Estimating Sheet Flow Velocities Using Quinine as a Fluorescent Tracer: Bare, Mulched, Vegetated and Paved Surfaces
Abstract
:1. Introduction
2. Materials and Methods
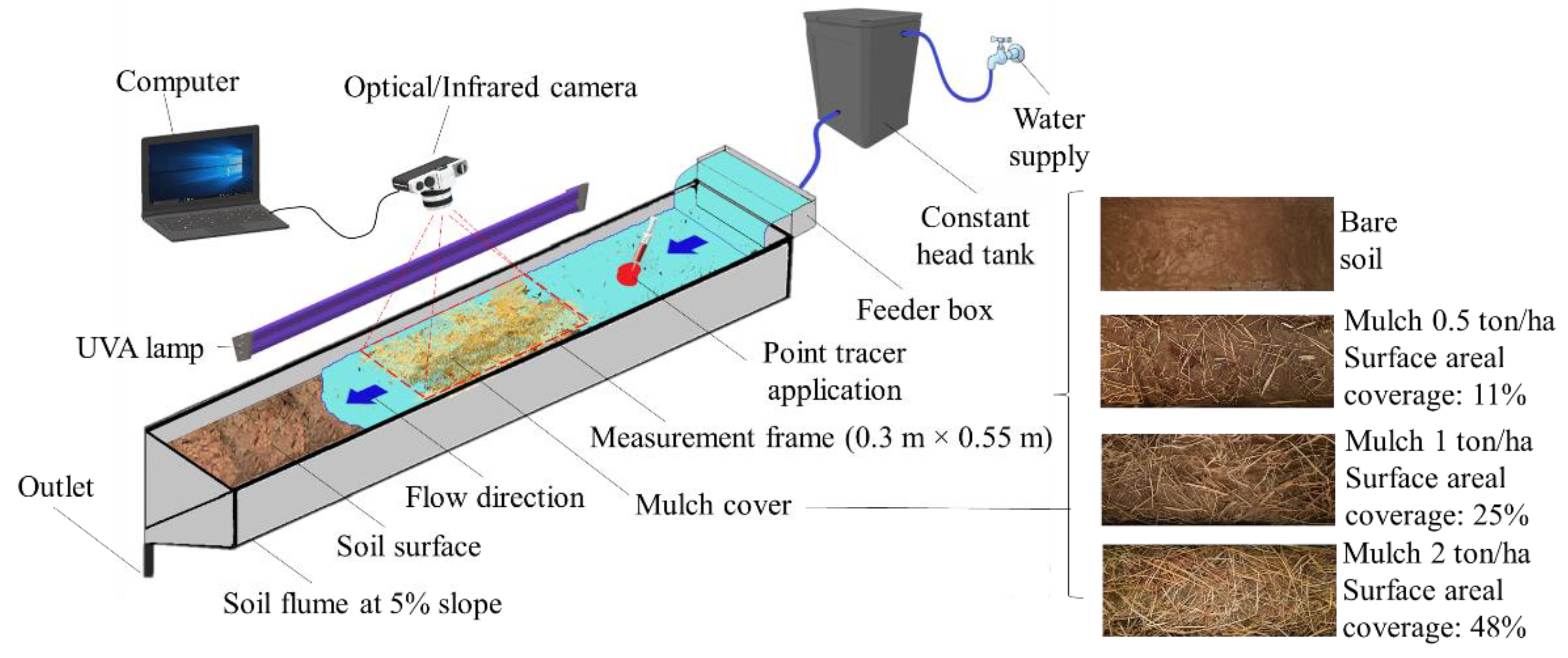
2.1. Laboratory Measurement Setup
- (a)
- A rectangular soil flume (3.0 m long and 0.3 m wide) adjusted to a constant slope of 5% (≈2.86°). The flume was prepared with a soil layer of 62 mm depth, with a bulk density of 1100 kg/m3.
- (b)
