Potential Strategies in the Biopesticide Formulations: A Bibliometric Analysis
Abstract
:1. Introduction
2. Bibliometric Analysis of Biopesticide Formulations
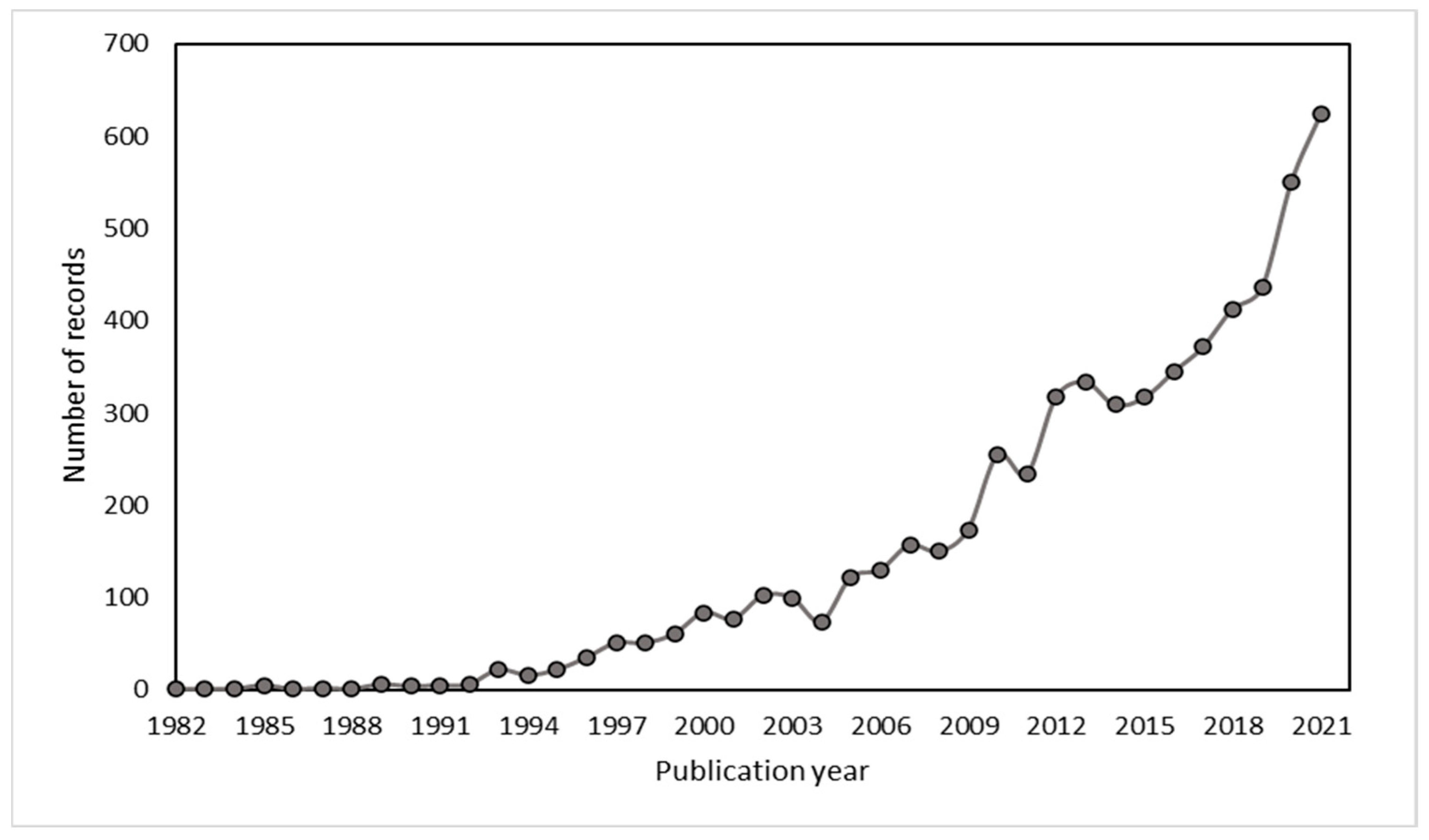
2.1. Scientific Production
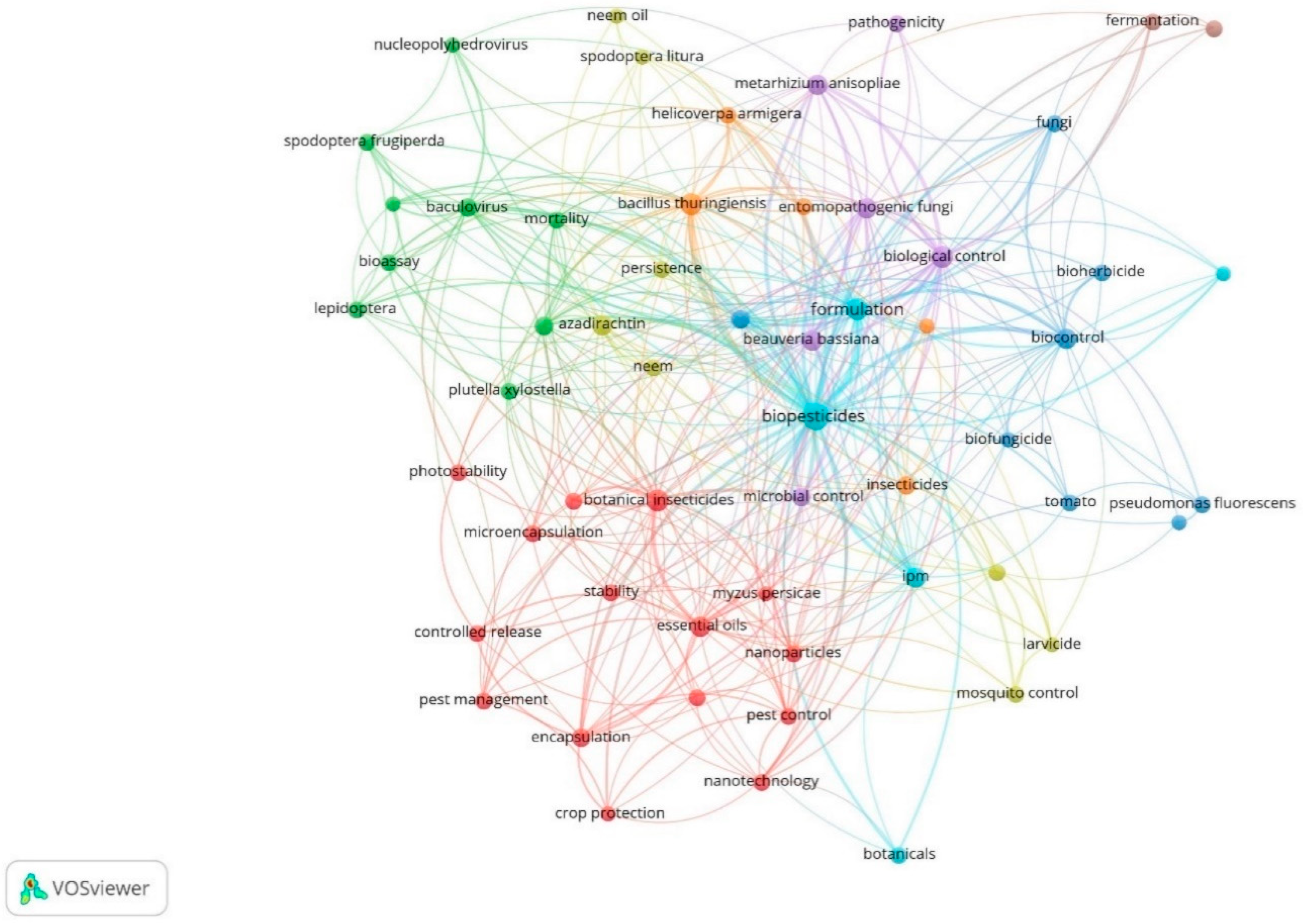
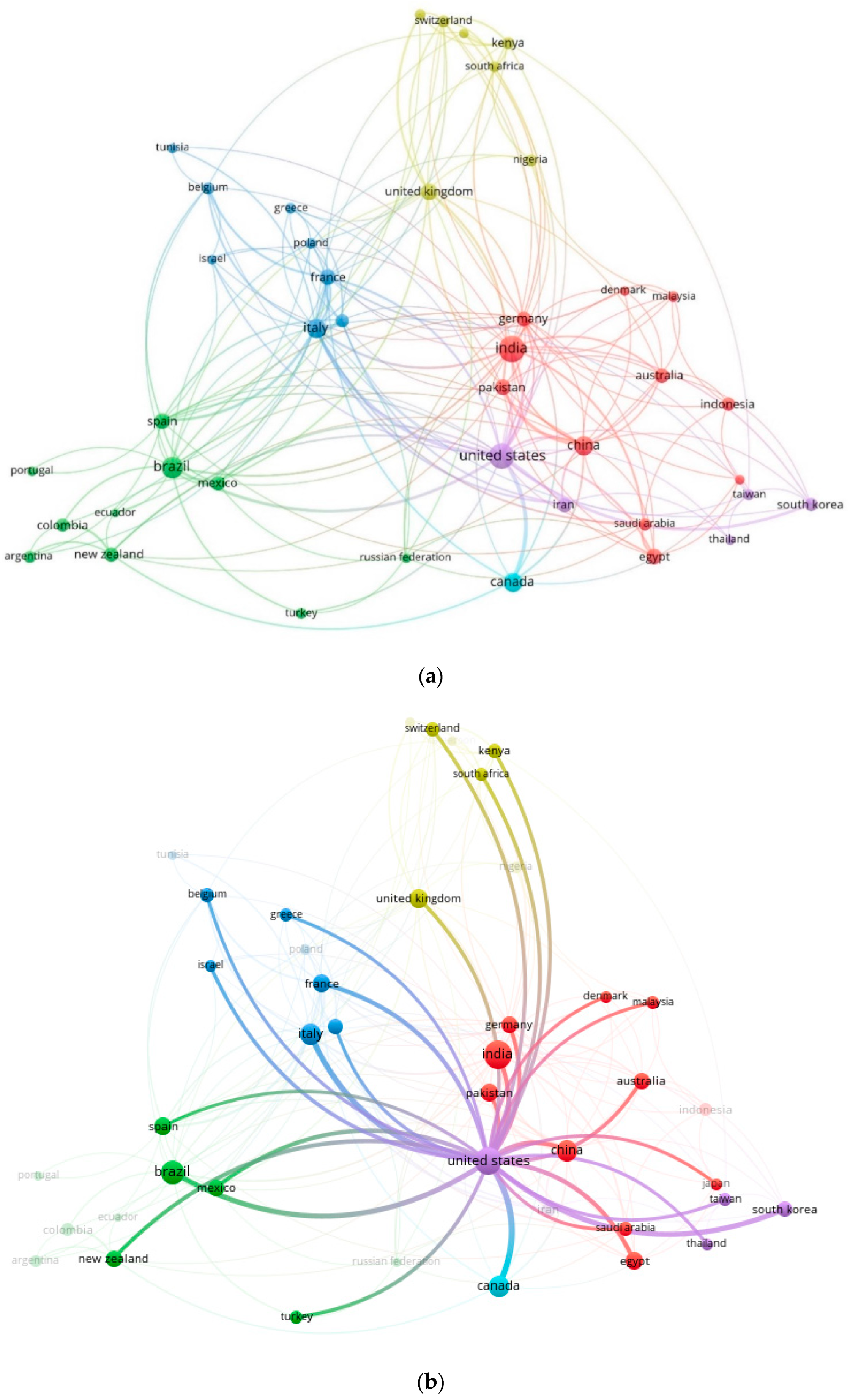
2.2. Co-Occurrence and Co-Authorship Analysis
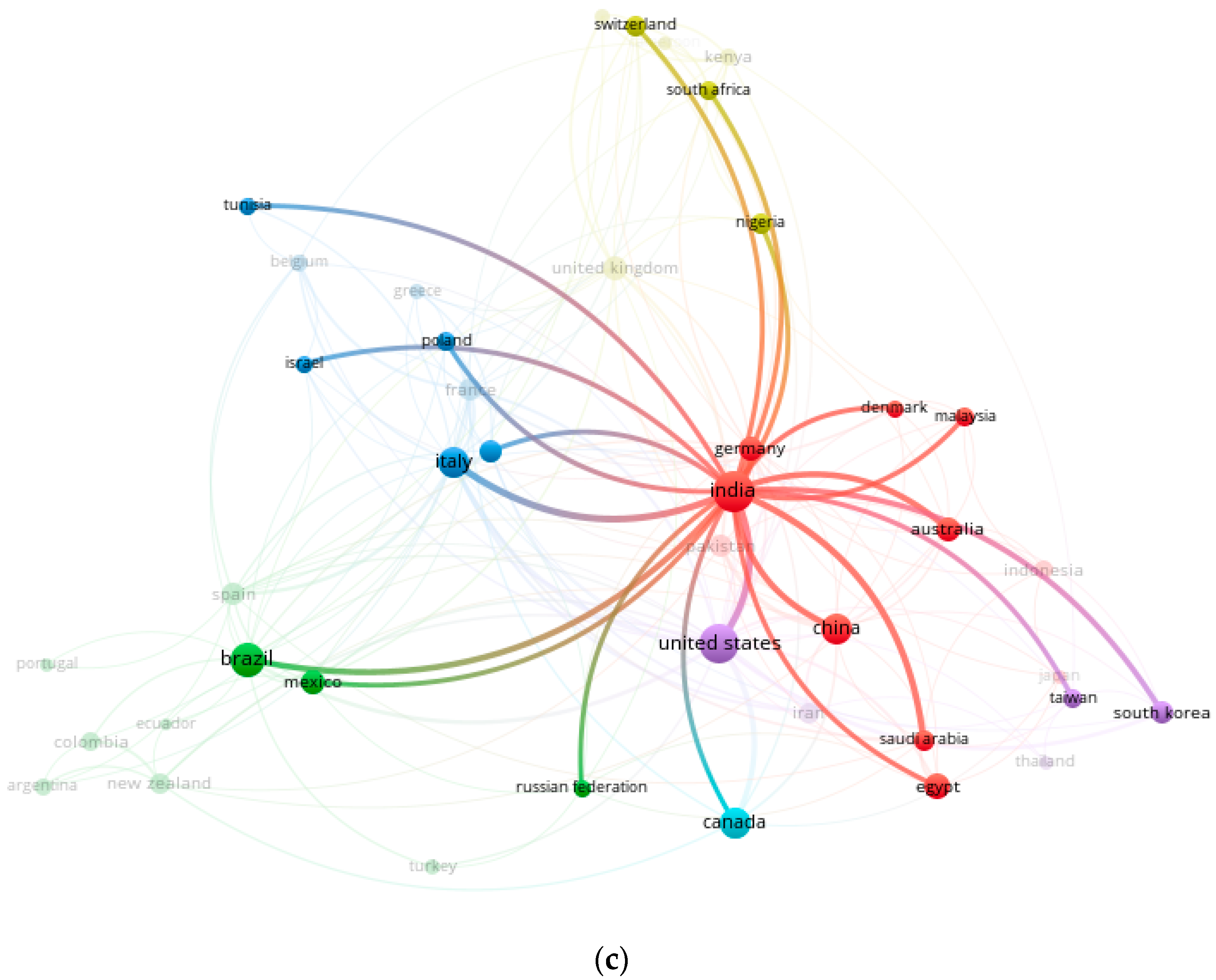
2.3. Contingency Matrix, Sankey Diagram, and Historical Map
3. Potential Strategies in the Biopesticide Formulations
3.1. Microbial and Botanical Biopesticides
| Compounds | Botanical Sources | Target Pests | Formulation | References |
|---|---|---|---|---|
| Terpenes | ||||
| β-caryophyllene, α-humulene, α-bergamotene/β-bergamotene, and α-santalene | Solanum habrochaites | Macrosiphum euphorbiae | Leafs extracts | [95] |
| Azadirachtin | Azadirachta indica | Drosophila melanogaster, Myzus persicae, Spodoptera litura, Bactrocera dorsalis, Anticarsia gemmatalis | Emulsions | [102,103,104,105,106] |
| Azadirachtin | Azadirachta indica | Not reported | Nanoemulsion | [107] |
| Azadirachtin | Azadirachta indica | Not reported | Encapsulation | [108] |
| α-pinene, linalool | Various spice plants | Spodoptera litura, Achaea Janata | Nanoparticles | [109] |
| Eugenol | clove essential oil | Sitophilus zeamais | Suspensions | [110] |
| Eugenol | Not reported | Sf9 cell line (Spodoptera frugiperda) | Suspensions | [111] |
| β-caryophyllene | Not reported | Hypothenemus hampei | Aqueous suspension | [112] |
| Limonene | Orange essential oil | Tribolium confusum and Cryptolestes ferrugineus | Nanoemulsions | [113] |
| Limonene and α-pinene | Baccharis reticularia | Tribolium castaneum | Nanoemulsions | [114] |
| Carvacrol, geraniol, eugenol, thymol | Not reported | Ditylenchus dipsaci | Biomass extracts | [115] |
| Sabinene, β-caryophyllene, terpinolene, pinene, limonene | Hyptis suaveolens, Hyptis spicigera | Sitophilus granirius | Emulsions | [116] |
| Oxygenated monoterpenes | Mentha pulegium, Mentha suaveolens | Toxoptera aurantii | Biomass extracts | [117] |
| β-caryophyllene, caryophyllene oxide, epiglobulol | Atalantia buxifolia | Tribolium castaneum, Lasioderma serricorne, Liposcelis bostrychophila | Biomass extracts | [118] |
| Flavonoids | ||||
| Naringenin, hesperidin | Not reported | Xylella fastidiosa | Syringe application | [119] |
| Pinocembrin | Fluorensia oolepis | Epilachna paenulata, Xanthogaleruca luteola, Spodoptera frugiperda | Ethanolics extracts | [97] |
| Miricitine, naringenina, quercetina | Cynara cardunculos | Trifolium incarnatum | Emulsions | [120] |
| Flavonoids from roots, stalks and fruits | Withania somnífera, Terminalia chebula | Furarium oxysporum | Biomass extracts | [121] |
| Naringine, naringenine, hesperidine and its Cu2+ complexes | Not reported | Spodoptera frugiperda | Suspensions | [122] |
| Tetrahydrocurcumin | Curcuma | Fusarium graminearum | Encapsulation | [123] |
| Quercetin, chlorogenic acid, rutin | Not reported | Helicoverpa argimera, Spodoptera lutira | Oral Infection | [124] |
| Flavonoids from plant tissue | Calotropis procera | Callosobruchus chinensis | Methanolic extracts | [125] |
| Alkaloids | ||||
| Lupanine | Lupinus | Arion vulgaris, Arion rufus, Deroceras reticulatum | Oral Infection | [126] |
| Berberine | Berberis | Bipolaris oryzae, Curvularia lunata, Pyricularia oryzae, Rhizoctonia solani | Aqueos extracts | [99] |
| Matrine | Sophora flavescens | Diaphorina citri, Panonychus citri, Sitophilus zeamais, Spodoptera frugiperda | Emulsions | [127] |
| Alkaloides N-Phenilsulfonylmatrinics and N-bencilmatrinics | Organic synthesis | Mythimna, Aphis citricola | Organic solvents Extracts | [128] |
| Sarmentine, sarmentosina | Piper sarmentosum | Echinochloa crusgalli, Amaranthus retroflexus | Emulsions | [129] |
| Berberine | Cotis chinensis | Bidens pilosa | Aqueos extracts | [130] |
| Palmatine, Jatrorrizine | Tinospora capillipes | Colletotrichum gloeosporioides, Fusarium oxysporum, Mycosphaerella sentina, Pestalotia mangiferae, Cercospora kaki, Gymnosporagium haraeanum, Rhizoctonia solani, Colletotrichum graminicola | Aqueos extracts | [131] |
| Tylophorine, tylophorinine, isotylocrebrine | Tylophora indica | Helicoverpa armígera | Organic solvents Extracts | [132] |
| Flindersine | Toddalia asiatica | Helicoverpa armígera, Spodoptera litura | Organic solvents Extracts | [133] |
3.2. Emulsions
3.3. Suspension Concentrates
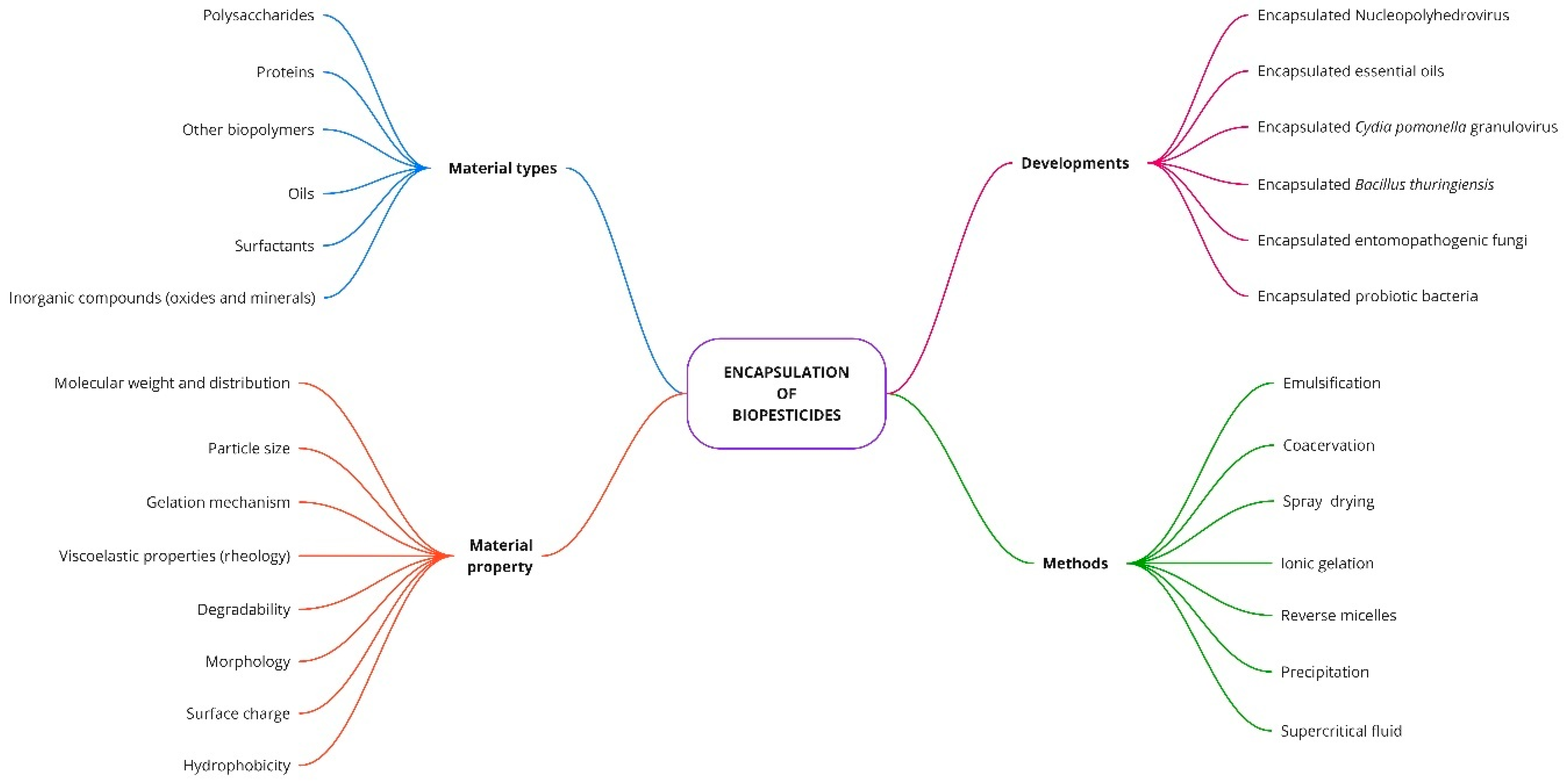
3.4. Encapsulation
3.5. Hydrogels
3.6. Nanoformulations
4. Overall Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, K.D.; Mobolade, A.J.; Bharali, R.; Sahoo, D.; Rajashekar, Y. Main Plant Volatiles as Stored Grain Pest Management Approach: A Review. J. Agric. Food Res. 2021, 4, 100127. [Google Scholar] [CrossRef]
- Memon, Q.U.A.; Wagan, S.A.; Chunyu, D.; Shuangxi, X.; Jingdong, L.; Damalas, C.A. Health Problems from Pesticide Exposure and Personal Protective Measures among Women Cotton Workers in Southern Pakistan. Sci. Total Environ. 2019, 685, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wafa, T.; Nadia, K.; Amel, N.; Ikbal, C.; Insaf, T.; Asma, K.; Hedi, M.A.; Mohamed, H. Oxidative Stress, Hematological and Biochemical Alterations in Farmers Exposed to Pesticides. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 1058–1069. [Google Scholar] [CrossRef]
- Sarker, S.; Akbor, M.A.; Nahar, A.; Hasan, M.; Islam, A.R.M.T.; Siddique, M.A.B. Level of Pesticides Contamination in the Major River Systems: A Review on South Asian Countries Perspective. Heliyon 2021, 7, e07270. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of Mechanisms and Uses of Biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Marcinkevičienė, A.; Čmukas, A.; Velicka, R.; Kosteckas, R.; Skinuliene, L. Effects of Biopesticides and Undersown Cover Crops on Soil Properties in the Organic Farming System. Agronomy 2022, 12, 2153. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Malinga, L.N.; Laing, M.D. Efficacy of Three Biopesticides against Cotton Pests under Field Conditions in South Africa. Crop Prot. 2021, 145, 105578. [Google Scholar] [CrossRef]
- Nyangau, P.; Muriithi, B.; Diiro, G.; Akutse, K.S.; Subramanian, S. Farmers’ Knowledge and Management Practices of Cereal, Legume and Vegetable Insect Pests, and Willingness to Pay for Biopesticides. Int. J. Pest Manag. 2020, 68, 204–216. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E. A Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Keswani, C. (Ed.) Bioeconomy for Sustainable Development; Springer: Pradesh, India, 2019; Volume 14, ISBN 9789811394300. [Google Scholar]
