The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Methods
2.1.1. Nematode Extraction and Analysis
2.1.2. Physicochemical Analyses
2.2. Statistical Analyses
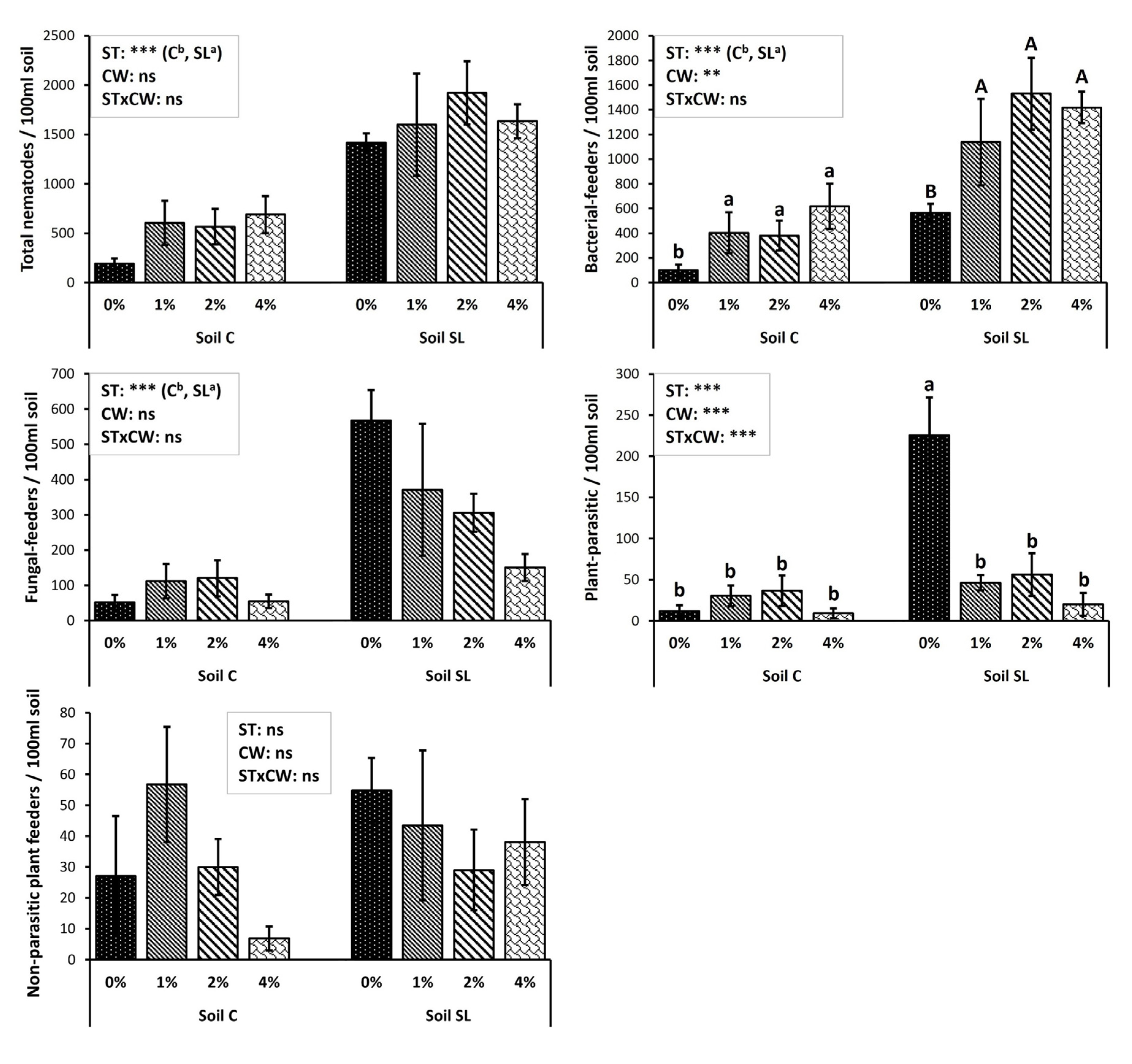
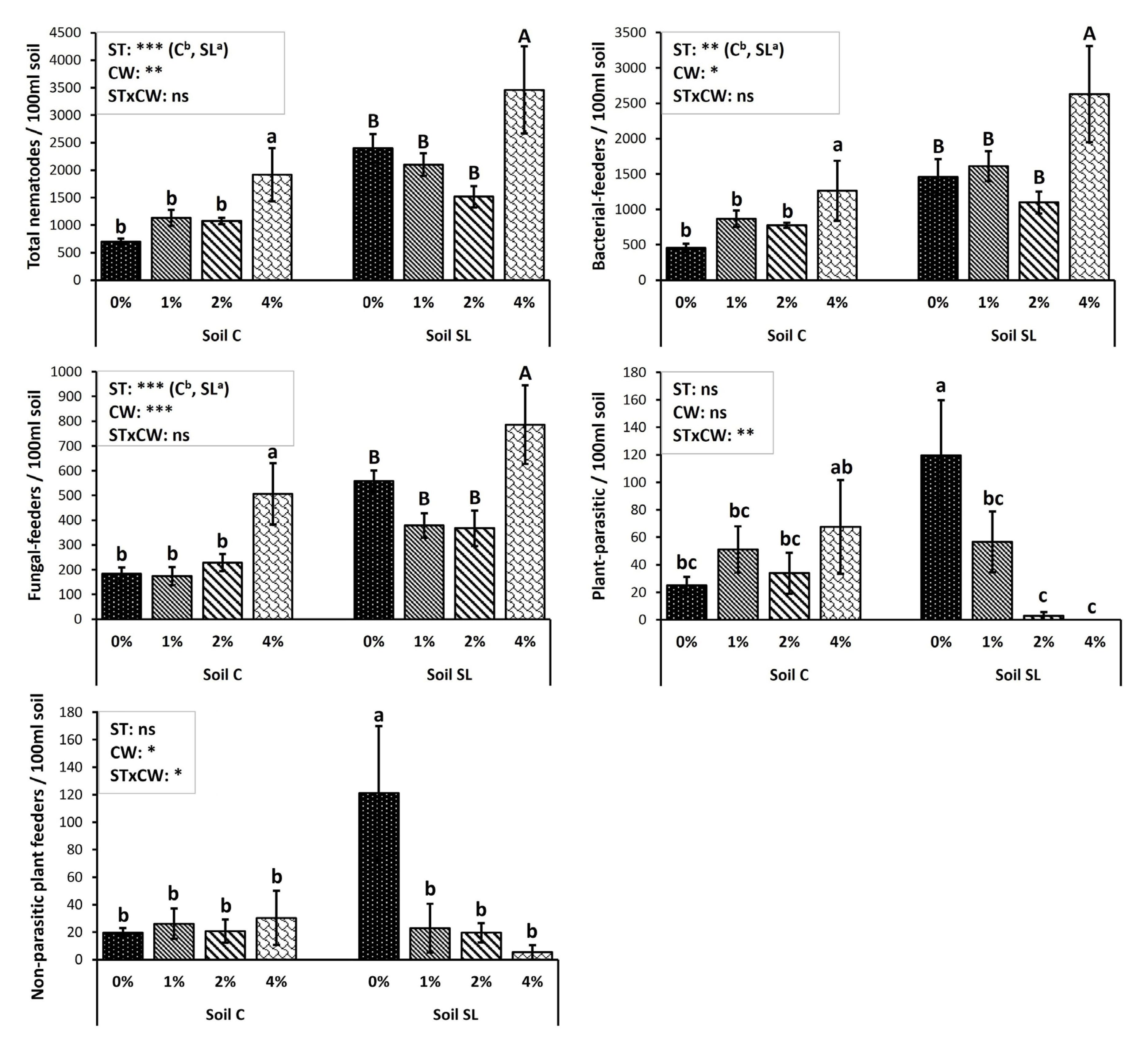