- An upstream water supply system that included a constant head tank and a feeder box, which applied a known constant upstream flow of approximately 0.14 L/s over the flume’s soil surface. The measuring frame was defined at 1.0 m distance from the upstream flume section (incoming flow section).
- (c)
- (d)
- Different flow velocity tracers (Section 2.3).
- (e)
- A tubular ultraviolet lamp UVA (BLB light bulb), used for the detection of the fluorescent quinine tracer, with properties: nominal power of 36 W; UVA irradiance @ 20 cm; 315–400 nm; 350 mW/cm2.
- (f)
- Optical and infrared sensors (Section 2.4).
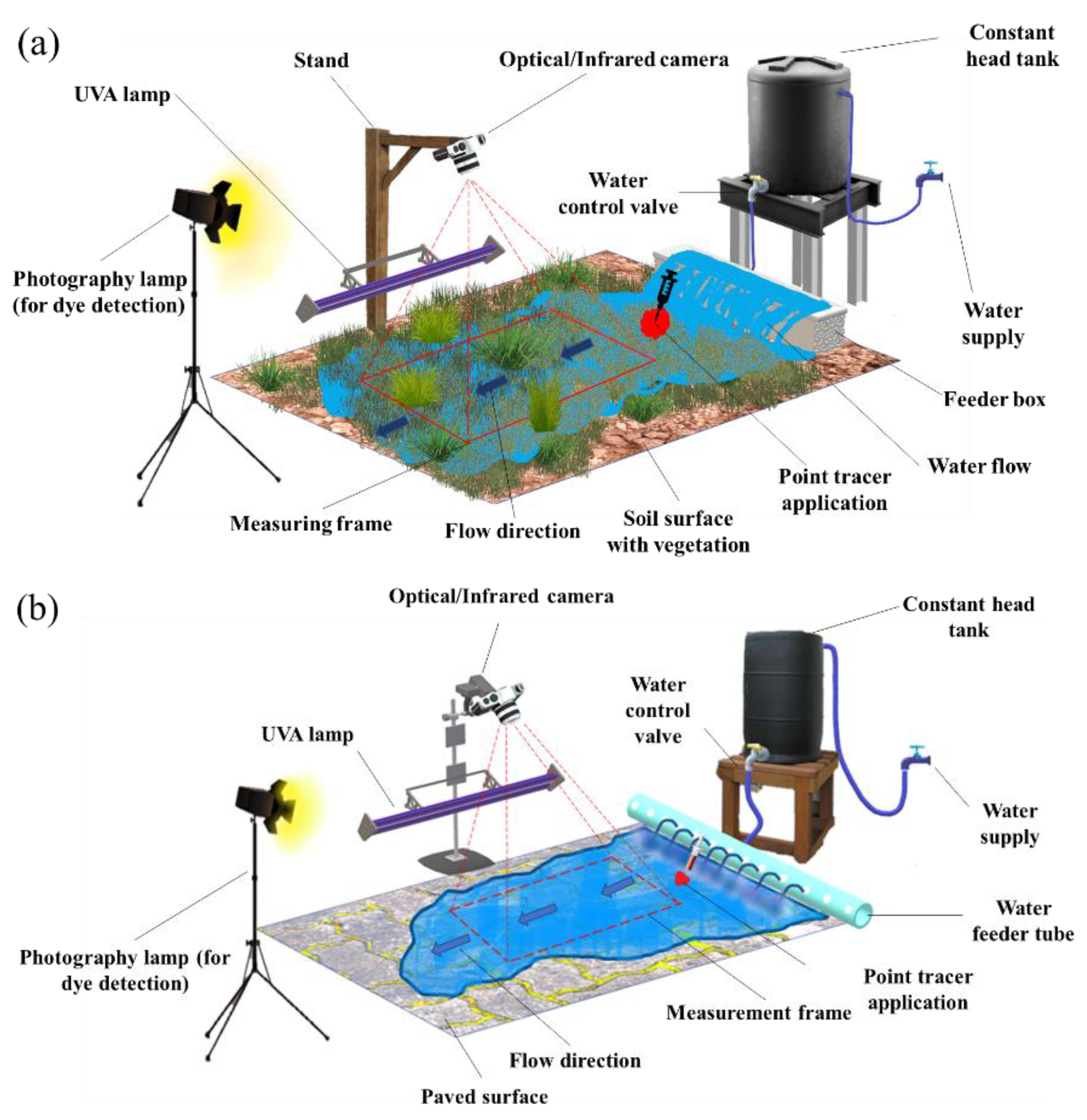
2.2. Field Measurement Setup
2.3. Tracers
2.3.1. Fluorescent Quinine
2.3.2. Colour Dye
2.3.3. Thermal
2.4. Video Recording Systems
2.5. Experimental Procedure
2.6. Velocity Estimation Method
2.7. Image Processing Method
3. Results and Discussion
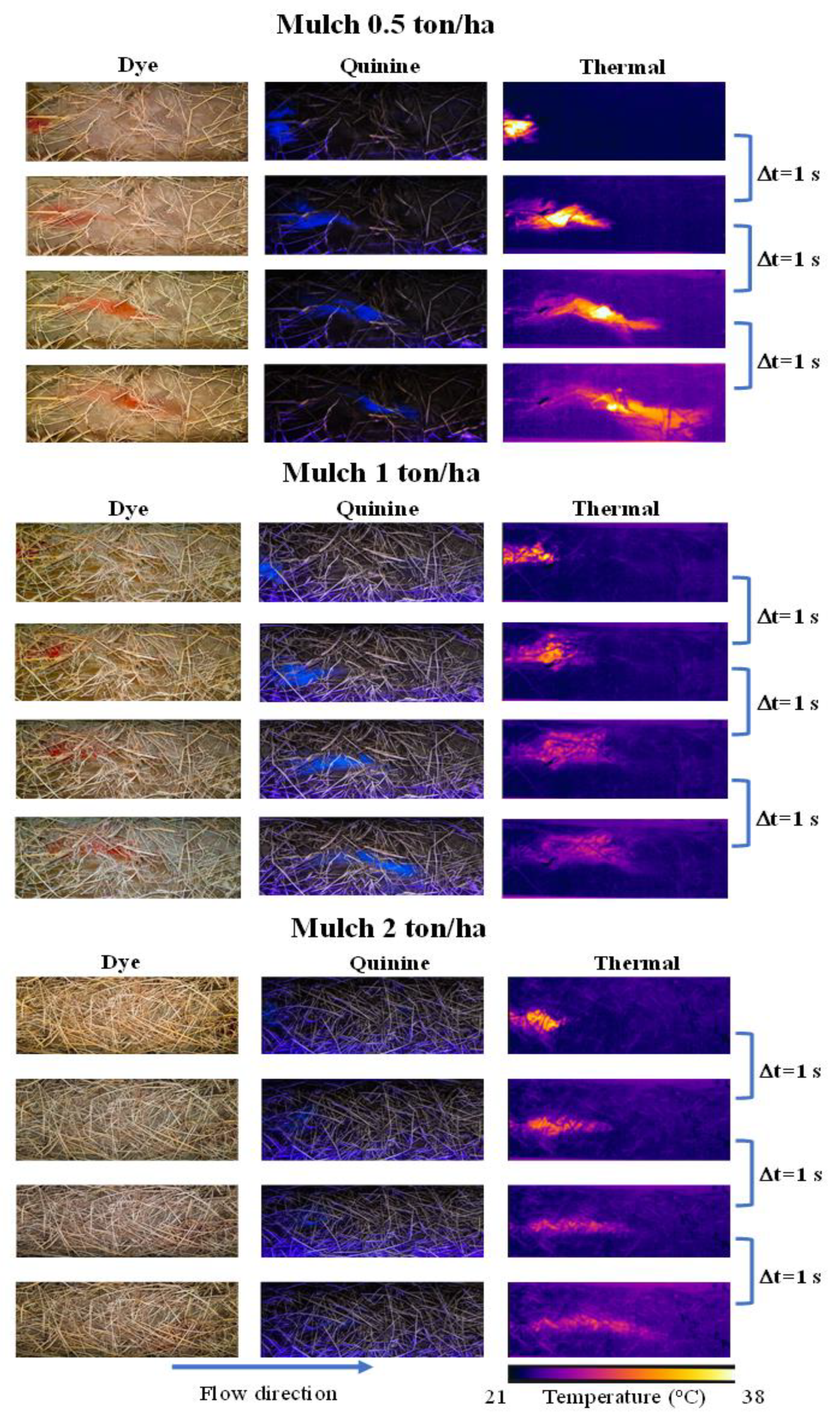
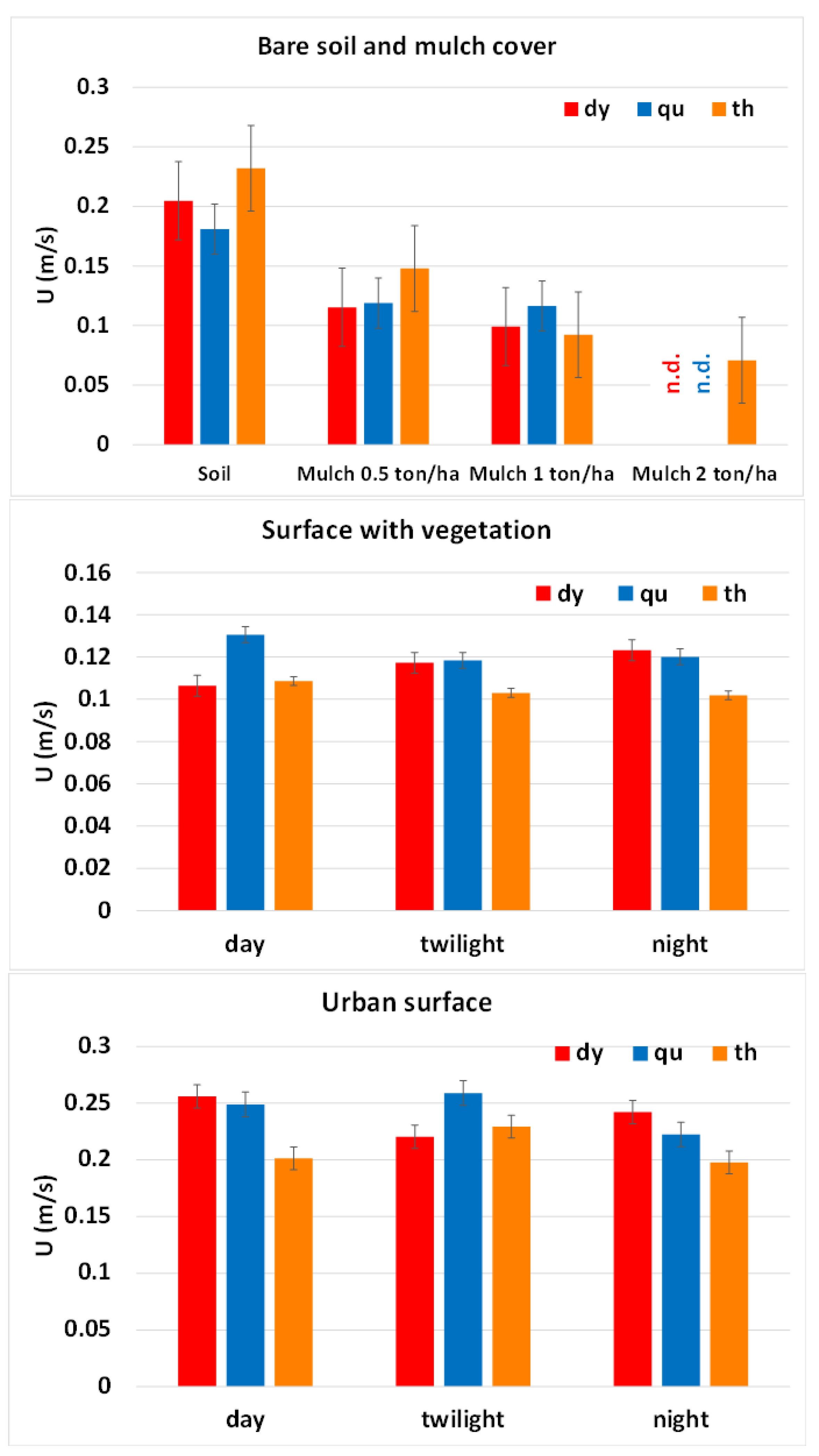