- Bashir, O.; Claverie, J.P.; Lemoyne, P.; Vincent, C. Controlled-Release of Bacillus Thurigiensis Formulations Encapsulated in Lightresistant Colloidosomal Microcapsules for the Management of Lepidopteran Pests of Brassica Crops. PeerJ 2016, 4, e2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akash, M.; Anfal, A.; Shraddha., P.M.; Madhu, B. Microbe-Based Biopesticide Formulation: A Tool for Crop Protection and Sustainable Agriculture Development. In Microbial Technology for the Welfare of Society; Springer: Berlin/Heidelberg, Germany, 2019; Volume 17, pp. 125–145. ISBN 978-981-13-8843-9. [Google Scholar]
- Kala, S.; Sogan, N.; Agarwal, A.; Naik, S.N.; Patanjali, P.K.; Kumar, J. Biopesticides: Formulations and Delivery Techniques. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2019; pp. 209–220. ISBN 9780128193044. [Google Scholar]
- Ndao, A.; Kumar, L.R.; Tyagi, R.D.; Valéro, J. Biopesticide and Formulation Processes Based on Starch Industrial Wastewater Fortified with Soybean Medium. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Tenorio, F.; Giraldo-Estrada, C. Characterization and Chemical Modification of Pullulan Produced from a Submerged Culture of Aureobasidium Pullulans ATCC 15233. Polym. Test 2022, 114, 107686. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Ocampo, D.; Vargas, G.J.; Sáez, A.A. Trends on CO2 Capture with Microalgae: A Bibliometric Analysis. Molecules 2022, 27, 4669. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for Nano-Biotechnology Enabled Protection and Nutrition of Plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Debnath, N.; Das, S.; Seth, D.; Chandra, R.; Bhattacharya, S.C.; Goswami, A. Entomotoxic Effect of Silica Nanoparticles against Sitophilus Oryzae (L.). J. Pest Sci. 2011, 84, 99–105. [Google Scholar] [CrossRef]
- Bateman, R.P.; Carey, M.; Moore, D.; Prior, C. The Enhanced Infectivity of Metarhizium Flavoviride in Oil Formulations to Desert Locusts at Low Humidities. Ann. Appl. Biol. 1993, 122, 145–152. [Google Scholar] [CrossRef]
- Lomer, C.J.; Bateman, R.P.; Johnson, D.L.; Langewald, J.; Thomas, M. Biological Control of Locusts and Grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Viljoen, A.M. Geraniol—A Review of a Commercially Important Fragrance Material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Faria, M.; Wraight, S.P. Biological Control of Bemisia Tabaci with Fungi. Crop Prot. 2001, 20, 767–778. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Aly Hassan, A.; Kim, K.-H. Nano-Based Smart Pesticide Formulations: Emerging Opportunities for Agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of Nanotechnology for the Encapsulation of Botanical Insecticides for Sustainable Agriculture: Prospects and Promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [Green Version]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The Science, Development, and Commercialization of Postharvest Biocontrol Products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Montesinos, E. Development, Registration and Commercialization of Microbial Pesticides for Plant Protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Villena de Francisco, E.; García-Estepa, R.M. Nanotechnology in the Agrofood Industry. J. Food Eng. 2018, 238, 1–11. [Google Scholar] [CrossRef]
- Ubando, A.T.; Conversion, A.; Barroca, R.B.; Enano, N.H.; Espina, R.U. Computational Fluid Dynamics on Solar Dish in a Concentrated Solar Power: A Bibliometric Review. Solar 2022, 2, 251–273. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.H.; Chang, J.S. Microalgal Biosorption of Heavy Metals: A Comprehensive Bibliometric Review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef]
- Hernandez-Tenorio, F.; Arroyave-Miranda, H.; Miranda, A.M.; González, S.M.; Rodríguez, C.A.; Sáez, A.A. Improving Deproteinization in Colombian Latex from Hevea Brasiliensis: A Bibliometric Approximation. Polymers 2022, 14, 4248. [Google Scholar] [CrossRef]
- Maniquiz-Redillas, M.; Robles, M.E.; Cruz, G.; Reyes, N.J.; Kim, L.H. First Flush Stormwater Runoff in Urban Catchments: A Bibliometric and Comprehensive Review. Hydrology 2022, 9, 63. [Google Scholar] [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial Biopesticides for Invertebrate Pests and Their Markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kamle, M.; Borah, R.; Mahato, D.K.; Sharma, B. Bacillus Thuringiensis as Microbial Biopesticide: Uses and Application for Sustainable Agriculture. Egypt. J. Biol. Pest Control 2021, 31, 1–7. [Google Scholar] [CrossRef]
- Frankenhuyzen, K. van Insecticidal Activity of Bacillus Thuringiensis Crystal Proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.L.B.; Linh, N.N.; Ha, N.H.; Van Dong, N.; Hien, L.T.T. Genome Sequence of a Vietnamese Bacillus Thuringiensis Strain TH19 Reveals Two Potential Insecticidal Crystal Proteins against Etiella Zinckenella Larvae. Biol. Control 2021, 152, 104473. [Google Scholar] [CrossRef]
- Isayama, S.; Suzuki, T.; Nakai, M.; Kunimi, Y. Influence of Tannic Acid on the Insecticidal Activity of a Bacillus Thuringiensis Serovar Aizawai Formulation against Spodoptera Litura Fabricius (Lepidoptera: Noctuidae). Biol. Control 2021, 157, 104558. [Google Scholar] [CrossRef]
- Torres-quintero, M.C.; Arenas-sosa, I.; Zuñiga-, F.; Hernández-velázquez, V.M.; Alvear-garcia, A. Characterization of Insecticidal Cry Protein from Bacillus Thuringiensis Toxic to Myzus Persicae (Sulzer). J. Invertebr. Pathol. 2022, 189, 107731. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, L.; He, J.; Fu, K.; Li, X.; Jia, L.; Wang, R.; Zhang, W. Characterization of a Novel Bacillus Thuringiensis Toxin Active against Aedes Aegypti Larvae. Acta Trop. 2021, 223, 106088. [Google Scholar] [CrossRef]
- Solano-Alvarez, N.; Valencia-Hernández, J.A.; Rico-García, E.; Torres-Pacheco, I.; Ocampo-Velázquez, R.V.; Escamilla-Silva, E.M.; Romero-García, A.L.; Alpuche-Solís, Á.G.; Guevara-González, R.G. A Novel Isolate of Bacillus Cereus Promotes Growth in Tomato and Inhibits Clavibacter Michiganensis Infection under Greenhouse Conditions. Plants 2021, 10, 506. [Google Scholar] [CrossRef]
- Kulimushi, P.Z.; Arias, A.A.; Franzil, L.; Steels, S.; Ongena, M. Stimulation of Fengycin-Type Antifungal Lipopeptides in Bacillus Amyloliquefaciens in the Presence of the Maize Fungal Pathogen Rhizomucor Variabilis. Front. Microbiol. 2017, 8, 850. [Google Scholar] [CrossRef]
