Effects of the Decomposition of Mixed Plant Residues in Ecological Tea Garden Soil
Abstract
1. Introduction
2. Materials and Methods
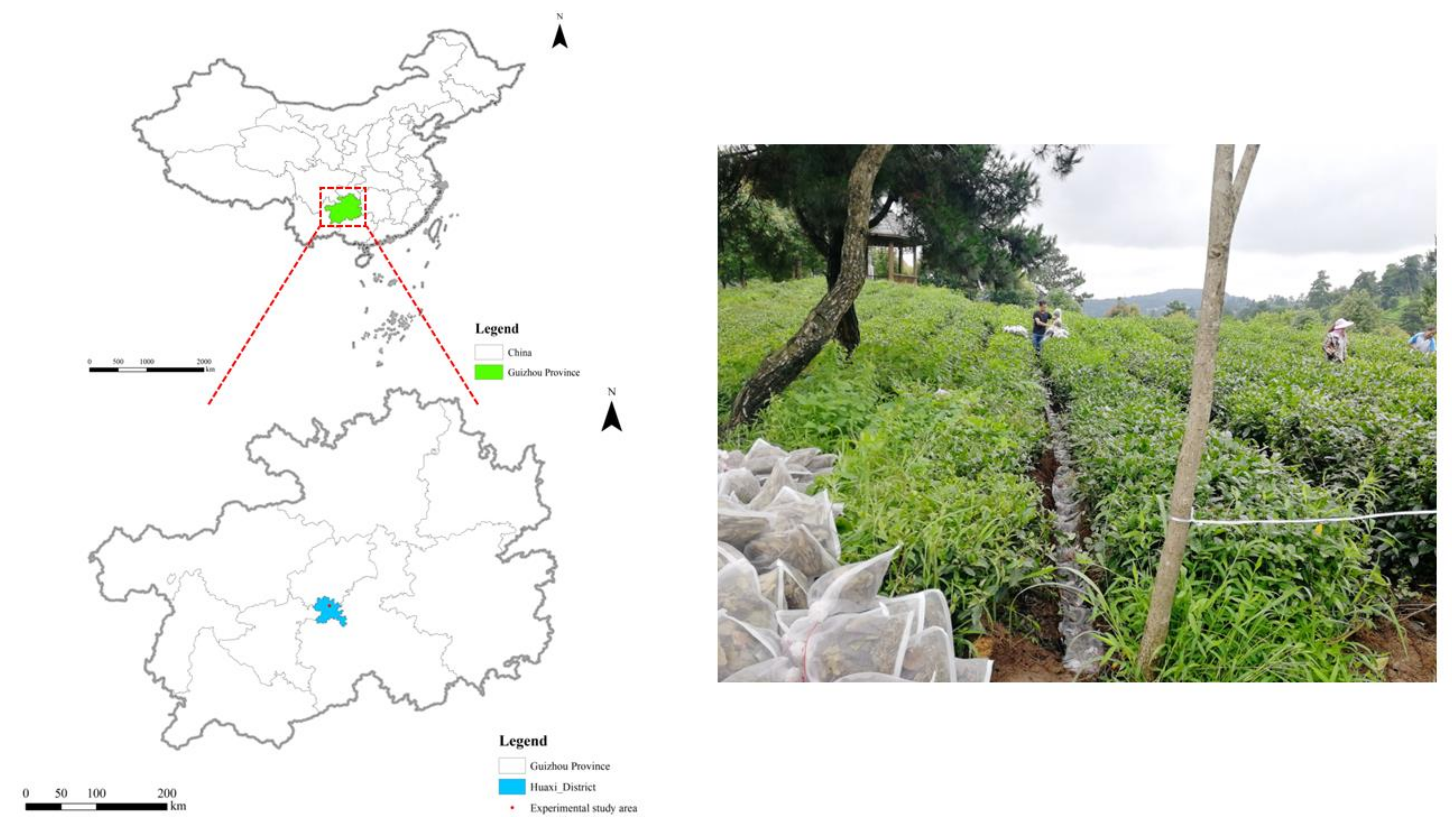
2.1. Study Area
2.2. Test Method
2.3. Decomposition Bag