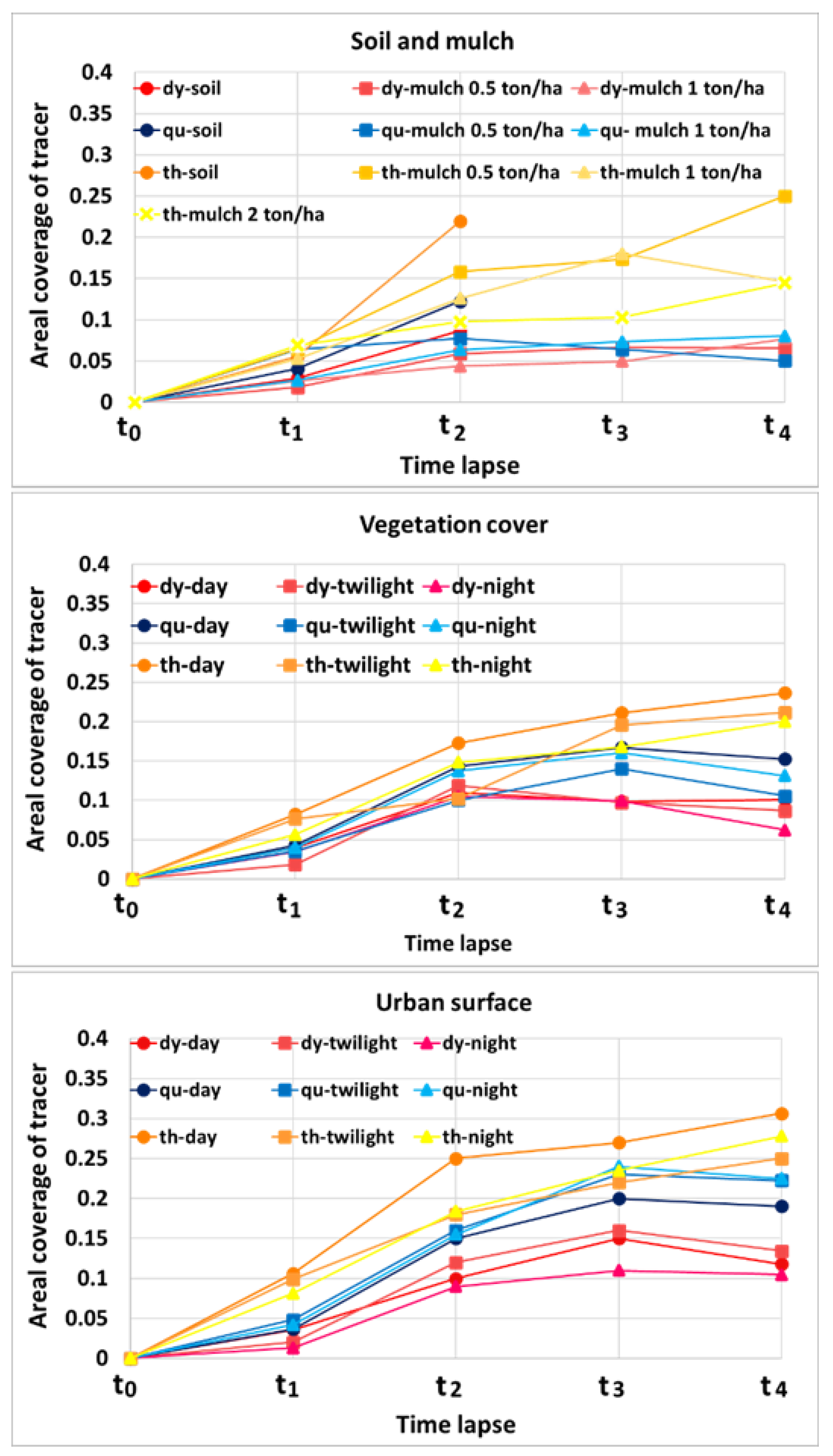
3.1. Laboratory Experiments
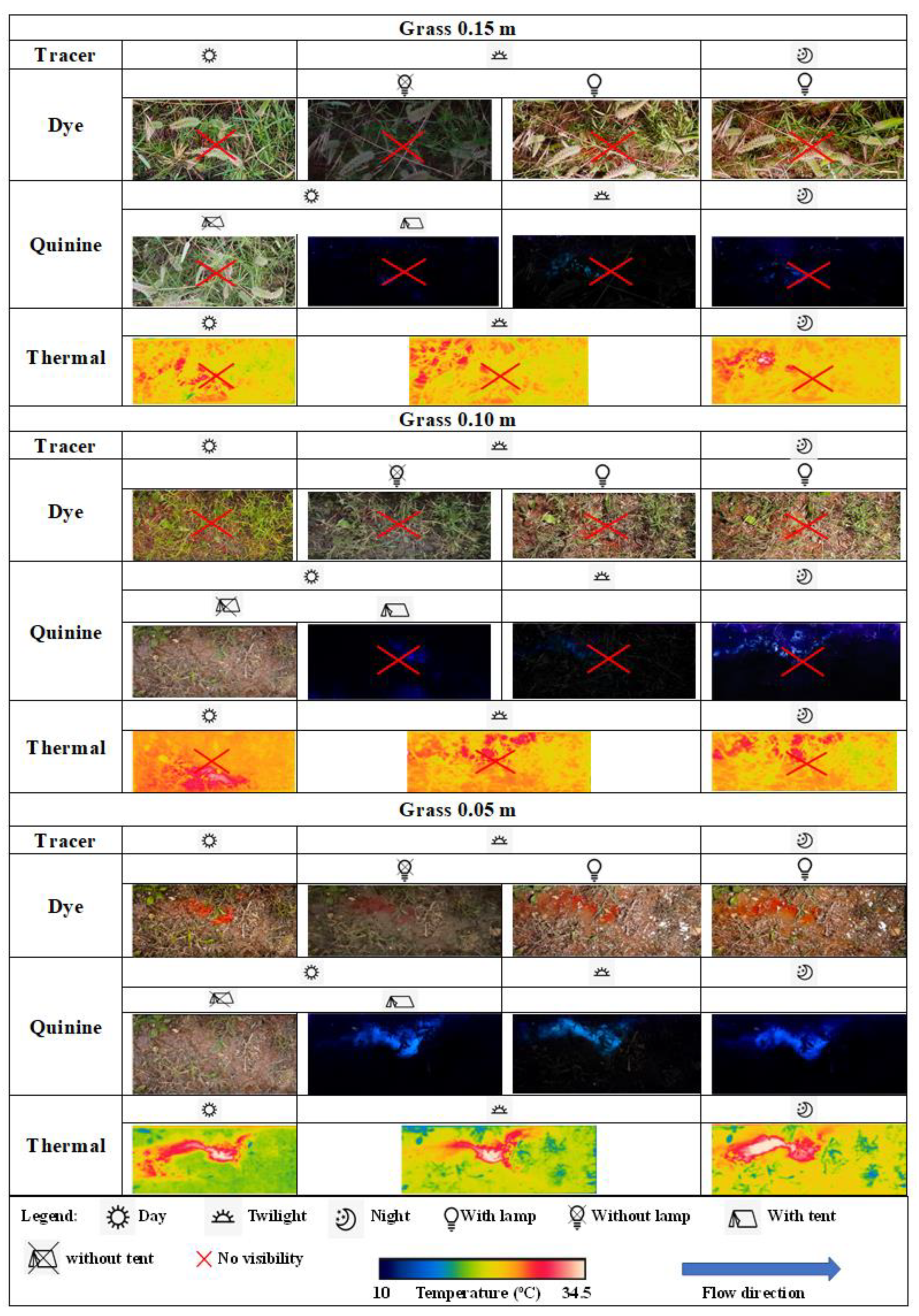
3.2. Field Experiments
3.3. Comparison of Flow Velocity Estimates
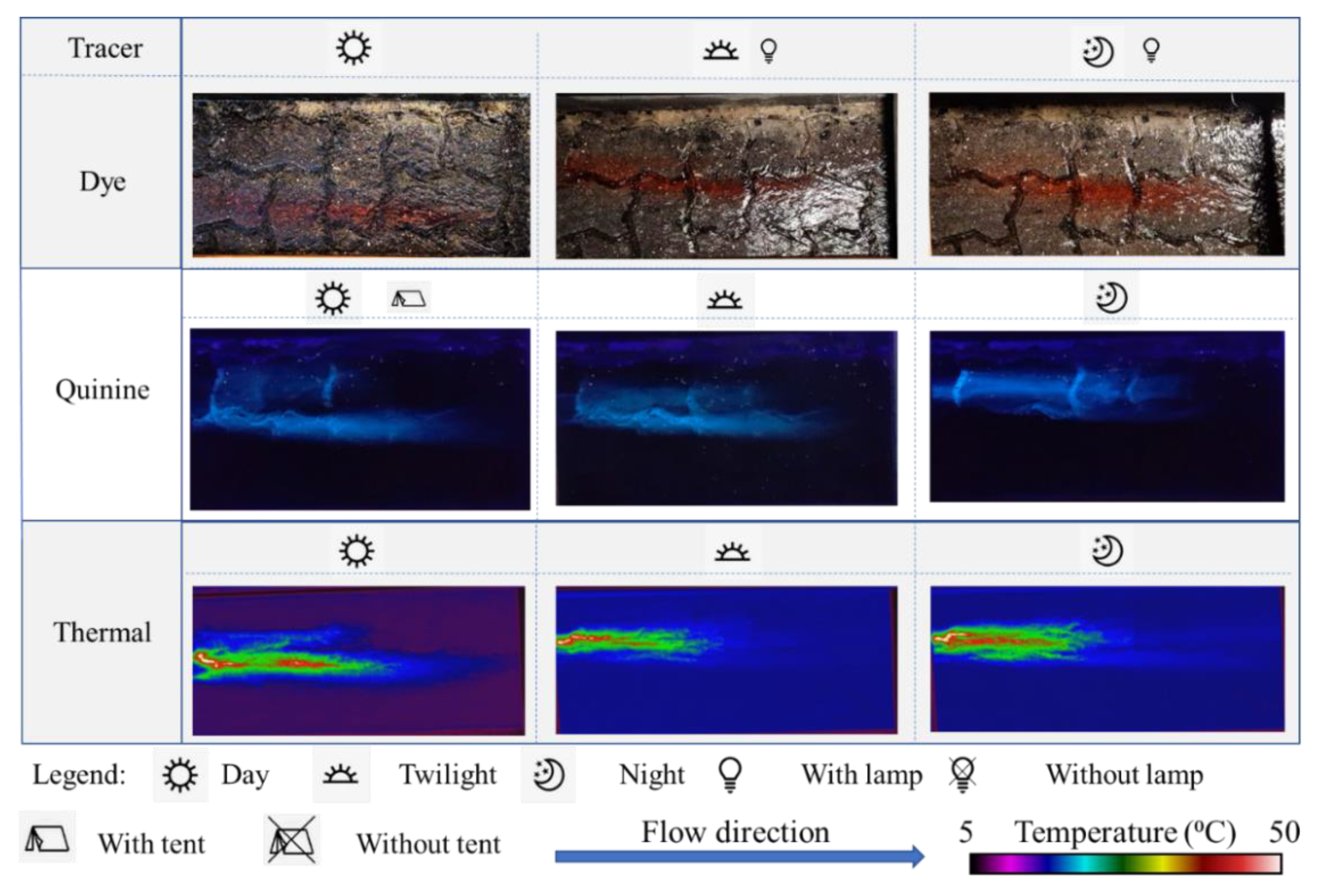
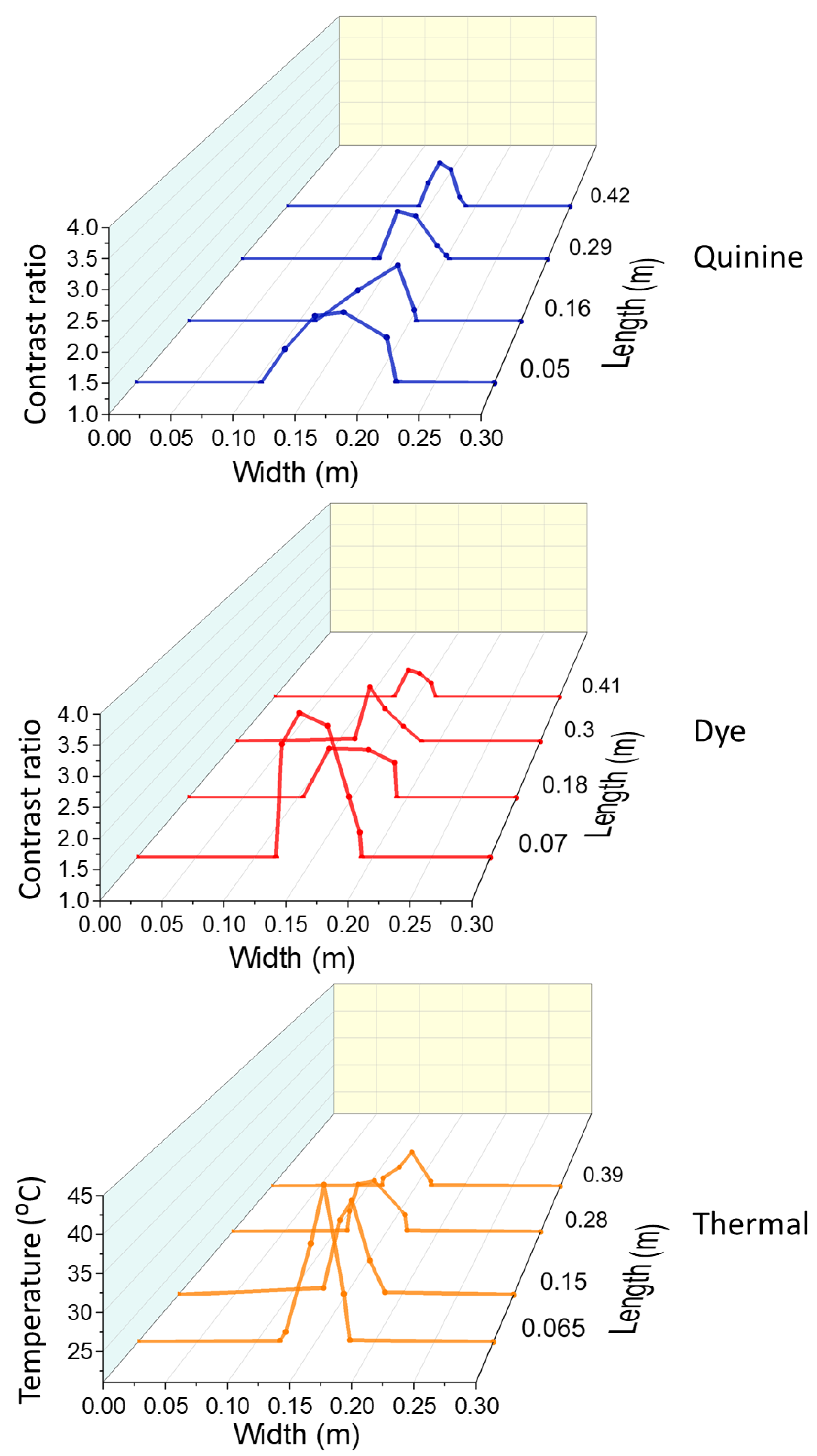
3.4. Comparison of the Tracer Visibility
3.5. Fluorescent Quinine Tracer: Main Advantages and Limitations
4. Conclusions
- Despite the quinine tracer’s fluorescence characteristics when exposed to UVA light, in daylight conditions the quinine tracer cannot be visually noticed unless a tent (i.e., a shading system, to dim the sunlight) is used. In dark conditions (twilight, night, etc.) the dye is not visible unless using an artificial light source. The thermal tracer can be used in all ambient light conditions due to the capability of infrared cameras for detecting heat; therefore, the performance of the thermal tracer technique is not affected by ambient light limitations.