- Rajula, J.; Rahman, A.; Krutmuang, P. Entomopathogenic Fungi in Southeast Asia and Africa and Their Possible Adoption in Biological Control. Biol. Control 2020, 151, 104399. [Google Scholar] [CrossRef]
- Dannon, H.F.; Dannon, A.E.; Douro-Kpindou, O.K.; Zinsou, A.V.; Houndete, A.T.; Toffa-Mehinto, J.; Elegbede, I.A.T.M.; Olou, B.D.; Tamò, M. Toward the Efficient Use of Beauveria Bassiana in Integrated Cotton Insect Pest Management. J. Cott. Res. 2020, 3, 1–21. [Google Scholar] [CrossRef]
- Harith-Fadzilah, N.; Abd Ghani, I.; Hassan, M. Omics-Based Approach in Characterising Mechanisms of Entomopathogenic Fungi Pathogenicity: A Case Example of Beauveria Bassiana. J. King Saud Univ.-Sci. 2021, 33, 101332. [Google Scholar] [CrossRef]
- Biryol, S.; Demirbağ, Z.; Erdoğan, P.; Demir, I. Development of Beauveria Bassiana (Ascomycota: Hypocreales) as a Mycoinsecticide to Control Green Peach Aphid, Myzus Persicae (Homoptera: Aphididae) and Investigation of Its Biocontrol Potential. J. Asia. Pac. Entomol. 2022, 25, 101878. [Google Scholar] [CrossRef]
- Schrank, A.; Vainstein, M.H. Metarhizium Anisopliae Enzymes and Toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Leemon, D.M.; Jonsson, N.N. Comparison of Bioassay Responses to the Potential Fungal Biopesticide Metarhizium Anisopliae in Rhipicephalus (Boophilus) Microplus and Lucilia Cuprina. Vet. Parasitol. 2012, 185, 236–247. [Google Scholar] [CrossRef]
- Riaz, T.; Masoom, A.; Virk, U.Y.; Raza, M.; Shakoori, F.R. Impacts of Metarhizium Anisopliae on Mortality, Energy Reserves, and Carbohydrase of Trogoderma Granarium. J. Stored Prod. Res. 2022, 99, 102013. [Google Scholar] [CrossRef]
- Olowe, O.M.; Nicola, L.; Asemoloye, M.D.; Akanmu, A.O.; Babalola, O.O. Trichoderma: Potential Bio-Resource for the Management of Tomato Root Rot Diseases in Africa. Microbiol. Res. 2022, 257, 126978. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Hendrich, C.G.; von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R.; Dalsing, B.L.; Ricca, P.; Naidoo, J.; Cook, D.; et al. Metabolomics of Tomato Xylem Sap during Bacterial Wilt Reveals Ralstonia Solanacearum Produces Abundant Putrescine, a Metabolite That Accelerates Wilt Disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef]
- Guo, Y.; Fan, Z.; Yi, X.; Zhang, Y.; Khan, R.A.A.; Zhou, Z. Sustainable Management of Soil-Borne Bacterium Ralstonia solanacearum In Vitro and In Vivo through Fungal Metabolites of Different Trichoderma spp. Sustainability 2021, 13, 1491. [Google Scholar] [CrossRef]
- Shehata, I.E.; Hammam, M.M.A.; El-Borai, F.E.; Duncan, L.W.; Abd-Elgawad, M.M.M. Traits of the Entomopathogenic Nematode, Heterorhabditis Bacteriophora (Hb-EG Strain), for Potential Biocontrol in Strawberry Fields. Egypt. J. Biol. Pest Control 2020, 30, 1–6. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, W.; Paul, B.; Paul, S.; Khan, Z.; Aggarwal, C. Entomopathogenic Nematodes for the Management of Subterranean Termites. In Plant, Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–36. ISBN 9783319274553. [Google Scholar]
- Kary, N.E.; Sanatipour, Z.; Mohammadi, D.; Dillon, A.B. Combination Effects of Entomopathogenic Nematodes, Heterorhabditis Bacteriophora and Steinernema Feltiae, with Abamectin on Developmental Stages of Phthorimaea Operculella (Lepidoptera, Gelechiidae). Crop Prot. 2021, 143, 105543. [Google Scholar] [CrossRef]
- Jakubowicz, V.; Taibo, C.B.; Sciocco-Cap, A.; Arneodo, J.D. Biological and Molecular Characterization of Rachiplusia Nu Single Nucleopolyhedrovirus, a Promising Biocontrol Agent against the South American Soybean Pest Rachiplusia Nu. J. Invertebr. Pathol. 2019, 166, 107211. [Google Scholar] [CrossRef]
- Jalali, E.; Maghsoudi, S.; Noroozian, E. Ultraviolet Protection of Bacillus Thuringiensis through Microencapsulation with Pickering Emulsion Method. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, N.; Kessel, T.; Skaljac, M.; Spohn, M.; Vilcinskas, A.; Lee, K.Z. The Gram-Positive Bacterium Leuconostoc Pseudomesenteroides Shows Insecticidal Activity against Drosophilid and Aphid Pests. Insects 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Radja Commare, R.; Nandakumar, R.; Kandan, A.; Suresh, S.; Bharathi, M.; Raguchander, T.; Samiyappan, R. Pseudomonas Fluorescens Based Bio-Formulation for the Management of Sheath Blight Disease and Leaffolder Insect in Rice. Crop Prot. 2002, 21, 671–677. [Google Scholar] [CrossRef]
- Amiri-Besheli, B. Efficacy of Bacillus Thuringiensis, Mineral Oil, Insecticidal Emulsion and Insecticidal Gel against Phyllocnistis Citrella Stainton (Lepidoptera: Gracillariidae). Plant Prot. Sci. 2008, 44, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Saberi-Rise, R.; Moradi-Pour, M. The Effect of Bacillus Subtilis Vru1 Encapsulated in Alginate—Bentonite Coating Enriched with Titanium Nanoparticles against Rhizoctonia Solani on Bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.; Chen, L.; Yu, X.; Borriss, R.; Gao, X. Difficidin and Bacilysin from Bacillus Amyloliquefaciens FZB42 Have Antibacterial Activity against Xanthomonas Oryzae Rice Pathogens. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the Formulation of Alginate-Gelatin Encapsulated Pseudomonas Fluorescens (VUPF5 and T17-4 Strains) for Controlling Fusarium Solani on Potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Llabot, J.M.; Cantoro, R.; Chiotta, M.L.; Allemandi, D.A.; Torres, A.M.; Chulze, S.N. Spray-Drying Process as a Suitable Tool for the Formulation of Bacillus Velezensis RC218, a Proved Biocontrol Agent to Reduce Fusarium Head Blight and Deoxynivalenol Accumulation in Wheat. Biocontrol Sci. Technol. 2020, 30, 329–338. [Google Scholar] [CrossRef]
- Aziz Qureshi, A.; Vineela, V.; Vimala Devi, P.S. Sodium Humate as a Promising Coating Material for Microencapsulation of Beauveria Bassiana Conidia Through Spray Drying. Dry. Technol. 2015, 33, 162–168. [Google Scholar] [CrossRef]
- Amatuzzi, R.F.; Poitevin, C.G.; Poltronieri, A.S.; Zawadneak, M.A.C.; Pimentel, I.C. Susceptibility of Duponchelia Fovealis Zeller (Lepidoptera: Crambidae) to Soil-Borne Entomopathogenic Fungi. Insects 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiriga, A.W.; Haukeland, S.; Kariuki, G.M.; Coyne, D.L.; Beek, N.V. Effect of Trichoderma Spp. and Purpureocillium Lilacinum on Meloidogyne Javanica in Commercial Pineapple Production in Kenya. Biol. Control 2018, 119, 27–32. [Google Scholar] [CrossRef]