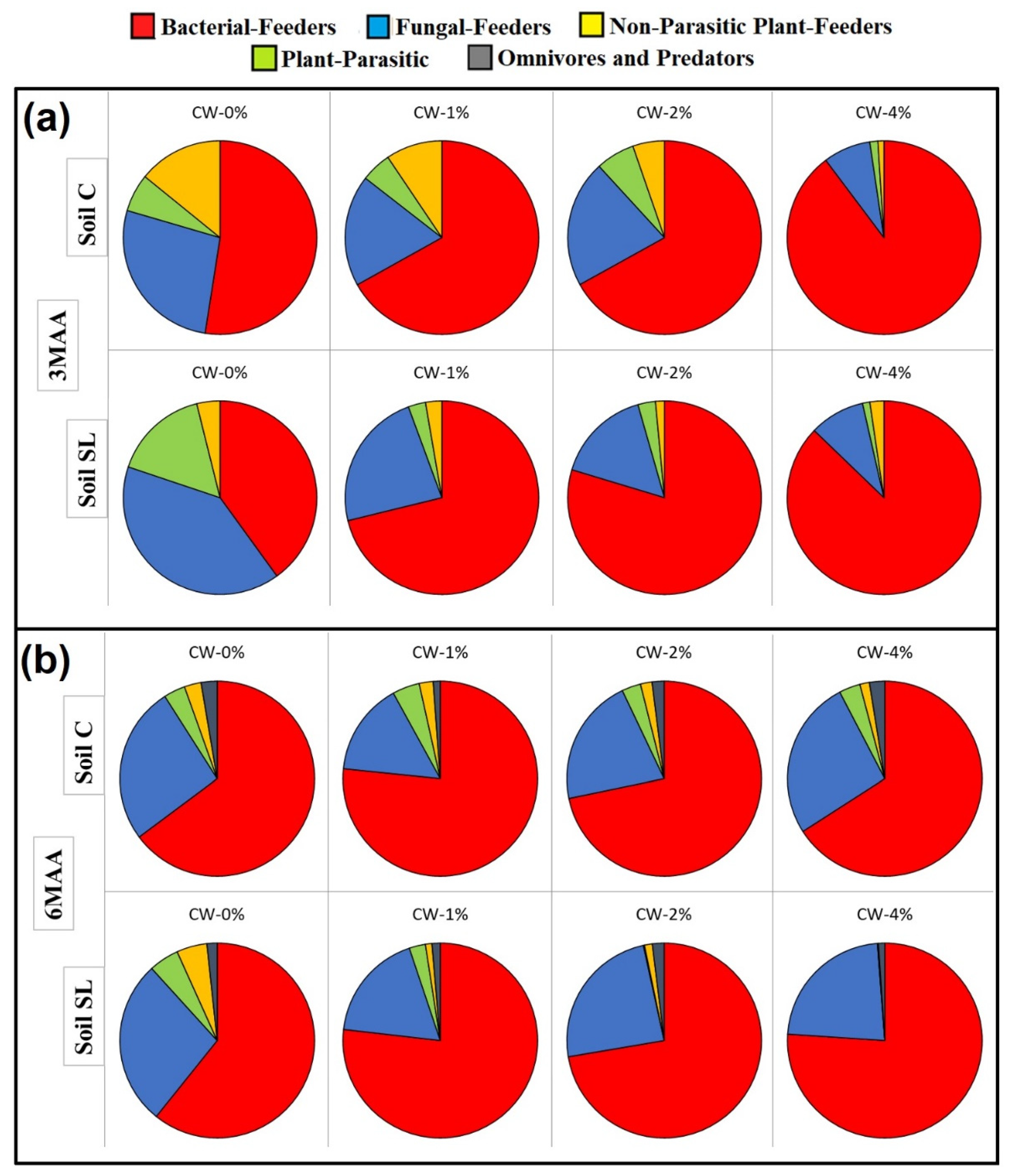
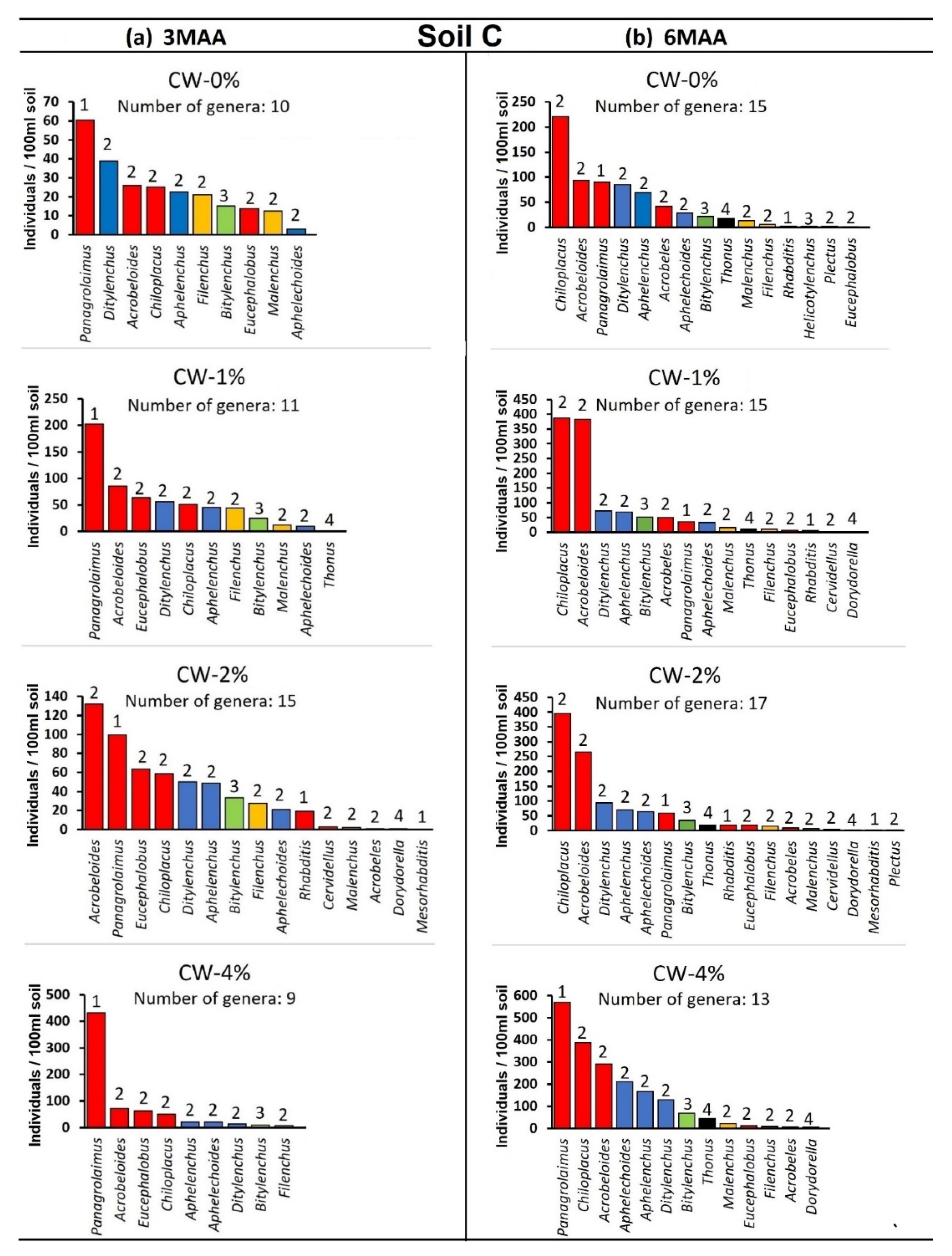