Collection and Index Testing
2.4. Data Analysis
2.5. Data Processing
3. Results
3.1. Dynamic Change in the Mixed Decomposition Rate
3.1.1. Decomposition Rate
3.1.2. Decomposition Rate (k)
3.2. Dynamics of Element Release in the Process of Mixed Decomposition
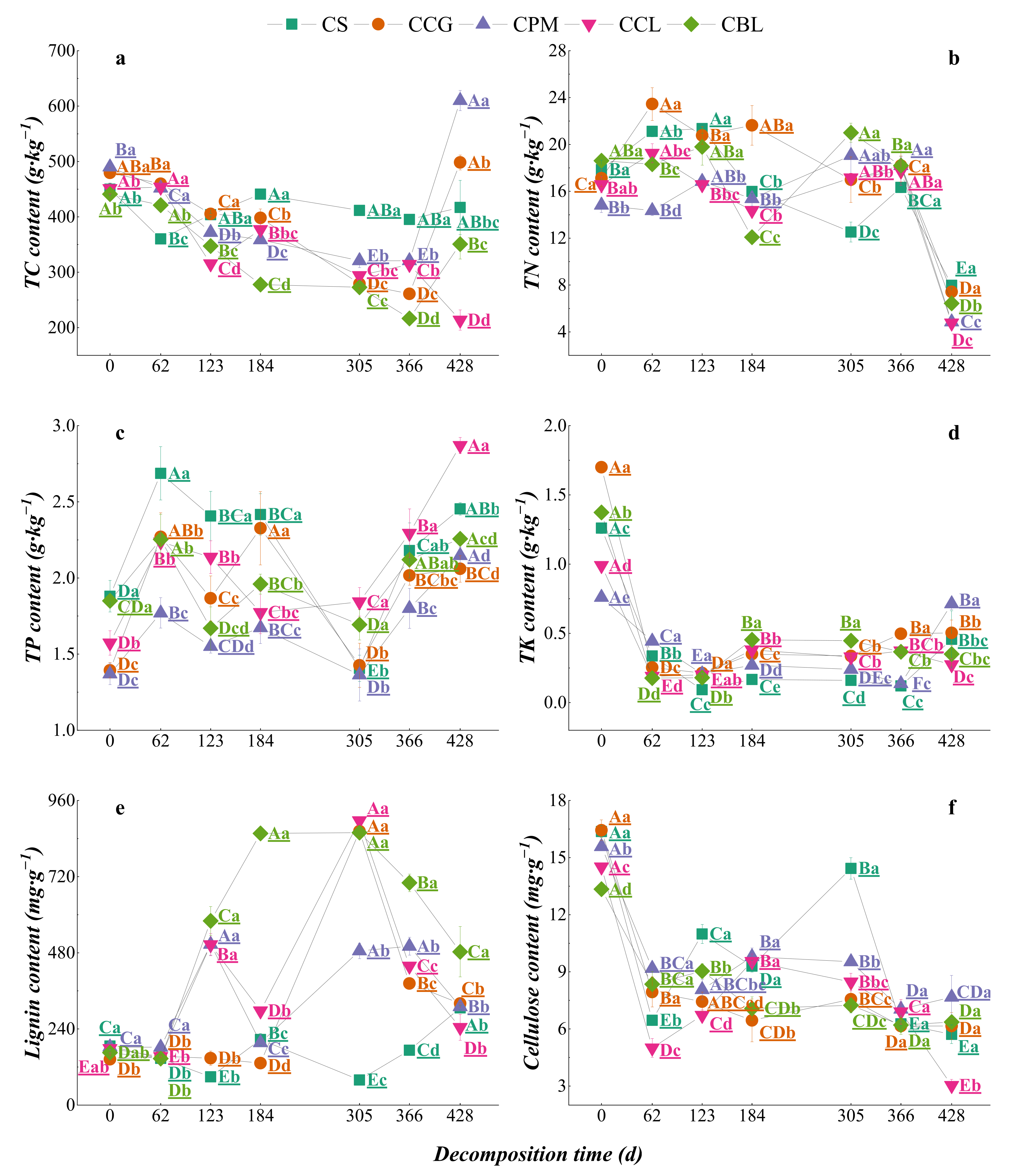
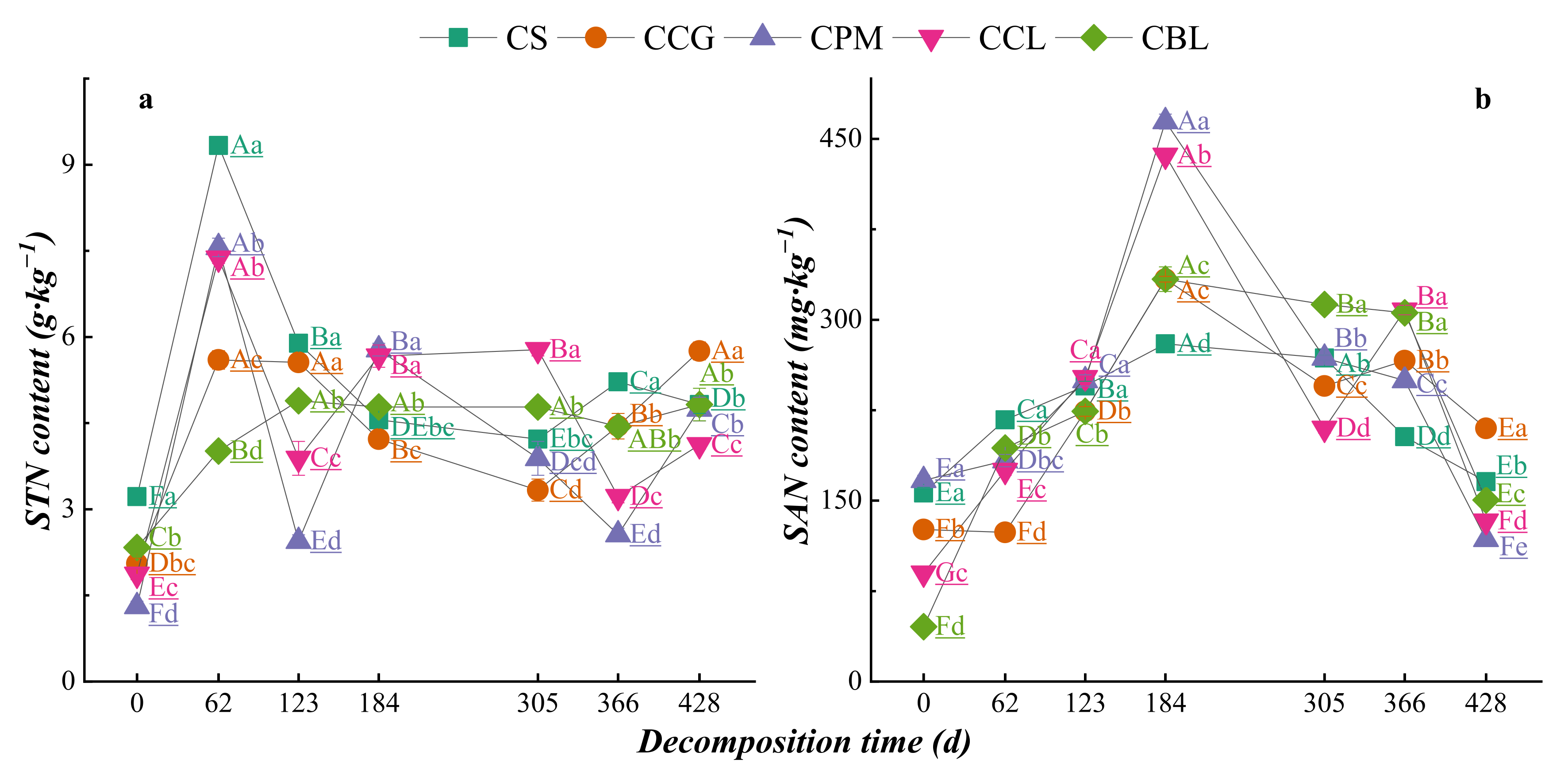