- Rodrigues, I.M.W.; Forim, M.R.; da Silva, M.F.G.F.; Fernandes, J.B.; Filho, A.B. Effect of Ultraviolet Radiation on Fungi Beauveria Bassiana and Metarhizium Anisopliae, Pure and Encapsulated, and Bio-Insecticide Action on Diatraea Saccharalis. Adv. Entomol. 2016, 4, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Du, C.; Zhang, J.; Yang, B.; Cuthbertson, A.G.S.; Ali, S. Synthesis of Metarhizium Anisopliae–Chitosan Nanoparticles and Their Pathogenicity against Plutella Xylostella (Linnaeus). Microorganisms 2021, 10, 1. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Malik, A. Preparation, Characterization, and Insecticidal Activity Evaluation of Three Different Formulations of Beauveria Bassiana against Musca Domestica. Parasitol. Res. 2013, 112, 3485–3495. [Google Scholar] [CrossRef]
- Friuli, M.; Nitti, P.; Aneke, C.I.; Demitri, C.; Cafarchia, C.; Otranto, D. Freeze-Drying of Beauveria Bassiana Suspended in Hydroxyethyl Cellulose Based Hydrogel as Possible Method for Storage: Evaluation of Survival, Growth and Stability of Conidial Concentration before and after Processing. Results Eng. 2021, 12, 100283. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Wu, S.; Kostromytska, O.S. Microsclerotial Granular Formulation of the Entomopathogenic Fungus Metarhizium Brunneum and Its Combinations with Hydrogel and Imidacloprid against the Annual Bluegrass Weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2020, 113, 1118–1128. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Bilesky-José, N.; de Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma Harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef] [Green Version]
- Chinnaperumal, K.; Govindasamy, B.; Paramasivam, D.; Dilipkumar, A.; Dhayalan, A.; Vadivel, A.; Sengodan, K.; Pachiappan, P. Bio-Pesticidal Effects of Trichoderma Viride Formulated Titanium Dioxide Nanoparticle and Their Physiological and Biochemical Changes on Helicoverpa Armigera (Hub.). Pestic. Biochem. Physiol. 2018, 149, 26–36. [Google Scholar] [CrossRef]
- Herrera, W.; Valbuena, O.; Pavone-Maniscalco, D. Formulation of Trichoderma Asperellum TV190 for Biological Control of Rhizoctonia Solani on Corn Seedlings. Egypt. J. Biol. Pest Control 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Swarnakumari, N.; Sindhu, R.; Thiribhuvanamala, G.; Rajaswaminathan, V. Evaluation of Oil Dispersion Formulation of Nematophagus Fungus, Pochonia Chlamydosporia against Root-Knot Nematode, Meloidogyne Incognita in Cucumber. J. Asia Pac. Entomol. 2020, 23, 1283–1287. [Google Scholar] [CrossRef]
- Mastore, M.; Arizza, V.; Manachini, B.; Brivio, M.F. Modulation of Immune Responses of Rhynchophorus Ferrugineus (Insecta: Coleoptera) Induced by the Entomopathogenic Nematode Steinernema Carpocapsae (Nematoda: Rhabditida). Insect Sci. 2015, 22, 748–760. [Google Scholar] [CrossRef]
- NanGong, Z.; Li, T.; Zhang, W.; Song, P.; Wang, Q. Capsule-C: An Improved Steinernema Carpocapsae Capsule Formulation for Controlling Agrotis Ipsilon Hufnagel (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Ebrahimi, L.; Niknam, G.; Dunphy, G.B.; Toorchi, M. Effect of an Entomopathogenic Nematode, Steinernema Carpocapsae on Haemocyte Profile and Phenoloxidase Activity of the Colorado Potato Beetle, Leptinotarsa Decemlineata. Biocontrol Sci. Technol. 2014, 24, 1383–1393. [Google Scholar] [CrossRef]
- Aquino-Bolaños, T.; Ruiz-Vega, J.; Ortiz Hernández, Y.D.; Jiménez Castañeda, J.C. Survival of Entomopathogenic Nematodes in Oil Emulsions and Control Effectiveness on Adult Engorged Ticks (Acari: Ixodida). J. Nematol. 2019, 51, e2019-01. [Google Scholar] [CrossRef] [Green Version]
- Jaffuel, G.; Sbaiti, I.; Turlings, T.C.J. Encapsulated Entomopathogenic Nematodes Can Protect Maize Plants from Diabrotica Balteata Larvae. Insects 2020, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.; Guevara, J.; Cuartas, P.; Espinel, C.; Villamizar, L. Microencapsulated Spodoptera Frugiperda Nucleopolyhedrovirus: Insecticidal Activity and Effect on Arthropod Populations in Maize. Biocontrol Sci. Technol. 2013, 23, 829–846. [Google Scholar] [CrossRef]
- Gifani, A.; Marzban, R.; Safekordi, A.; Ardjmand, M.; Dezianian, A. Ultraviolet Protection of Nucleopolyhedrovirus through Microencapsulation with Different Polymers. Biocontrol Sci. Technol. 2015, 25, 814–827. [Google Scholar] [CrossRef]
- Popham, H.J.R.; Rowley, D.L.; Harrison, R.L. Differential Insecticidal Properties of Spodoptera Frugiperda Multiple Nucleopolyhedrovirus Isolates against Corn-Strain and Rice-Strain Fall Armyworm, and Genomic Analysis of Three Isolates. J. Invertebr. Pathol. 2021, 183, 107561. [Google Scholar] [CrossRef]
- Ordóñez-García, M.; Rios-Velasco, C.; Ornelas-Paz, J.D.J.; Bustillos-Rodríguez, J.C.; Acosta-Muñiz, C.H.; Berlanga-Reyes, D.I.; Salas-Marina, M.Á.; Cambero-Campos, O.J.; Gallegos-Morales, G. Molecular and Morphological Characterization of Multiple Nucleopolyhedrovirus from Mexico and Their Insecticidal Activity against Spodoptera Frugiperda (Lepidoptera: Noctuidae). J. Appl. Entomol. 2020, 144, 123–132. [Google Scholar] [CrossRef]
- Rose, J.; Kleespies, R.G.; Wang, Y.; Wennmann, J.T.; Jehle, J.A. On the Susceptibility of the Box Tree Moth Cydalima Perspectalis to Anagrapha Falcifera Nucleopolyhedrovirus (AnfaNPV). J. Invertebr. Pathol. 2013, 113, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A.H. Botanical Insecticide as Simple Extractives for Pest Control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical Activity and Role of Botanical Pesticides in Pest Management for Sustainable Agricultural Crop Production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Ahmad, W.; Singh, S.; Kumar, S.; Waseem Ahmad, C. Phytochemical Screening and Antimicrobial Study of Euphorbia Hirta Extracts. J. Med. Plants Stud. 2017, 5, 183–186. [Google Scholar]
- Céspedes, A.C.L.; Avila, J.G.; Marin, J.C.; Domínguez, L.M.; Torres, P.; Aranda, E. Chapter 1 Natural Compounds as Antioxidant and Molting Inhibitors Can Play a Role as a Model for Search of New Botanical Pesticides. Adv. Phytomed. 2006, 3, 1–27. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, H.; Hou, R.; Wu, J.; Wang, Y.; Huang, S.; Cheng, D.; Xu, H.; Zhang, Z. Effects of Sublethal Azadirachtin on the Immune Response and Midgut Microbiome of Apis Cerana Cerana (Hymenoptera: Apidae). Ecotoxicol. Environ. Saf. 2022, 229, 113089. [Google Scholar] [CrossRef]