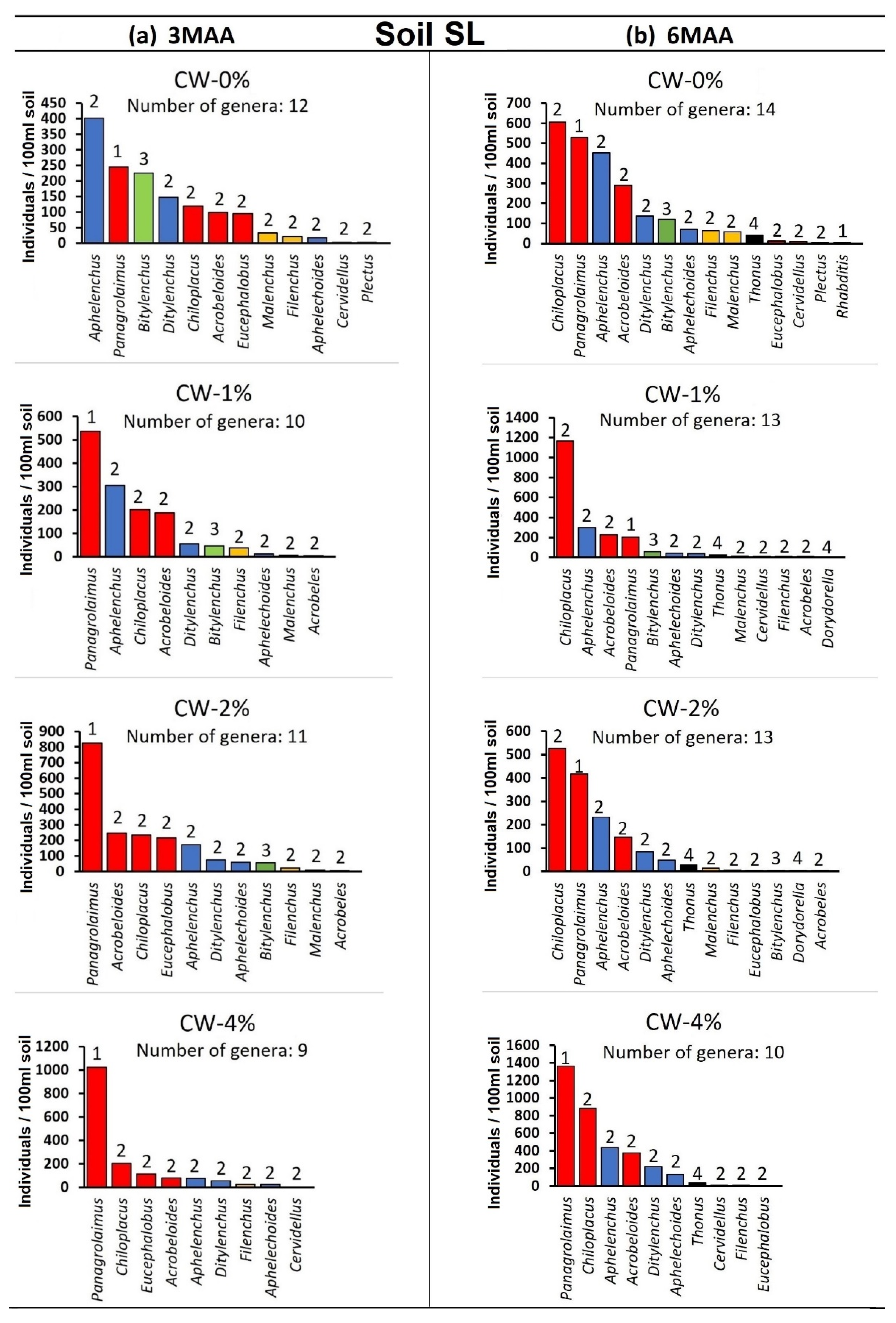
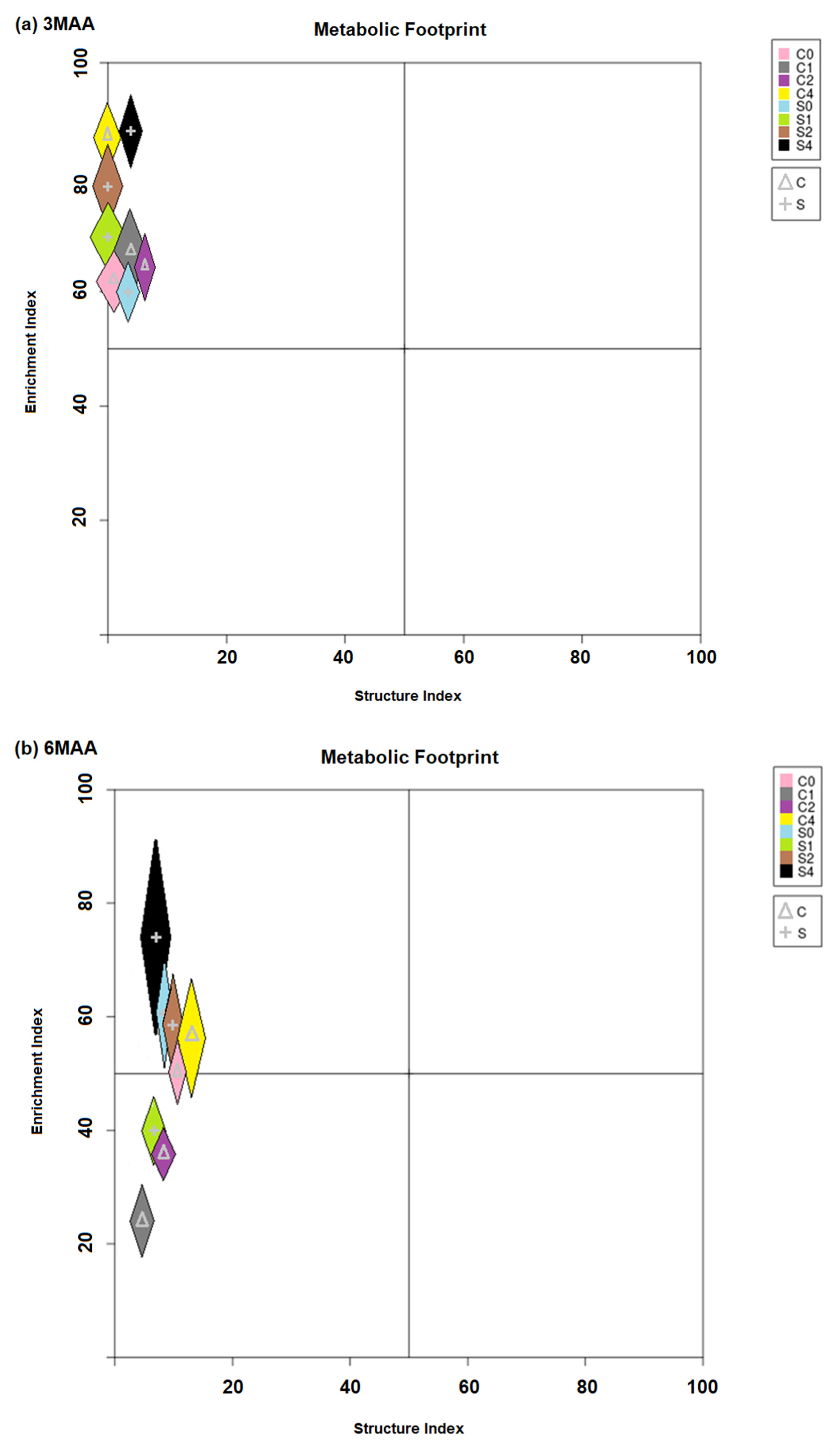
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization. World Coffee Consumption. 2022. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on 3 July 2022).
