 0.69 | 47.76 ± 0.58 | 30.29 ± 3.19 | 50.07 ± 4.02 |
| % Colonization | 42-8 | 20.00 | 100.00 | 71.75 ± 3.37 | 89.38 ± 4.95 | 74.57 ± 3.06 | 90.00 nd | 65.00 ± 35.00 |
| Colonization extent | 543-143 | 11.67 | 71.94 | 49.91 ± 0.54 | 40.71 ± 0.64 | 47.99 ± 0.47 | 39.93 ± 0.84 nd | 51.73 ± 3.36 |
| Colonization severity | 543-143 | 4.03 | 68.73 | 39.47 ± 0.64 | 37.29 ± 0.73 | 39.01 ± 0.53 | 35.94 ± 0.76 nd | 47.52 ± 4.63 |
| %BRI | LD | LS | %C | CExt | CS | |
|---|---|---|---|---|---|---|
| Principal factors | ||||||
| Year | ||||||
| 2014 | 73 a | 39.71 a | 30.22 a | 41 a | 40.11 | 19.80 a |
| 2015 | 94 b | 45.84 b | 43.48 b | 89 b | 40.71 | 37.29 b |
| Genotypes | ||||||
| AC-11 | 84 | 48.39 | 43.31 d | 69 | 45.85 c | 38.03 c |
| AC-24 | 65 | 40.84 | 29.17 a | 50 | 35.58 a | 24.57 a |
| AC-34 | 93 | 44.62 | 41.77 cd | 75 | 40.49 abc | 32.47 b |
| AC-35 | 86 | 44.00 | 38.69 bcd | 68 | 43.89 bc | 36.42 bc |
| AC-61 | 82 | 45.62 | 40.83 cd | 62 | 43.55 bc | 35.36 bc |
| AC-82 | 88 | 41.82 | 36.71 bc | 75 | 34.87 a | 26.45 a |
| AC-93 | 90 | 38.87 | 35.86 bc | 63 | 39.18 ab | 33.05 b |
| AC-104 | 83 | 41.87 | 34.59 b | 63 | 40.59 abc | 27.76 a |
| Interaction | ||||||
| 2014 | ||||||
| AC-11 | 69 | 45.79 | 31.48 bcd | 38 | 54.21 d | 20.33 cdef |
| AC-24 | 45 | 40.06 | 18.02 a | 20 | 32.46 ab | 6.49 a |
| AC-34 | 85 | 41.30 | 35.11 cde | 55 | 40.83 abc | 22.46 defg |
| AC-35 | 65 | 38.52 | 25.04 ab | 35 | 44.31 c | 15.51 bcd |
| AC-61 | 65 | 38.21 | 24.73 ab | 29 | 44.03 c | 12.95 abc |
| AC-82 | 95 | 39.98 | 37.98 de | 90 | 30.74 a | 27.67 fgh |
| AC-93 | 80 | 33.88 | 27.10 bc | 25 | 40.87 abc | 10.22 ab |
| AC-104 | 80 | 41.19 | 32.95 bcd | 40 | 46.83 cd | 18.73 cde |
| 2015 | ||||||
| AC-11 | 100 | 49.82 | 49.82 f | 100 | 43.34 c | 43.34 j |
| AC-24 | 85 | 41.26 | 35.07 cde | 80 | 36.36 abc | 29.09 gh |
| AC-34 | 100 | 47.73 | 47.43 f | 95 | 40.29 abc | 38.27 ij |
| AC-35 | 100 | 47.56 | 47.56 f | 100 | 43.74 c | 43.74 j |
| AC-61 | 100 | 49.69 | 49.69 f | 95 | 43.43 c | 41.26 j |
| AC-82 | 80 | 44.01 | 35.21 cde | 60 | 41.06 bc | 24.63 efgh |
| AC-93 | 100 | 42.86 | 42.86 ef | 100 | 38.76 abc | 38.76 ij |
| AC-104 | 85 | 42.50 | 36.13 de | 85 | 37.65 abc | 32.01 hi |
| Significance | ||||||
| Year | * | *** | *** | *** | ns | *** |
| Genotype | ns | ns | *** | ns | *** | *** |
| Year × Genotype | ns | *** | ** | *** |
| Progeny | ‘Andross’ | ‘Calante’ | ||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014–2015 | 2014–2015 | |||||
| Parameters * | N (2014–2015) | Minimum | Maximum | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Vitamin C | 84-23 | 0.72 | 19.53 | 10.50 ± 0.46 | 7.45 ± 0.56 | 9.84 ± 0.40 | 14.91 ± 1.11 | 10.72 ± 0.81 |