- Mullai, P.; Vishali, S.; Sobiya, E. Experiments and Adaptive-Network-Based Fuzzy Inference System Modelling in a Hybrid up-Flow Anaerobic Sludge Blanket Reactor to Assess Industrial Azadirachtin Effluent Quality. Bioresour. Technol. 2022, 358, 127395. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Barreiros, L.; Oliveira, R.F.; Cruz, A.; Prudêncio, C.; Oliveira, A.I.; Pinho, C.; Santos, N.; Morgado, J. Chemistry, Bioactivities, Extraction and Analysis of Azadirachtin: State-of-the-Art. Fitoterapia 2019, 134, 141–150. [Google Scholar] [CrossRef]
- Qin, D.; Zheng, Q.; Zhang, P.; Lin, S.; Huang, S.; Cheng, D.; Zhang, Z. Azadirachtin Directly or Indirectly Affects the Abundance of Intestinal Flora of Spodoptera Litura and the Energy Conversion of Intestinal Contents Mediates the Energy Balance of Intestine-Brain Axis, and along with Decreased Expression CREB in the Brain. Pestic. Biochem. Physiol. 2021, 173, 104778. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular Trichome-Derived Sesquiterpenes of Wild Tomato Accessions (Solanum Habrochaites) Affect Aphid Performance and Feeding Behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, L.; Segatto, M.L.; Olsson, O.; Zuin, V.G.; Kümmerer, K. Flavonoids as Biopesticides—Systematic Assessment of Sources, Structures, Activities and Environmental Fate. Sci. Total Environ. 2022, 824, 153781. [Google Scholar] [CrossRef] [PubMed]
- Diaz Napal, G.N.; Carpinella, M.C.; Palacios, S.M. Antifeedant Activity of Ethanolic Extract from Flourensia Oolepis and Isolation of Pinocembrin as Its Active Principle Compound. Bioresour. Technol. 2009, 100, 3669–3673. [Google Scholar] [CrossRef] [PubMed]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Kokkrua, S.; Ismail, S.I.; Mazlan, N.; Dethoup, T. Efficacy of Berberine in Controlling Foliar Rice Diseases. Eur. J. Plant Pathol. 2020, 156, 147–158. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant Isoquinoline Alkaloids: Advances in the Chemistry and Biology of Berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, D.; Li, W.; Yan, X.; Qiao, J.; Caiyin, Q. Research Progress on the Synthetic Biology of Botanical Biopesticides. Bioengineering 2022, 9, 207. [Google Scholar] [CrossRef]
- Nisbet, A.J.; Woodford, J.A.T.; Strang, R.H.C. The Effects of Azadirachtin on the Acquisition and Inoculation of Potato Leafroll Virus by Myzus Persicae. Crop Prot. 1996, 15, 9–14. [Google Scholar] [CrossRef]
- Zhao, T.; Lai, D.; Zhou, Y.; Xu, H.; Zhang, Z.; Kuang, S.; Shao, X. Azadirachtin A Inhibits the Growth and Development of Bactrocera Dorsalis Larvae by Releasing Cathepsin in the Midgut. Ecotoxicol. Environ. Saf. 2019, 183, 109512. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, T.; Sun, Z.; Li, H.; Qi, X.; Zhong, G.; Yi, X. Azadirachtin Acting as a Hazardous Compound to Induce Multiple Detrimental Effects in Drosophila Melanogaster. J. Hazard. Mater. 2018, 359, 338–347. [Google Scholar] [CrossRef]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Maroua, F.; Aribi, N. Azadirachtin Induced Larval Avoidance and Antifeeding by Disruption of Food Intake and Digestive Enzymes in Drosophila Melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Farder-Gomes, C.F.; Saravanan, M.; Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Azadirachtin-Based Biopesticide Affects the Respiration and Digestion in Anticarsia Gemmatalis Caterpillars. Toxin Rev. 2022, 41, 466–475. [Google Scholar] [CrossRef]
- Jerobin, J.; Sureshkumar, R.S.; Anjali, C.H.; Mukherjee, A.; Chandrasekaran, N. Biodegradable Polymer Based Encapsulation of Neem Oil Nanoemulsion for Controlled Release of Aza-A. Carbohydr. Polym. 2012, 90, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, F.M.R.; Polloni, A.E.; Junges, A.; da Silva, R.S.; Rubira, A.F.; Borges, G.R.; Dariva, C.; Franceschi, E. Encapsulation of Neem (Azadirachta Indica) Seed Oil in Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by SFEE Technique. J. Supercrit. Fluids 2019, 152, 104556. [Google Scholar] [CrossRef]
- Usha Rani, P.; Madhusudhanamurthy, J.; Sreedhar, B. Dynamic Adsorption of α-Pinene and Linalool on Silica Nanoparticles for Enhanced Antifeedant Activity against Agricultural Pests. J. Pest Sci. 2014, 87, 191–200. [Google Scholar] [CrossRef]
- Prates, L.H.F.; Faroni, L.R.D.A.; Heleno, F.F.; de Queiroz, M.E.L.R.; de Sousa, A.H.; de Assis Silva, M.V. Eugenol Diffusion Coefficient and Its Potential to Control Sitophilus Zeamais in Rice. Sci. Rep. 2019, 9, 11161. [Google Scholar] [CrossRef] [Green Version]
- Pinto, N.F.S.; Pereira, D.M.; Pereira, R.B.; Fortes, A.G.; Fernandes, M.J.G.; Castanheira, E.M.S.; Gonçalves, M.S.T. Synthesis of Amino Alcohols from Eugenol and Their Insecticidal Activity against Sf9 Cell Line. Chem. Proc. 2021, 3, 62. [Google Scholar] [CrossRef]
- Góngora, C.E.; Tapias, J.; Jaramillo, J.; Medina, R.; Gonzalez, S.; Casanova, H.; Ortiz, A.; Benavides, P. Evaluation of Terpene-Volatile Compounds Repellent to the Coffee Berry Borer, Hypothenemus Hampei (Ferrari) (Coleoptera: Curculionidae). J. Chem. Ecol. 2020, 46, 881–890. [Google Scholar] [CrossRef]
- Giunti, G.; Palermo, D.; Laudani, F.; Algeri, G.M.; Campolo, O.; Palmeri, V. Repellence and Acute Toxicity of a Nano-Emulsion of Sweet Orange Essential Oil toward Two Major Stored Grain Insect Pests. Ind. Crops Prod. 2019, 142, 111869. [Google Scholar] [CrossRef]
- Lima, L.A.; Ferreira-Sá, P.S.; Garcia, M.D.N.; Pereira, V.L.P.; Carvalho, J.C.T.; Rocha, L.; Fernandes, C.P.; Souto, R.N.P.; Araújo, R.S.; Botas, G.; et al. Nano-Emulsions of the Essential Oil of Baccharis Reticularia and Its Constituents as Eco-Friendly Repellents against Tribolium Castaneum. Ind. Crops Prod. 2021, 162, 113282. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Nasiou, E.; Skiada, P.; Giannakou, I.O. Effects of Four Terpenes on the Mortality of Ditylenchus Dipsaci (Kühn) Filipjev. Eur. J. Plant Pathol. 2021, 160, 137–146. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Cioni, P.L.; Flamini, G.; Rifici, A. Hyptis Suaveolens and Hyptis Spicigera (Lamiaceae) Essential Oils: Qualitative Analysis, Contact Toxicity and Repellent Activity against Sitophilus Granarius (L.) (Coleoptera: Dryophthoridae). J. Pest Sci. (2004) 2011, 84, 219–228. [Google Scholar] [CrossRef]
- Zekri, N.; Handaq, N.; El Caidi, A.; Zair, T.; Alaoui El Belghiti, M. Insecticidal Effect of Mentha Pulegium L. and Mentha Suaveolens Ehrh. Hydrosols against a Pest of Citrus, Toxoptera Aurantii (Aphididae). Res. Chem. Intermed. 2016, 42, 1639–1649. [Google Scholar] [CrossRef]