- As observed in the conducted laboratory and field experiments, the quinine tracer is better visualised than the dye tracer, which is confirmed by the relative luminance and contrast ratio revealed by these two tracers.
- By considering the specifications of the fluorescent tracer (quinine concentration and pH of the solution, volume of tracer used), the restrictions of using the fluorescent quinine as a tracer are: (i) impossible to use in bright light conditions; (ii) not suitable to use in presence of dense or tall vegetation cover or dense mulch, i.e., when the vegetation/mulch offers a percentage surface coverage that exceeds about 25–30%.
- The main advantages of using fluorescent quinine as a tracer are: (i) simple experimental setup requirement (e.g., UVA lamp, a normal optical camera, and water with quinine, which are all inexpensive and easily accessible materials); (ii) the high visibility of the injected tracer under low-luminosity conditions (e.g., field measurements in dark conditions, at night, twilight, shielded environments or close conduits); (iii) it is nontoxic to the environment, due to the very low concentration needed to produce high fluorescence (around 80 mg/L, which is also used, e.g., for human consumption in tonic water).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calkins, D.; Dunne, T. A salt tracing method for measuring channel velocities in small mountain streams. J. Hydrol. 1970, 11, 379–392. [Google Scholar] [CrossRef]
- Comiti, F.; Mao, L.; Wilcox, A.; Wohl, E.E.; Lenzi, M.A. Field-derived relationships for flow velocity and resistance in high-gradient streams. J. Hydrol. 2007, 340, 48–62. [Google Scholar] [CrossRef]
- Jodeau, M.; Hauet, A.; Paquier, A.; Le Coz, J.; Dramais, G. Application and evaluation of LS-PIV technique for the monitoring of river surface velocities in high flow conditions. Flow Meas. Instrum. 2008, 19, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Tazioli, A. Experimental methods for river discharge measurements: Comparison among tracers and current meter. Hydrol. Sci. J. 2011, 56, 1314–1324. [Google Scholar] [CrossRef]
- Tauro, F.; Grimaldi, S.; Petroselli, A.; Porfiri, M. Fluorescent particle tracers for surface flow measurements: A proof of concept in a natural stream. Water Resour. Res. 2012, 48, W06528. [Google Scholar] [CrossRef]
- Horton, R.E.; Leach, H.R.; Van Vliet, R. Laminar sheet-flow. Eos Trans. Am. Geophys. Union 1934, 15, 393–404. [Google Scholar] [CrossRef]
- Emmett, W.W. The Hydraulics of Overland Flow on Hillslopes; US Government Printing Office: Washington, DC, USA, 1970; Volume 662. [Google Scholar] [CrossRef]
- Abrahams, A.D.; Parsons, A.J.; Luk, S.H. Field measurement of the velocity of overland flow using dye tracing. Earth Surf. Process. Landf. 1986, 11, 653–657. [Google Scholar] [CrossRef]
- de Lima, J.L.M.P.; Abrantes, J.R.C.B. Using a thermal tracer to estimate overland and rill flow velocities. Earth Surf. Process. Landf. 2014, 30, 1293–1300. [Google Scholar] [CrossRef]
- Abrantes, J.R.; Moruzzi, R.B.; Silveira, A.; de Lima, J.L.M.P. Comparison of thermal, salt and dye tracing to estimate shallow flow velocities: Novel triple-tracer approach. J. Hydrol. 2018, 557, 362–377. [Google Scholar] [CrossRef]
- Tauro, F.; Grimaldi, S.; Petroselli, A.; Rulli, M.C.; Porfiri, M. Fluorescent particle tracers in surface hydrology: A proof of concept in a semi-natural hillslope. Hydrol. Earth Syst. Sci. 2012, 16, 2973. [Google Scholar] [CrossRef] [Green Version]
- Mujtaba, B.; de Lima, J.L. Laboratory testing of a new thermal tracer for infrared-Based PTV technique for shallow overland flows. Catena 2018, 169, 69–79. [Google Scholar] [CrossRef]
- Lei, T.; Chuo, R.; Zhao, J.; Shi, X.; Liu, L. An improved method for shallow water flow velocity measurement with practical electrolyte inputs. J. Hydrol. 2010, 390, 45–56. [Google Scholar] [CrossRef]
- Schuetz, T.; Weiler, M.; Lange, J.; Stoelzle, M. Two-dimensional assessment of solute transport in shallow waters with thermal imaging and heated water. Adv. Water Resour. 2012, 43, 67–75. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Meng, Y.; Xia, C.; Chen, K.; Chen, Y. Using stable isotopes as tracer to investigate hydrological condition and estimate water residence time in a plain region, Chengdu China. Sci. Rep. 2021, 11, 2812. [Google Scholar] [CrossRef]
- Abrahams, A.D.; Atkinson, J.F. Relation between grain velocity and sediment concentration in overland flow. Water Resour. Res. 1993, 29, 3021–3028. [Google Scholar] [CrossRef]
- Li, G.; Abrahams, A.D.; Atkinson, J.F. Correction factors in the determination of mean velocity of overland flow. Earth Surf. Process. Landf. 1996, 21, 509–515. [Google Scholar] [CrossRef]
- Giménez, R.; Govers, G. Flow detachment by concentrated flow on smooth and irregular beds. Soil Sci. Soc. Am. J. 2002, 66, 1475–1483. [Google Scholar] [CrossRef]
- Li, Z.B.; Lu, X.K.; Ding, F.W. Experimental study on dynamic processes of soil erosion on loess slope. J. Soil Water Conserv. 2002, 16, 5–7. [Google Scholar]
- Tatard, L.; Planchon, O.; Wainwright, J.; Nord, G.; Favis-Mortlock, D.; Silvera, N.; Ribolzi, O.; Esteves, M.; Huang, C.H. Measurement and modelling of high-resolution flow-velocity data under simulated rainfall on a low-slope sandy soil. J. Hydrol. 2008, 348, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, S.; Seeger, M.; Ries, J.B. Field experiments for understanding and quantification of rill erosion process. Catena 2012, 91, 21–34. [Google Scholar] [CrossRef]