- Tokimoto, T.; Kawasaki, N.; Nakamura, T.; Akutagawa, J.; Tanada, S. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid Interface Sci. 2005, 281, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Mello, F.V.C.; Filho, S.T.; Carpes, R.M.; Honório, J.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Randell, P.; Pickin, J.; Grant, B. Waste Generation and Resource Recovery in Australia. Reporting Period 2010/11. Final Report Version 2.6; Blue Environment Pty Ltd: Docklands, Australia, 2014. [Google Scholar]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.A.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Martín-García, J.M.; Delgado, R.; Sánchez-Marañón, M.; Delgado, G. Short-term effects of spent coffee grounds on the physical properties of two Mediterranean agricultural soils. Int. Agrophys. 2019, 33, 205–216. [Google Scholar] [CrossRef]
- Afriliana, A.; Hidayat, E.; Yoshiharu, M.; Taizo, M.; Harada, H. Evaluation of Potency Spent Coffee Grounds for Make Black Compost. E3s Web Conf. 2020, 142, 04002. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling coffee grounds and tea leaf wastes to improve the yield and mineral content of grains of paddy rice. J. Sci. Food Agric. 2011, 91, 2108–2111. [Google Scholar] [CrossRef]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field evaluation of coffee grounds application for crop growth enhancement, weed control, and soil improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankagote, P.; Baert, G.; Van Ranst, E. Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments. Soil Use Manag. 2011, 27, 94–102. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankogote, P.; Baert, G.; Van Ranst, E. Response of Italian ryegrass (Lolium multiflorum Lam.) to coffee waste application on a humid tropical sandy soil. Soil Use Manag. 2013, 29, 22–29. [Google Scholar] [CrossRef]
- Cruz, R.; Baptista, P.; Cunha, S.; Pereira, J.A.; Casal, S. Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules 2012, 17, 1535–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, R.; Gomes, T.; Ferreira, A.; Mendes, E.; Baptista, P.; Cunha, S.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Antioxidant activity and bioactive compounds of lettuce improved by espresso coffee residues. Food Chem. 2014, 145, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Morais, S.; Mendes, E.; Pereira, J.A.; Baptista, P.; Casal, S. Improvement of vegetables elemental quality by espresso coffee residues. Food Chem. 2014, 148, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Miłek, D.; Szewczyk, A.; Sławińska, I. Acute Toxicity of Experimental Fertilizers Made of Spent Coffee Grounds. Waste Biomass Valorization 2017, 9, 2157–2164. [Google Scholar] [CrossRef] [Green Version]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil–microorganism–plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Ntalli, N.; Adamski, Z.; Doula, M.; Monokrousos, N. Nematicidal amendments and soil remediation. Plants 2020, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Monokrousos, N.; Argyropoulou, M.D.; Tzani, K.; Menkissoglou-Spiroudi, U.; Boutsis, G.; D’Addabbo, T.; Ntalli, N. The Effect of Botanicals with Nematicidal Activity on the Structural and Functional Characteristics of the Soil Nematode Community. Agriculture 2021, 11, 326. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar]
- Ferris, H. Form and Function: Metabolic Footprints of Nematodes in the Soil Food Web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ntalli, N.; Menkissoglu-Spiroudi, U.; Doitsinis, K.; Kalomoiris, M.; Papadakis, E.N.; Boutsis, G.; Dimou, M.; Monokrousos, N. Mode of action and ecotoxicity of hexanoic and acetic acids on Meloidogyne javanica. J. Pest Sci. 2020, 93, 867–877. [Google Scholar] [CrossRef]