- Pang, X.; Almaz, B.; Qi, X.J.; Wang, Y.; Feng, Y.X.; Geng, Z.F.; Xi, C.; Du, S.S. Bioactivity of Essential Oil from Atalantia Buxifolia Leaves and Its Major Sesquiterpenes against Three Stored-Product Insects. J. Essent. Oil-Bearing Plants 2020, 23, 38–50. [Google Scholar] [CrossRef]
- da Silva, D.F.; Amaral, J.C.; Carlos, R.M.; Ferreira, A.G.; Forim, M.R.; Fernandes, J.B.; da Silva, M.F.d.G.F.; Filho, H.D.C.; de Souza, A.A. Octahedral Ruthenium and Magnesium Naringenin 5-Alkoxide Complexes: NMR Analysis of Diastereoisomers and in-Vivo Antibacterial Activity against Xylella fastidiosa. Talanta 2021, 225, 122040. [Google Scholar] [CrossRef]
- Kaab, S.B.; Rebey, I.B.; Hanafi, M.; Hammi, K.M.; Smaoui, A.; Fauconnier, M.L.; De Clerck, C.; Jijakli, M.H.; Ksouri, R. Screening of Tunisian Plant Extracts for Herbicidal Activity and Formulation of a Bioherbicide Based on Cynara Cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, P. In Vitro Biopesticide Effect of Alkaloids and Flavonoids of Some Plants against Fusarium Oxysporum. Arch. Phytopathol. Plant Prot. 2013, 46, 1236–1245. [Google Scholar] [CrossRef]
- Franceschini Sarria, A.L.; Matos, A.P.; Volante, A.C.; Bernardo, A.R.; Sabbag Cunha, G.O.; Fernandes, J.B.; Rossi Forim, M.; Vieira, P.C.; da Silva, M.F.D.G.F. Insecticidal Activity of Copper (II) Complexes with Flavanone Derivatives. Nat. Prod. Res. 2022, 36, 1342–1345. [Google Scholar] [CrossRef]
- Loron, A.; Navikaitė-šnipaitienė, V.; Rosliuk, D.; Rutkaitė, R.; Gardrat, C.; Coma, V. Polysaccharide Matrices for the Encapsulation of Tetrahydrocurcumin—Potential Application as Biopesticide against Fusarium Graminearum. Molecules 2021, 26, 3873. [Google Scholar] [CrossRef]
- Jadhav, D.R.; Mallikarjuna, N.; Rathore, A.; Pokle, D. Effect of Some Flavonoids on Survival and Development of Helicoverpa Armigera (Hübner) and Spodoptera Litura (Fab) (Lepidoptera: Noctuidae). Asian J. Agric. Sci. 2012, 4, 298–307. [Google Scholar]
- Salunke, B.K.; Kotkar, H.M.; Mendki, P.S.; Upasani, S.M.; Maheshwari, V.L. Efficacy of Flavonoids in Controlling Callosobruchus Chinensis (L.) (Coleoptera: Bruchidae), a Post-Harvest Pest of Grain Legumes. Crop Prot. 2005, 24, 888–893. [Google Scholar] [CrossRef]
- Kozłowski, J.; Jaskulska, M.; Kozłowska, M. The Role of Alkaloids in the Feeding Behaviour of Slugs (Gastropoda: Stylommatophora) as Pests of Narrow-Leafed Lupin Plants. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 263–269. [Google Scholar] [CrossRef]
- Zanardi, O.Z.; Ribeiro, L.; do, P.; Ansante, T.F.; Santos, M.S.; Bordini, G.P.; Yamamoto, P.T.; Vendramim, J.D. Bioactivity of a Matrine-Based Biopesticide against Four Pest Species of Agricultural Importance. Crop Prot. 2015, 67, 160–167. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Z.; Hao, M.; Lv, M.; Xu, H. Evaluation of Biological Activities, and Exploration on Mechanism of Action of Matrine–Cholesterol Derivatives. Bioorg. Chem. 2020, 94, 103439. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Chen, M.; Ye, H.C.; Zhang, Z.K.; Li, H.; Chen, L.L.; Chen, X.L.; Yan, C.; Zhang, J. Herbicidal Activities of Compounds Isolated from the Medicinal Plant Piper Sarmentosum. Ind. Crops Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- Wu, J.; Ma, J.J.; Liu, B.; Huang, L.; Sang, X.Q.; Zhou, L.J. Herbicidal Spectrum, Absorption and Transportation, and Physiological Effect on Bidens Pilosa of the Natural Alkaloid Berberine. J. Agric. Food Chem. 2017, 65, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, M.; Luo, H. Identification and Antimicrobial Activity of Two Alkaloids from Traditional Chinese Medicinal Plant Tinospora Capillipes. Ind. Crops Prod. 2012, 37, 298–302. [Google Scholar] [CrossRef]
- Kathuria, V.; Ruhl, S.; Kaushik, N.; Edrada-Ebel, R.A.; Proksch, P. Evaluation of Bio Efficacy of Tylophora Indica Leaf Extracts, Fractions and Pure Alkaloids against Helicoverpa Armigera (Hübner). Ind. Crops Prod. 2013, 46, 274–282. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Baskar, K.; Muthu, C.; Ignacimuthu, S.; Naif Abdullah, A.-D. Bioefficacy of Flindersine against Helicoverpa Armigera Hübner, Spodoptera Litura Fabricius, Anopheles Stephensis. Braz. Arch. Biol. Technol. 2015, 58, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, M.J.; Rees, G.D. Microemulsion-Based Media as Novel Drug Delivery Systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Gasic, S.; Tanovic, B. Biopesticide Formulations, Possibility of Application and Future Trends. Pestic. I Fitomedicina 2013, 28, 97–102. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for Delivery of Apiaceae Essential Oils—Towards Highly Effective and Eco-Friendly Mosquito Larvicides? Ind. Crops Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Lucia, A.; Guzmán, E. Emulsions Containing Essential Oils, Their Components or Volatile Semiochemicals as Promising Tools for Insect Pest and Pathogen Management. Adv. Colloid Interface Sci. 2021, 287, 102330. [Google Scholar] [CrossRef] [PubMed]
- Saroj, A.; Chanotiya, C.S.; Maurya, R.; Pragadheesh, V.S.; Yadav, A.; Samad, A. Antifungal Action of Lippia Alba Essential Oil in Rhizoctonia Solani Disease Management. SN Appl. Sci. 2019, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mollakhalili Meybodi, N.; Mohammadifar, M.A.; Naseri, A.R. Effective Factors on the Stability of Oil-in-Water Emulsion Based Beverage: A Review. J. Food Qual. Hazards Control 2014, 1, 67–71. [Google Scholar]
- Kale, S.; Deore, S. Emulsion Microemulsion and Nanoemulsion. Syst. Rev. Pharm. 2017, 8, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Ramadhany, P.; Witono, J.R.B.; Rosaria, R. Neem-Based Oil-in-Water (O/W) Emulsion as a Biopesticide. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012047. [Google Scholar] [CrossRef]
- Iqbal, N.; Kumar, N.; Saini, M.K.; Dubey, S.; Agrawal, A.; Kumar, J. Role of High Shear Mixing in Improving Stability and Bio-Efficacy of Botanical Oil in Water Formulation for Early Stage Mosquito Eradication. Heliyon 2020, 6, e03380. [Google Scholar] [CrossRef] [Green Version]
- Yegya Raman, A.K.; Venkataramani, D.; Bhagwat, S.; Martin, T.; Clark, P.E.; Aichele, C.P. Emulsion Stability of Surfactant and Solid Stabilized Water-in-Oil Emulsions after Hydrate Formation and Dissociation. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 607–621. [Google Scholar] [CrossRef]