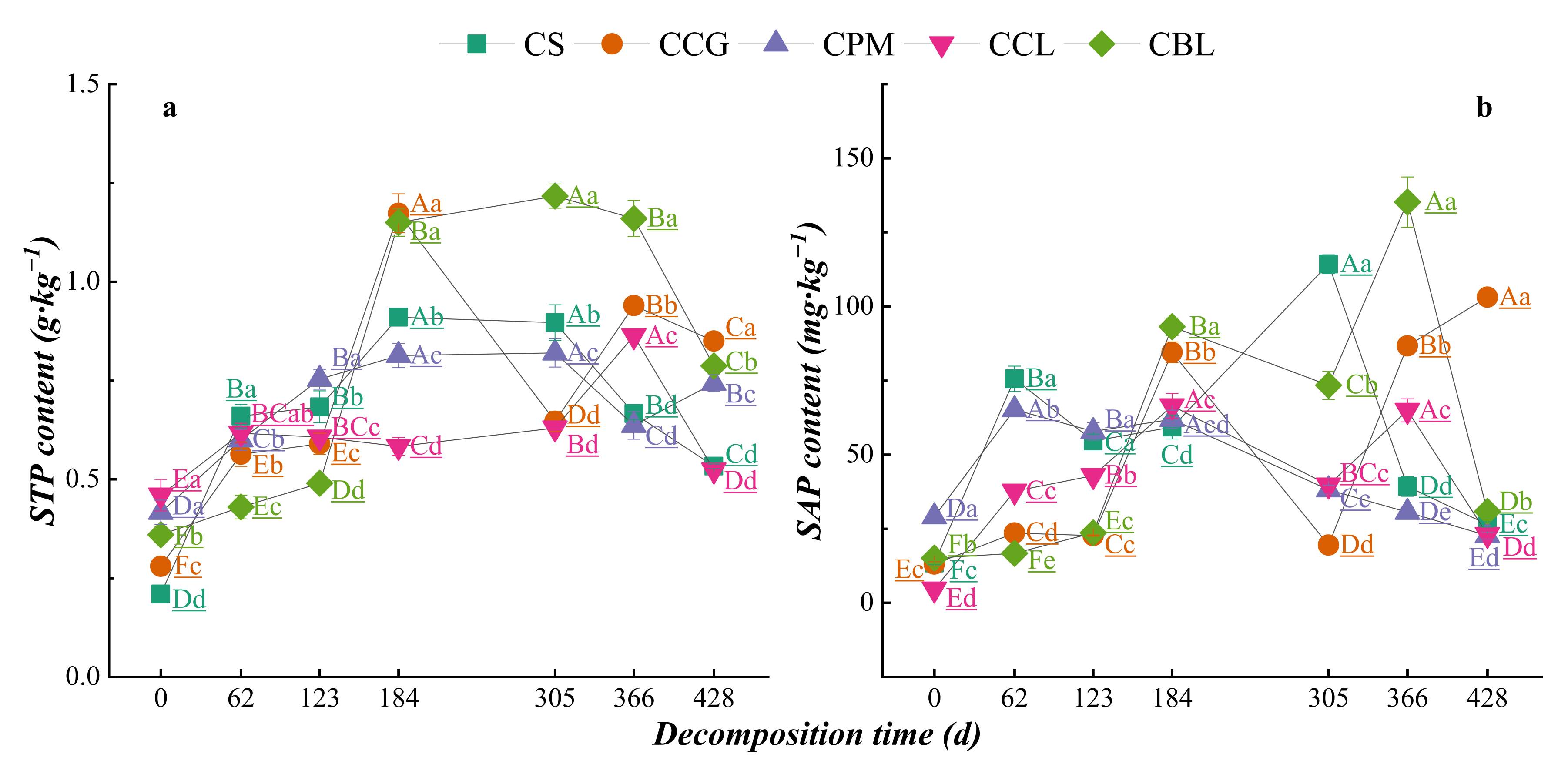
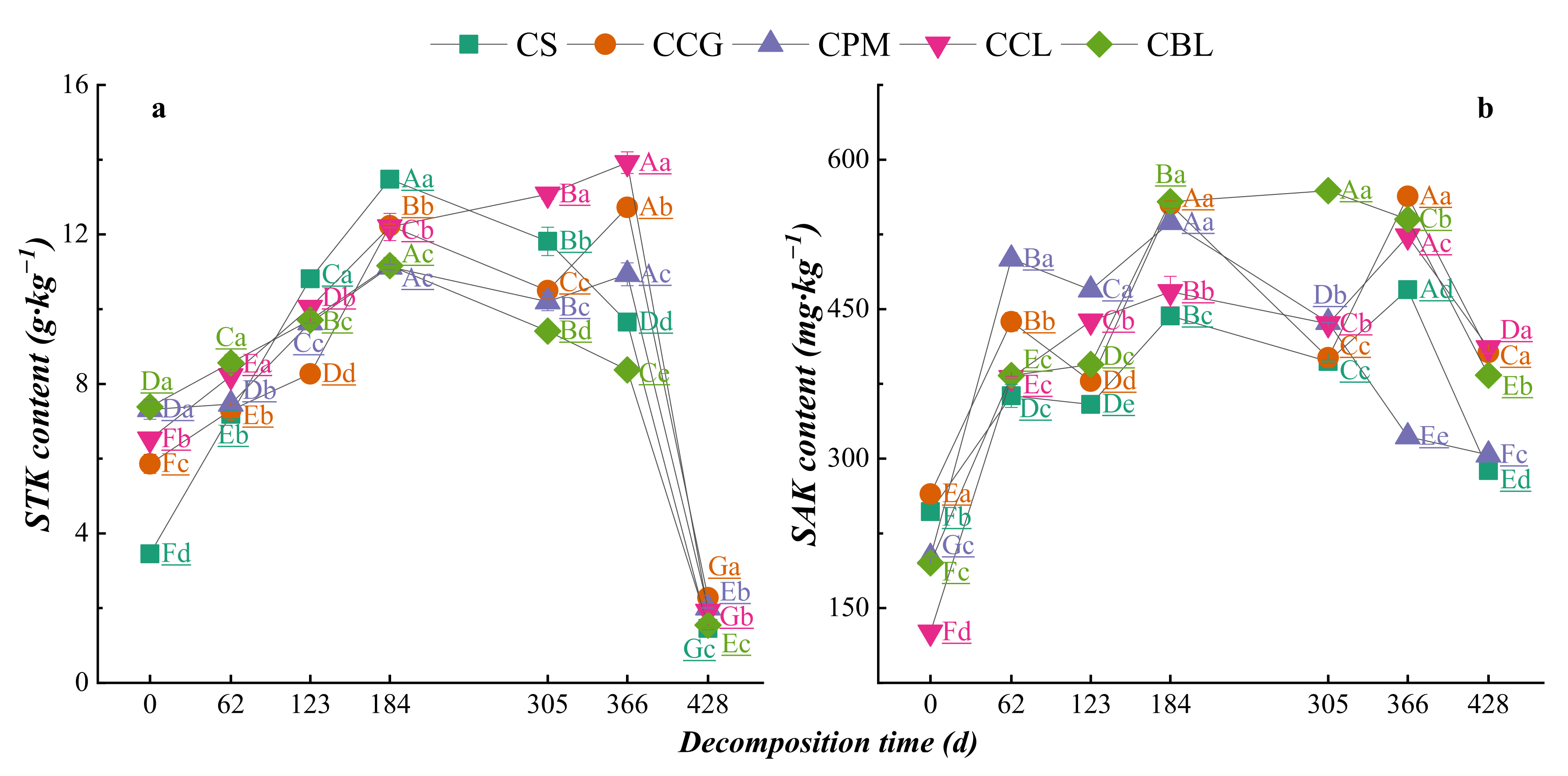
3.2.1. Dynamic Changes in Nutrient Contents of Plant Residues