- de Lima, R.L.; Abrantes, J.R.; de Lima, J.L.M.P.; de Lima, M.I.P. Using thermal tracers to estimate flow velocities of shallow flows: Laboratory and field experiments. J. Hydrol. Hydromech. 2015, 63, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Abrantes, J.R.; Moruzzi, R.B.; de Lima, J.L.M.P.; Silveira, A.; Montenegro, A.A. Combining a thermal tracer with a transport model to estimate shallow flow velocities. Phys. Chem. Earth Parts A/B/C 2019, 109, 59–69. [Google Scholar] [CrossRef]
- Zhang, G.H.; Luo, R.T.; Cao, Y.; Shen, R.C.; Zhang, X.C. Correction factor to dye-measured flow velocity under varying water and sediment discharges. J. Hydrol. 2010, 389, 205–213. [Google Scholar] [CrossRef]
- Li, G.; Abrahams, A.D. Effect of saltating sediment load on the determination of the mean velocity of overland flow. Water Resour. Res. 1997, 33, 341–347. [Google Scholar] [CrossRef]
- Gilley, J.E.; Finkner, S.C.; Doran, J.W.; Kottwitz, E.R. Adsorption of bromide tracers onto sediment. Appl. Eng. Agric. 1990, 6, 35–38. [Google Scholar] [CrossRef]
- Buzády, A.; Erostyák, J.; Paál, G. Determination of uranine tracer dye from underground water of Mecsek Hill, Hungary. J. Biochem. Biophys. Methods 2006, 69, 207–214. [Google Scholar] [CrossRef]
- Leibundgut, C.; Maloszewski, P.; Külls, C. Tracers in Hydrology; Wiley-Blackwell: Hoboken, NJ, USA; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- de Lima, J.L.M.P.; Zehsaz, S.; de Lima, M.I.P.; Isidoro, J.M.; Jorge, R.G.; Martins, R.G. Using Quinine as a Fluorescent Tracer to Estimate Overland Flow Velocities on Bare Soil: Proof of Concept under Controlled Laboratory Conditions. Agronomy 2021, 11, 1444. [Google Scholar] [CrossRef]
- Jones, W.K. Water tracing in karst aquifers. In Encyclopedia of Caves; Academic Press: Cambridge, MA, USA, 2019; pp. 1144–1155. [Google Scholar] [CrossRef]
- Aley, T.; Fletcher, M.W. The water tracer’s cookbook. Mo. Speleol. 1976, 16, 1–32. [Google Scholar]
- Montenegro, A.A.A.; Abrantes, J.R.C.B.; de Lima, J.L.M.P.; Singh, V.P.; Santos, T.E.M. Impact of mulching on soil and water dynamics under intermittent simulated rainfall. Catena 2013, 109, 139–149. [Google Scholar] [CrossRef]
- Abrantes, J.R.C.B.; Prats, S.A.; Keizer, J.J.; de Lima, J.L.M.P. Effectiveness of the application of rice straw mulching strips in reducing runoff and soil loss: Laboratory soil flume experiments under simulated rainfall. Soil Tillage Res. 2018, 180, 238–249. [Google Scholar] [CrossRef]
- de Lima, J.L.M.P.; Singh, V.P.; de Lima, M.I.P. The influence of storm movement on water erosion: Storm direction and velocity effects. Catena 2003, 52, 39–56. [Google Scholar] [CrossRef]
- Gelotte, K. Image Color Extract, Cool PHP Tools. Available online: https://www.coolphptools.com/color_extract (accessed on 21 August 2021).
- Abrantes, J.R.; de Lima, J.L.M.P.; Prats, S.A.; Keizer, J.J. Assessing soil water repellency spatial variability using a thermographic technique: An exploratory study using a small-scale laboratory soil flume. Geoderma 2017, 287, 98–104. [Google Scholar] [CrossRef]
- Diener, H.C.; Dethlefsen, U.; Dethlefsen-Gruber, S.; Verbeek, P. Effectiveness of quinine in treating muscle cramps: A double-blind, placebo-controlled, parallel-group, multicentre trial. Int. J. Clin. Pract. 2002, 56, 243–246. [Google Scholar] [PubMed]
- Geto, A.; Amare, M.; Tessema, M.; Admassie, S. Polymer-modified glassy carbon electrode for the electrochemical detection of quinine in human urine and pharmaceutical formulations. Anal. Bioanal. Chem. 2012, 404, 525–530. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Food and Drugs. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.575 (accessed on 11 June 2020).
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Flavouring Group Evaluation 35, Revision 1 (FGE. 35Rev1): Three quinine salts from the Priority list from chemical group 30. EFSA J. 2015, 13, 4245. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, J.N.; Shevchenko, T.; Magon, A.; Bohne, C. Paper acidity estimation: Application of pH-dependent fluorescence probes. J. Photochem. Photobiol. A Chem. 1998, 113, 189–195. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Safety Data Sheet Quinine Monohydrochloride Dihydrate 99%. Available online: https://www.alfa.com/en/msds/?language=EN&subformat=AGHS&sku=H33474 (accessed on 18 February 2021).
- Bunte, K.; Poesen, J. Effects of rock fragment size and cover on overland flow hydraulics, local turbulence and sediment yield on an erodible soil surface. Earth Surf. Process. Landf. 1994, 19, 115–135. [Google Scholar] [CrossRef]
- Aziz, N.M.; Scott, D.E. Experiments on sediment transport in shallow flows in high gradient channels. Hydrol. Sci. J. 1989, 34, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Dunkerley, D. Estimating the mean speed of laminar overland flow using dye injection-uncertainty on rough surfaces. Earth Surf. Process. Landf. 2001, 26, 363–374. [Google Scholar] [CrossRef]
- Ali, M.; Sterk, G.; Seeger, M.; Stroosnijder, L. Effect of flow discharge and median grain size on mean flow velocity under overland flow. J. Hydrol. 2012, 452, 150–160. [Google Scholar] [CrossRef]