- S’Jacob, J.J.; van Bezooijen, J. A Manual for Practical Work in Nematology; Department of Nematology, Wageningen Agricultural University: Wageningen, The Netherlands, 1984. [Google Scholar]
- Bongers, T. Systematisch gedeelte. In De Nematoden van Nederland: Vormgeving en technische realisatie, 2nd ed.; Uitgeverij Pirola: Schoorl, The Netherlands, 1994; pp. 67–383. [Google Scholar]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Chyla, M.A.; Zyrnicki, W. Determination of metal concentrations in animal hair by the ICP method: Comparison of various washing procedures. Biol. Trace Elem. Res. 2000, 75, 187–194. [Google Scholar] [CrossRef]
- Wolf, B. Communications in Soil Science and Plant Analysis Improvements in the azomethine—H method for the determination of boron. Commun. Soil Sci. Plant Anal. 1974, 5, 37–41. [Google Scholar] [CrossRef]
- Mangalassery, S.; Sjögersten, S.; Sparkes, D.L.; Sturrock, C.J.; Mooney, S.J. The effect of soil aggregate size on pore structure and its consequence on emission of greenhouse gases. Soil Tillage Res. 2013, 132, 39–46. [Google Scholar] [CrossRef]
- Schlüter, S.; Gil, E.; Doniger, T.; Applebaum, I.; Steinberger, Y. Abundance and Community Composition of Free-Living Nematodes as a Function of Soil Structure under Different Vineyard Managements. Appl. Soil Ecol. 2022, 170, 104291. [Google Scholar] [CrossRef]
- Talavera, M.; Thoden, T.C.; Vela-Delgado, M.D.; Verdejo-Lucas, S.; Sánchez-Moreno, S. The impact of fluazaindolizine on free-living nematodes and the nematode community structure in a root-knot nematode infested vegetable production system. Pest Manag. Sci. 2021, 77, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.; Tsiafouli, M.A.; Tzani, K.; Mavridi, O.; Oplos, C.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Whey: The soil bio-community enhancer that selectively controls root-knot nematodes. Plants 2019, 8, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, R.N.; Moens, M. Survival of parasitic nematodes outside the host. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A. review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Xu, J.; Yan, X.; Li, H.; Whalen, J.K.; Hu, F. Bacterial traits and quality contribute to the diet choice and survival of bacterial-feeding nematodes. Soil Biol. Biochem. 2017, 115, 467–474. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.; Scow, K. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation function. Appl. Soil Ecol. 2004, 25, 19–35. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bodenhausen, N.; Bunemann, E.K.; Brussaard, L.; Geisen, S.; Mader, P.; Quist, C.W.; Walser, J.C.; de Goede, R.G.M. Reduced tillage, but not organic matter input, increased nematode diversity and food web stability in European long-term field experiments. Mol. Ecol. 2019, 28, 4987–5005. [Google Scholar] [CrossRef] [Green Version]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- de Graaff, M.-A.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Vries, F.T.; de Bloem, J.; Quirk, H.; Stevens, C.J.; Bol, R.; Bardgett, R.D. Extensive management promotes plant and microbial nitrogen retention in temperate grassland. PLoS ONE 2012, 7, e51201. [Google Scholar] [CrossRef] [Green Version]
- Ugarte, C.M.; Zaborski, E.R.; Wander, M.M. Nematode indicators as integrative measures of soil condition in organic cropping systems. Soil Biol. Biochem. 2013, 64, 103–113. [Google Scholar] [CrossRef]