- Vandergheynst, J.; Scher, H.; Guo, H.Y.; Schultz, D. Water-in-Oil Emulsions That Improve the Storage and Delivery of the Biolarvacide Lagenidium Giganteum. Bio. Control 2007, 52, 207–229. [Google Scholar] [CrossRef]
- Yaakov, N.; Kottakota, C.; Mani, K.A.; Naftali, S.M.; Zelinger, E.; Davidovitz, M.; Ment, D.; Mechrez, G. Encapsulation of Bacillus Thuringiensis in an Inverse Pickering Emulsion for Pest Control Applications. Colloids Surfaces B Biointerfaces 2022, 213, 112427. [Google Scholar] [CrossRef] [PubMed]
- Vimala Devi, P.S.; Vineela, V. Suspension Concentrate Formulation of Bacillus Thuringiensis Var. Kurstaki for Effective Management of Helicoverpa Armigera on Sunflower (Helianthus Annuus). Biocontrol Sci. Technol. 2014, 25, 329–336. [Google Scholar] [CrossRef]
- Vineela, V.; Nataraj, T.; Reddy, G.; Vimala Devi, P.S. Enhanced Bioefficacy of Bacillus Thuringiensis Var. Kurstaki against Spodoptera Litura (Lepidoptera: Noctuidae) through Particle Size Reduction and Formulation as a Suspension Concentrate. Biocontrol Sci. Technol. 2017, 27, 58–69. [Google Scholar] [CrossRef]
- Do Nascimento Junior, D.R.; Tabernero, A.; Cabral Albuquerque, E.C.d.M.; Vieira de Melo, S.A.B. Biopesticide Encapsulation Using Supercritical CO2: A Comprehensive Review and Potential Applications. Molecules 2021, 26, 4003. [Google Scholar] [CrossRef] [PubMed]
- Vemmer, M.; Patel, A.V. Review of Encapsulation Methods Suitable for Microbial Biological Control Agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Milićević, Z.; Krnjajić, S.; Stević, M.; Ćirković, J.; Jelušić, A.; Pucarević, M.; Popović, T. Encapsulated Clove Bud Essential Oil: A New Perspective as an Eco-Friendly Biopesticide. Agriculture 2022, 12, 338. [Google Scholar] [CrossRef]
- Yaakov, N.; Ananth Mani, K.; Felfbaum, R.; Lahat, M.; Da Costa, N.; Belausov, E.; Ment, D.; Mechrez, G. Single Cell Encapsulation via Pickering Emulsion for Biopesticide Applications. ACS Omega 2018, 3, 14294–14301. [Google Scholar] [CrossRef] [Green Version]
- Amar Feldbaum, R.; Yaakov, N.; Ananth Mani, K.; Yossef, E.; Metbeev, S.; Zelinger, E.; Belausov, E.; Koltai, H.; Ment, D.; Mechrez, G. Single Cell Encapsulation in a Pickering Emulsion Stabilized by TiO2 Nanoparticles Provides Protection against UV Radiation for a Biopesticide. Colloids Surfaces B Biointerfaces 2021, 206, 111958. [Google Scholar] [CrossRef]
- Ment, D.; Shikano, I.; Glazer, I. Abiotic Factors. In Ecology of Invertebrate Diseases; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 143–186. [Google Scholar]
- Pemsel, M.; Schwab, S.; Scheurer, A.; Freitag, D.; Schatz, R.; Schlücker, E. Advanced PGSS Process for the Encapsulation of the Biopesticide Cydia Pomonella Granulovirus. J. Supercrit. Fluids 2010, 53, 174–178. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, B.; Luo, Y. Chitosan-Based Hydrogel Beads: Preparations, Modifications and Applications in Food and Agriculture Sectors—A Review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional Cross Linked Hydrophilic Polymeric Network “Hydrogels”: An Agriculture Boom. Agric. Water Manag. 2021, 253, 106939. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Nasser, S.; da Costa, M.P.M.; Ferreira, I.L.d.M.; Lima, J.B.P. K-Carrageenan-Bacillus Thuringiensis Israelensis Hydrogels: A Promising Material to Combat Larvae of the Aedes Aegypti Mosquito. Carbohydr. Polym. Technol. Appl. 2021, 2, 100125. [Google Scholar] [CrossRef]
- Flores-Céspedes, F.; Martínez-Domínguez, G.P.; Villafranca-Sánchez, M.; Fernández-Pérez, M. Preparation and Characterization of Azadirachtin Alginate-Biosorbent Based Formulations: Water Release Kinetics and Photodegradation Study. J. Agric. Food Chem. 2015, 63, 8391–8398. [Google Scholar] [CrossRef]
- Shen, Y.; Cui, B.; Wang, Y.; Cui, H. Marketing Strategy and Environmental Safety of Nano-Biopesticides. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Woodhead Publishing, Ltd.: Cambridge, UK, 2021; pp. 265–279. [Google Scholar]
- Hernandez-Tenorio, F.; Orozco-Sánchez, F. Nanoformulaciones de Bioinsecticidas Botánicos Para El Control de Plagas Agricolas. Rev. la Fac. Ciencias 2020, 9, 72–91. [Google Scholar] [CrossRef]
- Margulis-Goshen, K.; Magdassi, S. Nanotechnology: An Advanced Approach to the Development of Potent Insecticides. In Advanced Technologies for Managing Insect Pests; Springer: Dordrecht, The Netherlands, 2013; pp. 295–314. [Google Scholar]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the Preparation and the Recent Advances in Their Food Applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation Optimization of D-Limonene-Loaded Nanoemulsions as a Natural and Efficient Biopesticide. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 596, 124746. [Google Scholar] [CrossRef]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and Physical Properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Choupanian, M.; Omar, D.; Basri, M.; Asib, N. Preparation and Characterization of Neem Oil Nanoemulsion Formulations against Sitophilus Oryzae and Tribolium Castaneum Adults. J. Pestic. Sci. 2017, 42, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Nandini, B.; Puttaswamy, H.; Prakash, H.S.; Adhikari, S.; Jogaiah, S.; Nagaraja, G. Elicitation of Novel Trichogenic-Lipid Nanoemulsion Signaling Resistance against Pearl Millet Downy Mildew Disease. Biomolecules 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Sarabia, M.; Maspoch, D. Nanoencapsulation. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2016; pp. 2356–2369. [Google Scholar]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Sendi, J.J.; Aliakbar, A. Efficacy of Nanoencapsulated Thymus Eriocalyx and Thymus Kotschyanus Essential Oils by a Mesoporous Material MCM-41 Against Tetranychus Urticae (Acari: Tetranychidae). J. Econ. Entomol. 2017, 110, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wang, Z.; Yin, Y.; Zeng, D.; Xia, Y. Field Trials of Metarhizium Anisopliae Var. Acridum (Ascomycota: Hypocreales) against Oriental Migratory Locusts, Locusta Migratoria Manilensis (Meyen) in Northern China. Crop Prot. 2008, 27, 1244–1250. [Google Scholar] [CrossRef]
- Mullié, W.C.; Cheke, R.A.; Young, S.; Ibrahim, A.B.; Murk, A.J. Increased and Sex-Selective Avian Predation of Desert Locusts Schistocerca Gregaria Treated with Metarhizium Acridum. PLoS ONE 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aynalem, B.; Muleta, D.; Jida, M.; Shemekite, F.; Aseffa, F. Biocontrol Competence of Beauveria Bassiana, Metarhizium Anisopliae and Bacillus Thuringiensis against Tomato Leaf Miner, Tuta Absoluta Meyrick 1917 under Greenhouse and Field Conditions. Heliyon 2022, 8, e09694. [Google Scholar] [CrossRef]