- Pan, C.; Shangguan, Z.; Ma, L. Assessing the dye-tracer correction factor for documenting the mean velocity of sheet flow over smooth and grassed surfaces. Hydrol. Process. 2015, 29, 5369–5382. [Google Scholar] [CrossRef]
- Polyakov, V.; Li, L.; Nearing, M.A. Correction factor for measuring mean overland flow velocities on stony surfaces under rainfall using dye tracer. Geoderma 2021, 390, 114975. [Google Scholar] [CrossRef]
- Stone, M.C. A Field Guide to Digital Color; AK Peters: Netik, MA, USA, 2016. [Google Scholar] [CrossRef]
- Caldwell, B.; Cooper, M.; Reid, L.G.; Vanderheiden, G.; Chisholm, W.; Slatin, J.; White, J. Web Content Accessibility Guidelines (WCAG) 2.0; WWW Consortium (W3C): Cambridge, MA, USA, 2008; Volume 290. [Google Scholar]
- Poynton, C. Digital Video and HD: Algorithms and Interfaces, 2nd ed.; Morgan Kaufmann Publishers: San Francisco, CA, USA, 2012. [Google Scholar] [CrossRef]
- Mag-isa, A.E.; Lee, C.K.; Kim, S.M.; Kim, J.H.; Oh, C.S. Rapid determination of the number of graphene layers by using relative luminance. Carbon 2015, 94, 646–649. [Google Scholar] [CrossRef]
- FLIR Systems. Infrared Training Center (ITC): Infrared Thermography Manual for the Construction and Renewable Energy Market; FLIR Systems AB: Wilsonville, OR, USA, 2011. [Google Scholar]







| Surface | Day | Vegetation Height (m) | Discharge (L/s) | Slope Gradient (%) | Air Temperature (°C) | Flow Water Temperature (°C) | Sunset Time (hh:mm) |
|---|---|---|---|---|---|---|---|
| Vegetated soil | 1 | 0.15 | 0.40 | 25 | 23 | 20 | 20:51 |
| 2 | 0.10 | 0.39 | 25 | 23 | 21 | 20:52 | |
| 3 | 0.05 | 0.40 | 25 | 22 | 21 | 20:53 | |
| Paved | 1 | - | 0.21 | 20 | 25 | 20 | 20:51 |
| Surface | Q (L/s) | S (%) | Re | α |
|---|---|---|---|---|
| Bare soil and mulched surface | 0.14 | 5 | 474 | 0.62 |
| Vegetated surface (vegetation height of 0.05 m) | 0.40 | 25 | 1395 | 0.60 |
| Urban paved surface | 0.21 | 20 | 702 | 0.57 |
| Bare Soil and Mulch Cover | Surface with 0.05 m Vegetation Cover | Urban Surface | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface | Test number | Mean Velocity U (m/s) | Observation time | Test number | Mean Velocity U (m/s) | Observation time | Test number | Mean Velocity U (m/s) | ||||||
| Dye | Quinine | Thermal | Dye | Quinine (with tent) | Thermal | Dye | Quinine (with tent) | Thermal | ||||||
| Bare soil | 1 | 0.205 | 0.179 | 0.230 | Day (1 h before the sunset) | 1 | 0.103 | 0.128 | 0.104 | Day (1 h before the sunset) | 1 | 0.257 | 0.257 | 0.197 |
| 2 | 0.193 | 0.183 | 0.234 | 2 | 0.108 | 0.132 | 0.101 | 2 | 0.255 | 0.262 | 0.205 | |||
| 3 | 0.216 | 0.181 | - | 3 | 0.108 | 0.131 | 0.120 | 3 | 0.257 | 0.228 | - | |||
| Mean | 0.205 | 0.181 | 0.232 | Mean | 0.106 | 0.131 | 0.109 | Mean | 0.256 | 0.249 | 0.201 | |||
| S.D. | 0.010 | 0.002 | 0.002 | S.D. | 0.002 | 0.002 | 0.008 | S.D. | 0.001 | 0.015 | 0.004 | |||
| Dye (with light) | Quinine | Thermal | Dye (with light) | Quinine | Thermal | |||||||||
| 0.5 ton/ha mulch | 1 | 0.117 | 0.133 | 0.175 | Twilight (during the 30 min after the sunset) | 1 | 0.116 | 0.120 | 0.097 | Twilight (during the 30 min after the sunset) | 1 | 0.209 | 0.254 | 0.222 |
| 2 | 0.121 | 0.143 | 0.140 | 2 | 0.117 | 0.118 | 0.102 | 2 | 0.227 | 0.257 | 0.236 | |||
| 3 | 0.108 | 0.081 | 0.128 | 3 | 0.119 | 0.118 | 0.110 | 3 | 0.224 | 0.265 | - | |||
| Mean | 0.115 | 0.119 | 0.148 | Mean | 0.117 | 0.118 | 0.103 | Mean | 0.220 | 0.259 | 0.229 | |||
| S.D. | 0.006 | 0.027 | 0.020 | S.D. | 0.001 | 0.001 | 0.005 | S.D. | 0.008 | 0.005 | 0.007 | |||
| Dye (with light) | Quinine | Thermal | Dye (with light) | Quinine | Thermal | |||||||||
| 1 ton/ha mulch | 1 | 0.079 | 0.110 | 0.098 | Night (1 h after the sunset) | 1 | 0.124 | 0.119 | 0.101 | Night (1 h after the sunset) | 1 | 0.233 | 0.204 | 0.188 |
| 2 | 0.114 | 0.125 | 0.103 | 2 | 0.120 | 0.119 | 0.105 | 2 | 0.247 | 0.229 | 0.188 | |||
| 3 | 0.105 | 0.115 | 0.076 | 3 | 0.125 | 0.123 | 0.100 | 3 | 0.246 | 0.234 | 0.217 | |||
| Mean | 0.099 | 0.116 | 0.092 | Mean | 0.123 | 0.120 | 0.102 | Mean | 0.242 | 0.222 | 0.198 | |||
| S.D. | 0.015 | 0.006 | 0.012 | S.D. | 0.002 | 0.002 | 0.002 | S.D. | 0.006 | 0.013 | 0.013 | |||
| 2 ton/ha mulch | 1 | - | - | 0.071 | ||||||||||
| 2 | - | - | 0.070 | |||||||||||
| 3 | - | - | 0.071 | |||||||||||
| Mean | - | - | 0.071 | |||||||||||
| S.D. | - | - | 0.001 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehsaz, S.; de Lima, J.L.M.P.; de Lima, M.I.P.; Isidoro, J.M.G.P.; Martins, R. Estimating Sheet Flow Velocities Using Quinine as a Fluorescent Tracer: Bare, Mulched, Vegetated and Paved Surfaces. Agronomy 2022, 12, 2687. https://doi.org/10.3390/agronomy12112687
Zehsaz S, de Lima JLMP, de Lima MIP, Isidoro JMGP, Martins R. Estimating Sheet Flow Velocities Using Quinine as a Fluorescent Tracer: Bare, Mulched, Vegetated and Paved Surfaces. Agronomy. 2022; 12(11):2687. https://doi.org/10.3390/agronomy12112687
Chicago/Turabian StyleZehsaz, Soheil, João L. M. P. de Lima, M. Isabel P. de Lima, Jorge M. G. P. Isidoro, and Ricardo Martins. 2022. "Estimating Sheet Flow Velocities Using Quinine as a Fluorescent Tracer: Bare, Mulched, Vegetated and Paved Surfaces" Agronomy 12, no. 11: 2687. https://doi.org/10.3390/agronomy12112687