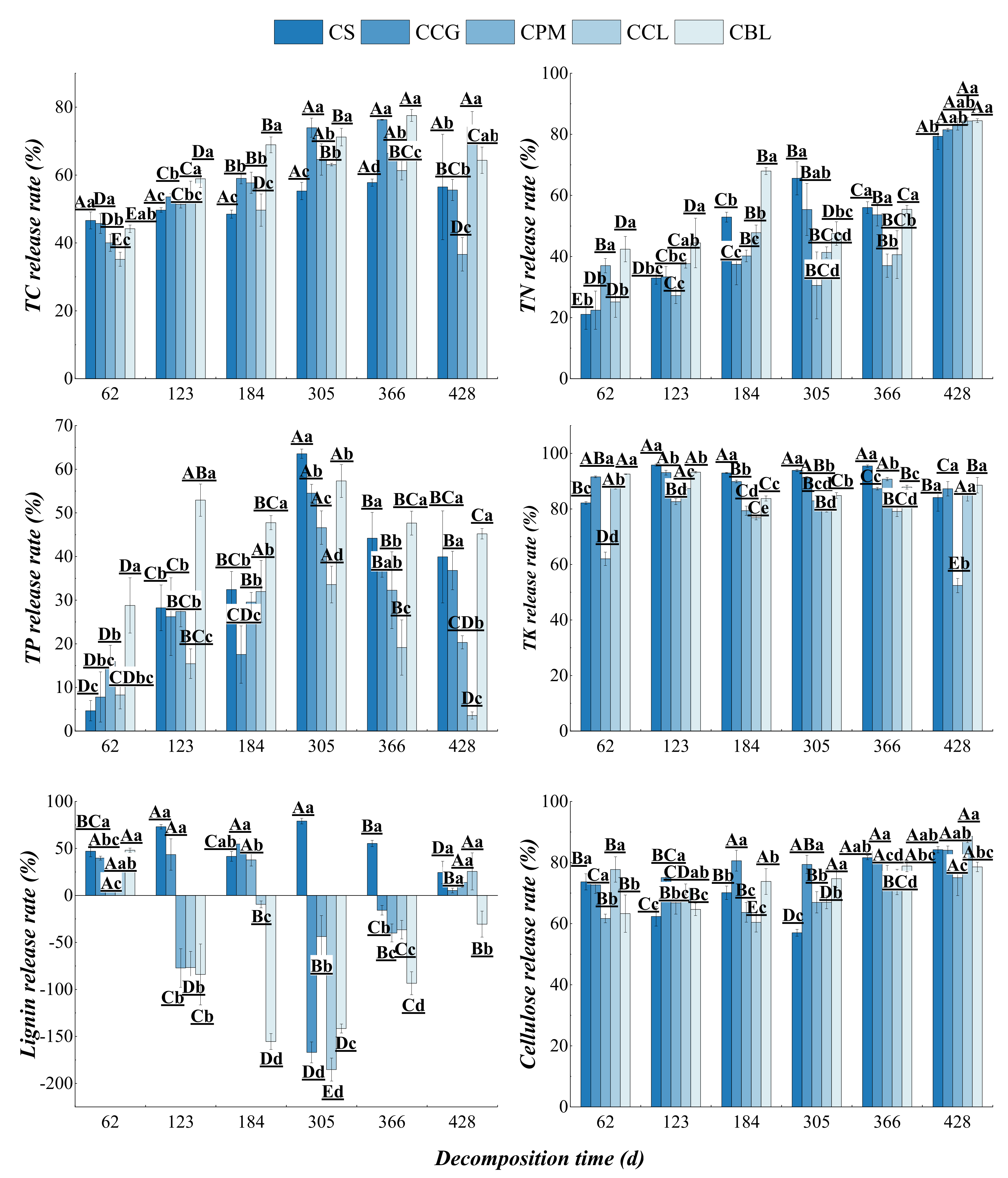
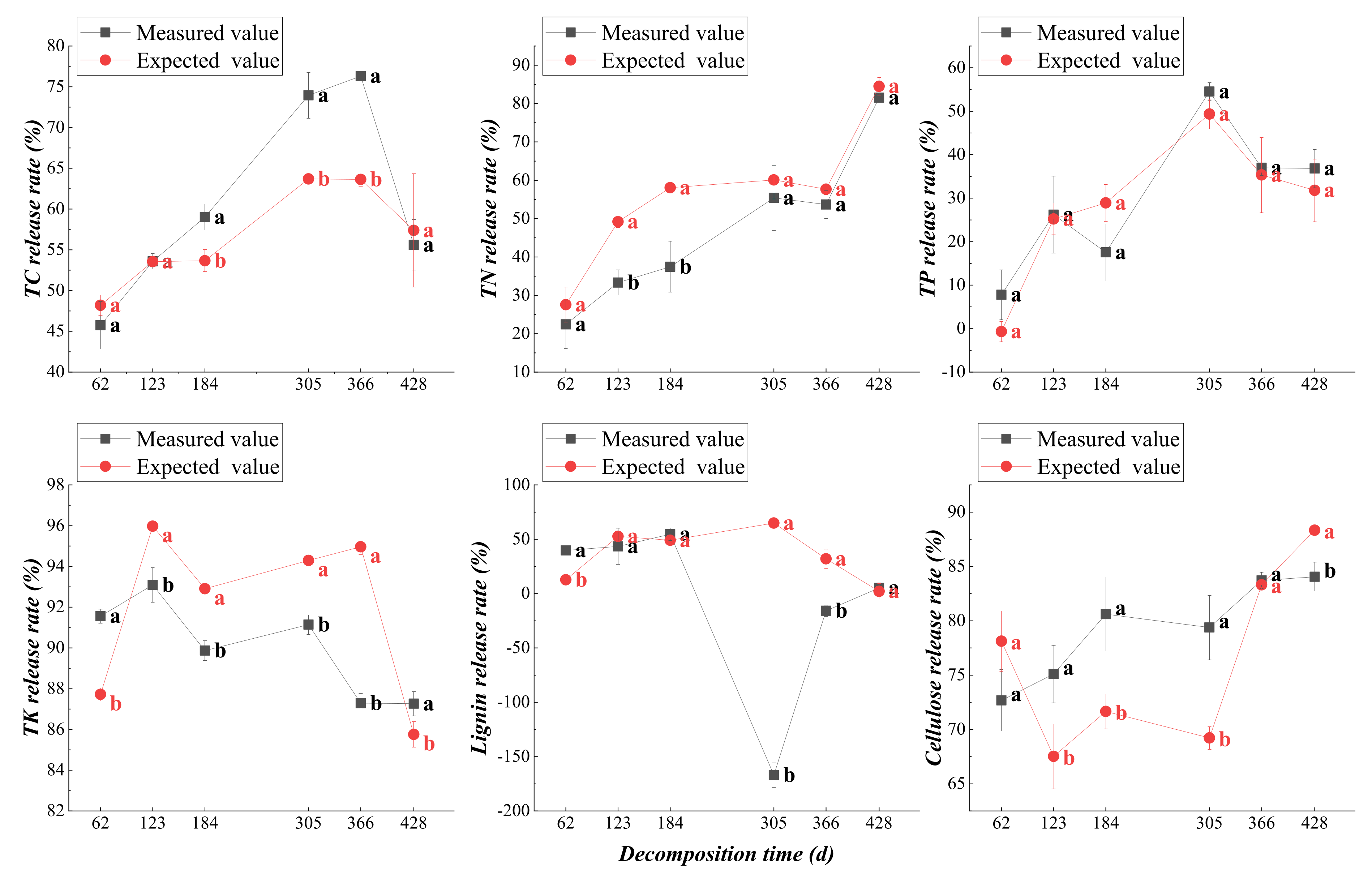
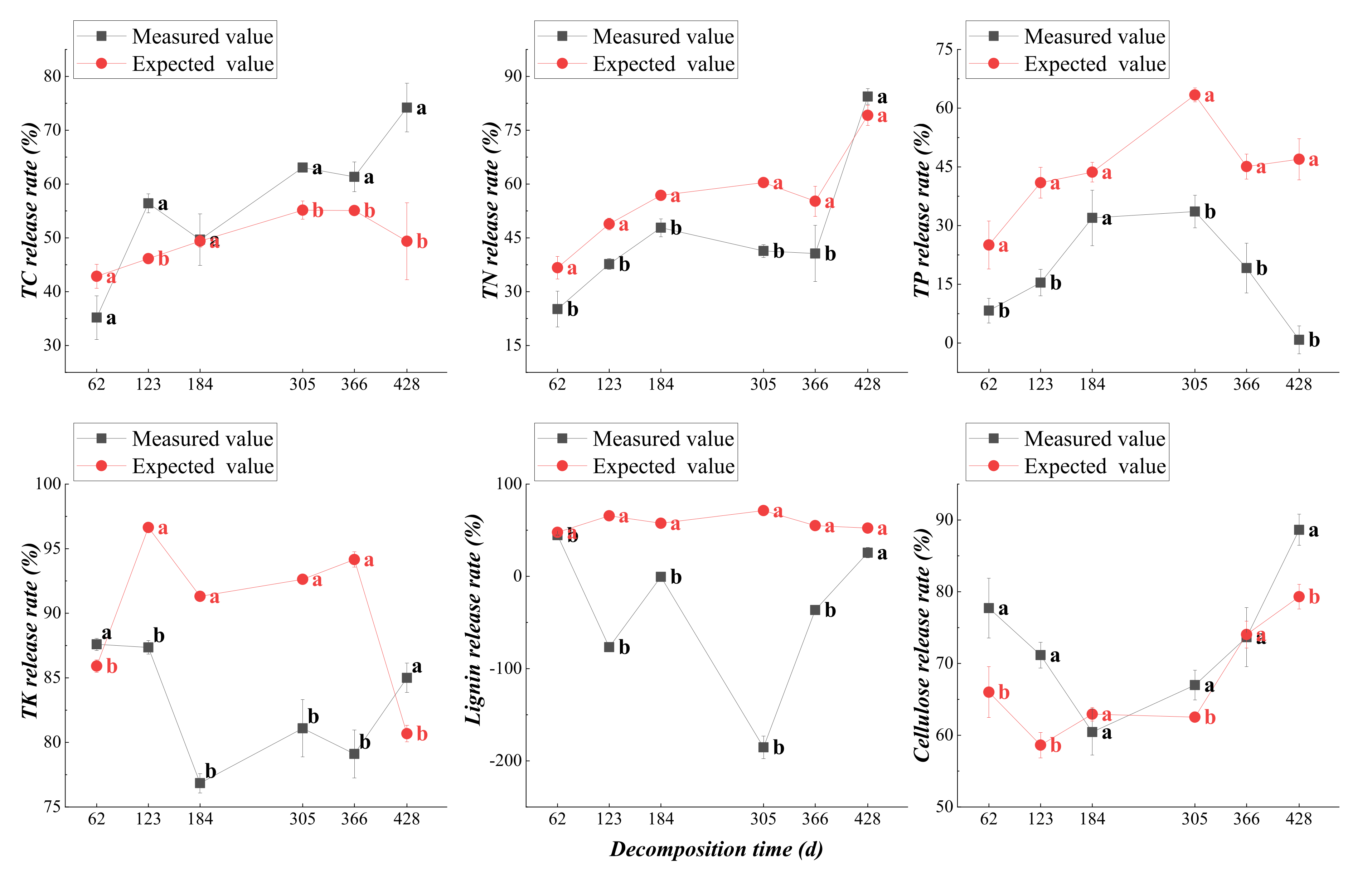