| Total phenolics | 84-24 | 77.64 | 96.27 | 58.80 ± 1.74 | 51.25 ± 2.44 | 57.12 ± 1.49 | 77.09 ± 3.77 | 93.65 ± 16.80 |
| Flavonoids | 115-22 | 11.45 | 58.90 | 28.29 ± 0.94 | 22.77 ± 1.41 | 27.40 ± 0.84 | 53.92 ± 7.56 | 87.90 ± 16.46 |
| RAC | 84-24 | 14.83 | 117.91 | 43.72 ± 2.04 | 91.74 ± 2.63 | 54.39 ± 2.55 | 89.41 ± 2.71 | 122.87 ± 13.94 |
| Vitamin C | Total Phenolics | Flavonoids | RAC | |
|---|---|---|---|---|
| Principal factors | ||||
| Year | ||||
| 2014 | 8.52 b | 65.88 b | 28.72 b | 51.43 a |
| 2015 | 7.45 a | 51.25 a | 22.77 a | 91.74 b |
| Genotype | ||||
| AC-11 | 6.41 a | 60.04 c | 24.39 bc | 83.80 c |
| AC-24 | 6.34 a | 58.16 c | 26.50 bc | 77.11 bc |
| AC-34 | 5.72 a | 57.41 c | 23.25 b | 71.21 ab |
| AC-35 | 8.38 b | 66.44 d | 31.45 de | 75.72 bc |
| AC-61 | 10.44 c | 65.71 d | 35.00 e | 70.95 ab |
| AC-82 | 11.41 c | 42.95 b | 22.95 b | 65.33 a |
| AC-93 | 7.80 b | 68.22 d | 29.05 cd | 94.76 d |
| AC-104 | 6.58 a | 37.89 a | 15.62 a | 66.03 a |
| Interaction | ||||
| 2014 | ||||
| AC-11 | 7.03 cd | 64.05 fg | 25.39 cd | 59.81 cd |
| AC-24 | 6.99 cd | 88.21 i | 36.93 e | 86.53 ef |
| AC-34 | 6.90 cd | 63.26 fg | 26.46 d | 36.86 ab |
| AC-35 | 9.21 ef | 63.54 fg | 36.42 e | 40.10 ab |
| AC-61 | 9.86 fg | 71.78 h | 34.65 e | 48.60 bc |
| AC-82 | 10.67 fgh | 44.93 c | 23.45 bcd | 30.53 a |
| AC-93 | 11.27 gh | 87.00 i | 35.06 e | 72.09 d |
| AC-104 | 6.23 bcd | 44.23 bc | 15.50 a | 36.91 ab |
| 2015 | ||||
| AC-11 | 6.00 abcd | 57.37 def | 23.39 bcd | 99.80 fg |
| AC-24 | 5.92 abc | 38.13 ab | 16.07 ab | 70.84 d |
| AC-34 | 4.93 abc | 53.51 d | 18.44 abc | 94.12 ef |
| AC-35 | 7.82 de | 68.38 gh | 26.49 d | 99.47 fg |
| AC-61 | 10.82 fgh | 61.66 efg | 35.23 e | 85.85 ef |
| AC-82 | 11.90 h | 41.62 bc | 22.45 abcd | 88.53 ef |
| AC-93 | 4.32 a | 55.70 de | 23.04 bcd | 109.87 g |
| AC-104 | 6.82 cd | 33.66 a | 15.73 a | 85.45 e |
| Significance | ||||
| Year | *** | *** | *** | *** |
| Genotype | *** | *** | *** | *** |
| Year × Genotype | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, J.; Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Identification of ‘Calanda’-Type Peach Genotypes Tolerant to Monilinia laxa (Aderh. & Ruhland) Honey. Agronomy 2022, 12, 2662. https://doi.org/10.3390/agronomy12112662
Montenegro J, Obi VI, Barriuso JJ, Gogorcena Y. Identification of ‘Calanda’-Type Peach Genotypes Tolerant to Monilinia laxa (Aderh. & Ruhland) Honey. Agronomy. 2022; 12(11):2662. https://doi.org/10.3390/agronomy12112662
Chicago/Turabian StyleMontenegro, Joaquín, Vitus Ikechukwu Obi, Juan Jose Barriuso, and Yolanda Gogorcena. 2022. "Identification of ‘Calanda’-Type Peach Genotypes Tolerant to Monilinia laxa (Aderh. & Ruhland) Honey" Agronomy 12, no. 11: 2662. https://doi.org/10.3390/agronomy12112662