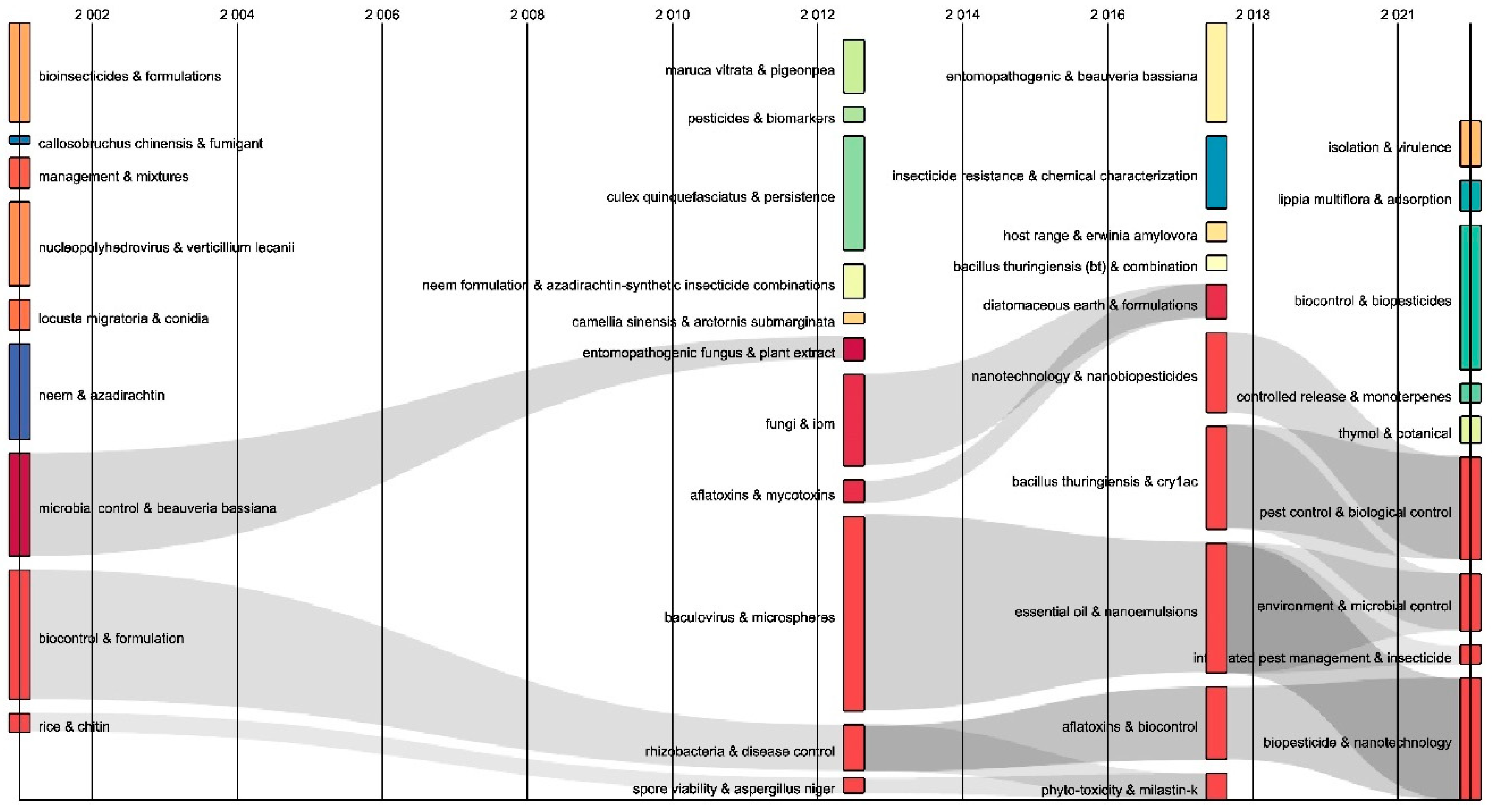
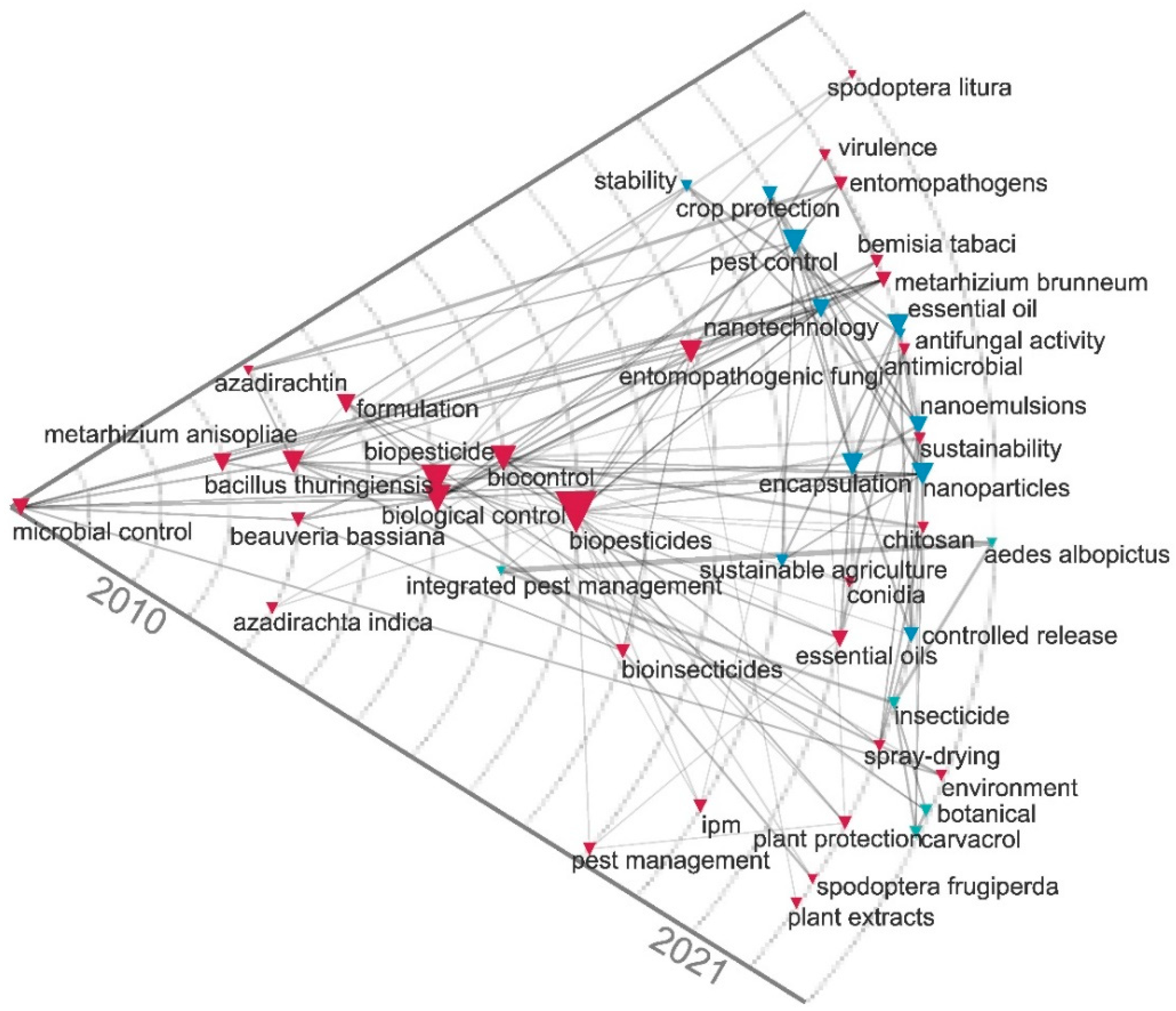

| Rank | Country | Number of Citations | Average Article Citations | Number of Publications |
|---|---|---|---|---|
| 1 | United States | 4080 | 25.98 | 157 |
| 2 | India | 3491 | 20.29 | 172 |
| 3 | Brazil | 2198 | 21.76 | 101 |
| 4 | Canada | 1730 | 27.46 | 63 |
| 5 | Italy | 1709 | 26.29 | 65 |
| 6 | United Kingdom | 1106 | 29.10 | 38 |
| 7 | Spain | 884 | 24.55 | 36 |
| 8 | France | 847 | 24.91 | 34 |
| 9 | Czech Republic | 772 | 42.88 | 18 |
| 10 | Germany | 720 | 28.8 | 25 |
| Title | Journals | Authors Affiliation Countries | Number of Citations | Number of Citations Per Year | References |
|---|---|---|---|---|---|
| Perspectives for nano-biotechnology enabled protection and nutrition of plants | Biotechnology advances | India | 567 | 47.25 | [18] |
| Biological control of locusts and grasshoppers | Annual review of entomology | Canada, Benin, United Kingdom | 353 | 16.04 | [21] |
| Geraniol-A review of a commercially important fragrance material | South African Journal of Botany | South Africa | 299 | 23.00 | [22] |
| Biological control of Bermisia tabaci with fungi | Crop Protection | Brazil, United States | 248 | 11.27 | [23] |
| Nano-based smart pesticide formulations: Emerging opportunities for agriculture | Journal of Controlled Release | India, Italy, United States, South Korea | 244 | 61.00 | [24] |
| The enhanced infectivity of Metarhizium flavoviride in oil formulations to desert locusts at low humidities | Annals of Applied Biology | United Kingdom | 237 | 7.90 | [20] |
| Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises | Biotechnology Advances | Brazil, India | 232 | 25.77 | [25] |
| Microbial inoculation of seed for improved crop performance: issues and opportunities | Applied Microbiology and Biotechnology | New Zealand | 188 | 26.85 | [26] |
| The science, development, and commercialization of postharvest biocontrol products | Posthasvest Biology and Technology | Israel, United, States, Spain, Italy, Belgium | 180 | 25.71 | [27] |
| Development, registration, and commercialization of microbial pesticides for plant protection | International Microbiology | Spain | 179 | 8.95 | [28] |
| Microorganism (Strain) | Target Pests | Formulation | References |
|---|---|---|---|
| Bacteria | |||
| Bacillus cereaus | Clavibacter michiganensis | Aqueous suspension | [41] |
| Bacillus thuringiensis | Ephestia kuehniella | Encapsulation | [57] |
| Leuconostoc pseudomesenteroides | Drosophila suzukii, Drosophila melanogaster, Acyrthosiphon pisum | Suspensions | [58] |
| Pseudomonas fluorescens | Rhizoctonia solani, Cnaphalocrosis medinalis | Suspensions | [59] |
| Bacillus thuringiensis | Phyllocnistis citrella | Emulsion | [60] |
| Bacillus subtilis Vru1 | Rhizoctonia solani | Nanoencapsulation | [61] |
| Bacillus amyloliquefaciens FZB42 | Xanthomonas oryzae | Suspensions | [62] |
| Bacillus thuringiensis | Artogeia rapae L. Trichoplusia ni, T. ni Hübner, Plutella xylostella L, Autographa californica Spreyer | Encapsulation | [12] |
| Pseudomonas fluorescens (VUPF5 and T17-4 strains) | Fusarium solani | Nanoencapsulation | [63] |
| Bacillus velezensis RC218 | Fusarium | Spray drying | [64] |
| Fungi | |||
| Beauveria bassiana | Myzus persicae | Emulsion | [46] |
| Beauveria bassiana | Helicoverpa armigera | Encapsulation | [65] |
| Beauveria, Metarhizium, Isaria, and Lecanicillium | Duponchelia fovealis | Suspensions | [66] |
| Purpureocillium lilacinum and Trichoderma spp | Meloidogyne javanica | Suspensions | [67] |
| Beauveria bassiana and Metarhizium anisopliae | Diatraea saccharalis | Encapsulation | [68] |
| Metarhizium anisopliae | Plutella xylostella | Nanoparticles | [69] |
| Beauveria bassiana | Musca domestica | Encapsulation and emulsion | [70] |
| Beauveria bassiana | Nor reported | Hydrogel | [71] |
| Metarhizium brunneum | Annual Bluegrass Weevil | Hydrogel | [72] |
| Trichoderma harzianum | Sclerotinia sclerotiorum | Encapsulation | [73] |
| Trichoderma viride | Helicoverpa armigera | Nanoparticles | [74] |
| Trichoderma asperellum TV190 | Rhizoctonia solani | Emulsion | [75] |
| Pochonia chlamydosporia | Meloidogyne incognita | Emulsion | [76] |
| Nematodes | |||
| Steinernema carpocapsae | Rhynchophorus ferrugineus | Encapsulation | [77] |
| Steinernema carpocapsae | Agrotis ipsilon Hufnagel | Encapsulation | [78] |
| Steinernema carpocapsae | Leptinotarsa decemlineata | Encapsulation | [79] |
| Heterorhabditis bacteriophora, Steinernema carpocapsae, and Steinernema websteri | Ixodes scapularis Say | Emulsion | [80] |
| Heterorhabditis bacteriophora | Diabrotica balteata | Encapsulation | [81] |
| Virus | |||
| Nucleopolyhedrovirus of S. frugiperda (SfMNPV) | Spodoptera frugiperda | Encapsulation | [82] |
| Helicoverpa armigera nuclear polyhedrosis virus (HaNPV) | Helicoverpa armigera | Encapsulation | [83] |
| VPN of Spodoptera frugiperda | Spodoptera frugiperda | Viral suspensions | [84] |
| VPN SfCH15, SfCH32 | Spodoptera frugiperda | Viral suspensions | [85] |
| VPN of Anagrapha falcifera | Cydalima perpectalis | Viral suspensions | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Tenorio, F.; Miranda, A.M.; Rodríguez, C.A.; Giraldo-Estrada, C.; Sáez, A.A. Potential Strategies in the Biopesticide Formulations: A Bibliometric Analysis. Agronomy 2022, 12, 2665. https://doi.org/10.3390/agronomy12112665
Hernandez-Tenorio F, Miranda AM, Rodríguez CA, Giraldo-Estrada C, Sáez AA. Potential Strategies in the Biopesticide Formulations: A Bibliometric Analysis. Agronomy. 2022; 12(11):2665. https://doi.org/10.3390/agronomy12112665
Chicago/Turabian StyleHernandez-Tenorio, Fabian, Alejandra M. Miranda, Carlos A. Rodríguez, Catalina Giraldo-Estrada, and Alex A. Sáez. 2022. "Potential Strategies in the Biopesticide Formulations: A Bibliometric Analysis" Agronomy 12, no. 11: 2665. https://doi.org/10.3390/agronomy12112665
APA StyleHernandez-Tenorio, F., Miranda, A. M., Rodríguez, C. A., Giraldo-Estrada, C., & Sáez, A. A. (2022). Potential Strategies in the Biopesticide Formulations: A Bibliometric Analysis. Agronomy, 12(11), 2665. https://doi.org/10.3390/agronomy12112665