- Holtkamp, R.; Kardol, P.; Van der Wal, A.; Dekker, S.C.; Van der Putten, W.H.; de Ruiter, P.C. Soil food web structure during ecosystem development after land abandonment. Appl. Soil Ecol. 2008, 39, 23–34. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, X.K.; Xu, M.G.; Zhang, S.Q.; Huang, S.M.; Liang, W.J. Responses of soil micro-food web to long-term fertilization in a wheat-maize rotation system. Appl. Soil Ecol. 2016, 98, 56–64. [Google Scholar] [CrossRef]
- Guan, P.T.; Zhang, X.K.; Yu, J.; Cheng, Y.Y.; Li, Q.; Andriuzzi, W.S.; Liang, W.J. Soil microbial food web channels associated with biological soil crusts in desertification restoration: The carbon flow from microbes to nematodes. Soil Biol. Biochem. 2018, 116, 82–90. [Google Scholar] [CrossRef]
- Szili-Kovács, T.; Török, K.; Tilston, E.L.; Hopkins, D.W. Promoting microbial immobilization of soil nitrogen during restoration of abandoned agricultural fields by organic additions. Biol. Fertil. Soils 2007, 43, 823–828. [Google Scholar] [CrossRef]
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L.; Shah, A.A. Influence of ecological and edaphic factors on biodiversity of soil nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059. [Google Scholar] [CrossRef]
- Badra, T.; Elgindi, D.M. The relationship between phenolic content and Tylenchulus semipenetrans populations in nitrogen-amended citrus plants. Rev. De Nematol. 1979, 2, 161–164. [Google Scholar]
| Variable | Soil C | Soil SL |
|---|---|---|
| Sand (%) | 10.8 ± 1.1 | 54.4 ± 3.3 |
| Clay (%) | 58 ± 2.4 | 13.6 ± 1.7 |
| Silt (%) | 31.2 ± 1.8 | 32.1 ± 2.4 |
| pH | 7.5 ± 0.1 | 7.4 ± 0.1 |
| EC (mS/cm) | 0.5 ± 0.1 | 0.8 ± 0.1 |
| Organic Matter (%) | 2.4 ± 0.3 | 1.9 ± 0.1 |
| CaCO3 (%) | 6.4 ± 0.6 | 0.6 ± 0.4 |
| NO3 (ppm) | 35.9 ± 18.6 | 60.5 ± 14.9 |
| NH4 (ppm) | 54.3 ± 2.2 | 68.9 ± 16.9 |
| P (ppm) | 105.4 ± 9 | 53.6 ± 8.4 |
| K (ppm) | 771.2 ± 160.4 | 561.4 ± 26.1 |
| Ex. Mg (ppm) | 1051.4 ± 39.1 | 267.1 ± 16.6 |
| Ex. Ca (ppm) | 6835.4 ± 1350.1 | 3312.1 ± 246.8 |
| Fe (ppm) | 12.3 ± 3.1 | 7.1 ± 0.6 |
| Zn (ppm) | 1.6 ± 0.6 | 1.9 ± 0.3 |
| Mn (ppm) | 8.2 ± 1.4 | 15.1 ± 0.8 |
| Cu (ppm) | 3.1 ± 0.4 | 12.1 ± 0.8 |
| Variable | Value | Variable | Value |
|---|---|---|---|
| Total N (%) | 2.5 | B (ppm) | 6.3 |
| C (%) | 42.1 | P (ppm) | 1232.1 |
| Cu (ppm) | 19.6 | S (ppm) | 281.1 |
| Fe (ppm) | 48.4 | Zn (ppm) | 8.8 |
| K (ppm) | 5882.1 | Cd (ppm) | 0.01 |
| Mg (ppm) | 1518.2 | Co (ppm) | 0.2 |
| Mn (ppm) | 33.3 | Cr (ppm) | 0.2 |
| Na (ppm) | 254.2 | Pb (ppm) | 0.1 |
| Ca (ppm) | 1536.1 | Ni (ppm) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kekelis, P.; Papatheodorou, E.M.; Terpsidou, E.; Dimou, M.; Aschonitis, V.; Monokrousos, N. The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied. Agronomy 2022, 12, 2702. https://doi.org/10.3390/agronomy12112702
Kekelis P, Papatheodorou EM, Terpsidou E, Dimou M, Aschonitis V, Monokrousos N. The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied. Agronomy. 2022; 12(11):2702. https://doi.org/10.3390/agronomy12112702
Chicago/Turabian StyleKekelis, Panagiotis, Efimia M. Papatheodorou, Eleni Terpsidou, Maria Dimou, Vassilis Aschonitis, and Nikolaos Monokrousos. 2022. "The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied" Agronomy 12, no. 11: 2702. https://doi.org/10.3390/agronomy12112702
APA StyleKekelis, P., Papatheodorou, E. M., Terpsidou, E., Dimou, M., Aschonitis, V., & Monokrousos, N. (2022). The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied. Agronomy, 12(11), 2702. https://doi.org/10.3390/agronomy12112702