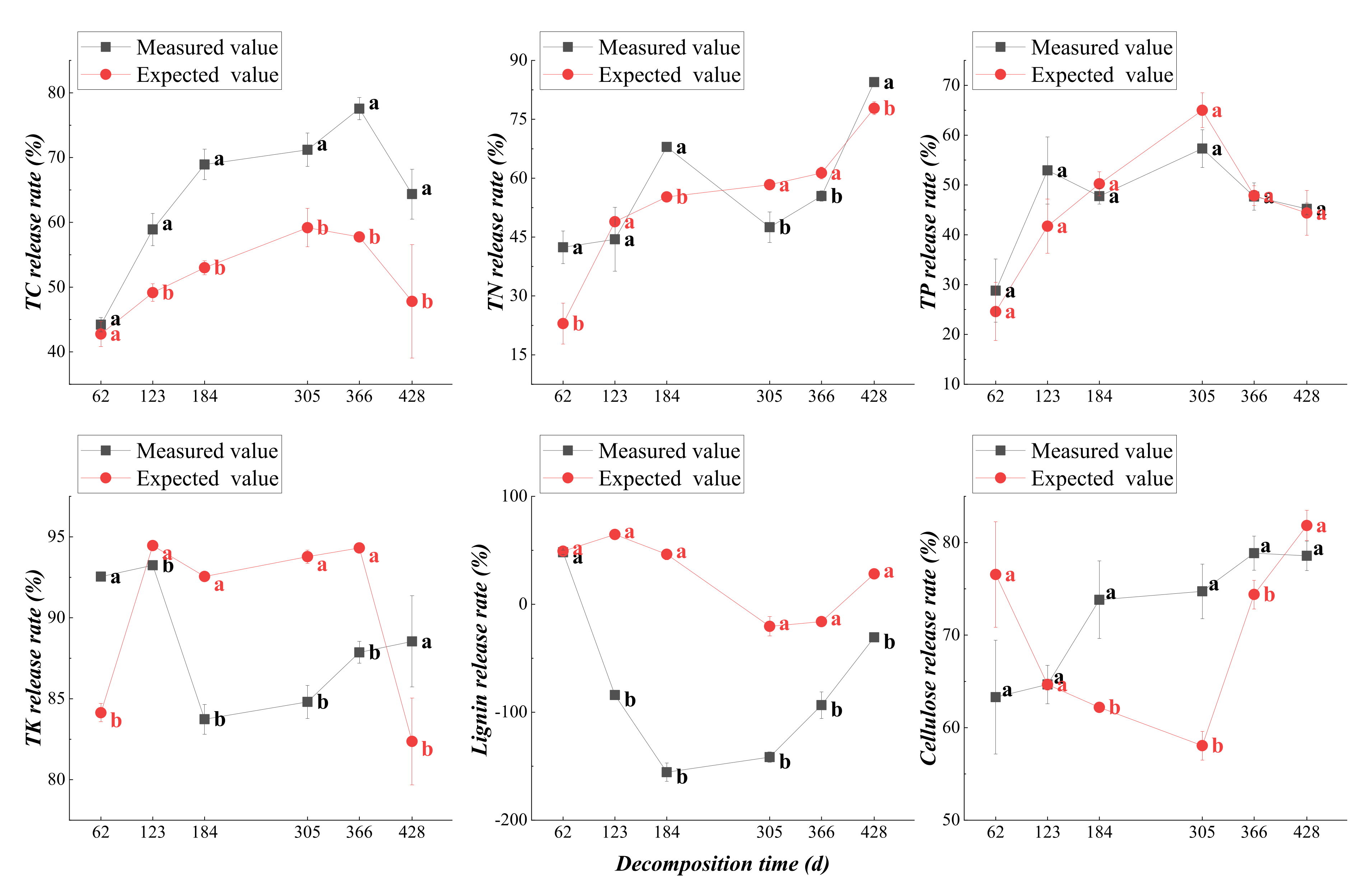
3.2.2. Dynamic Changes in Nutrient Release from Plant Residues
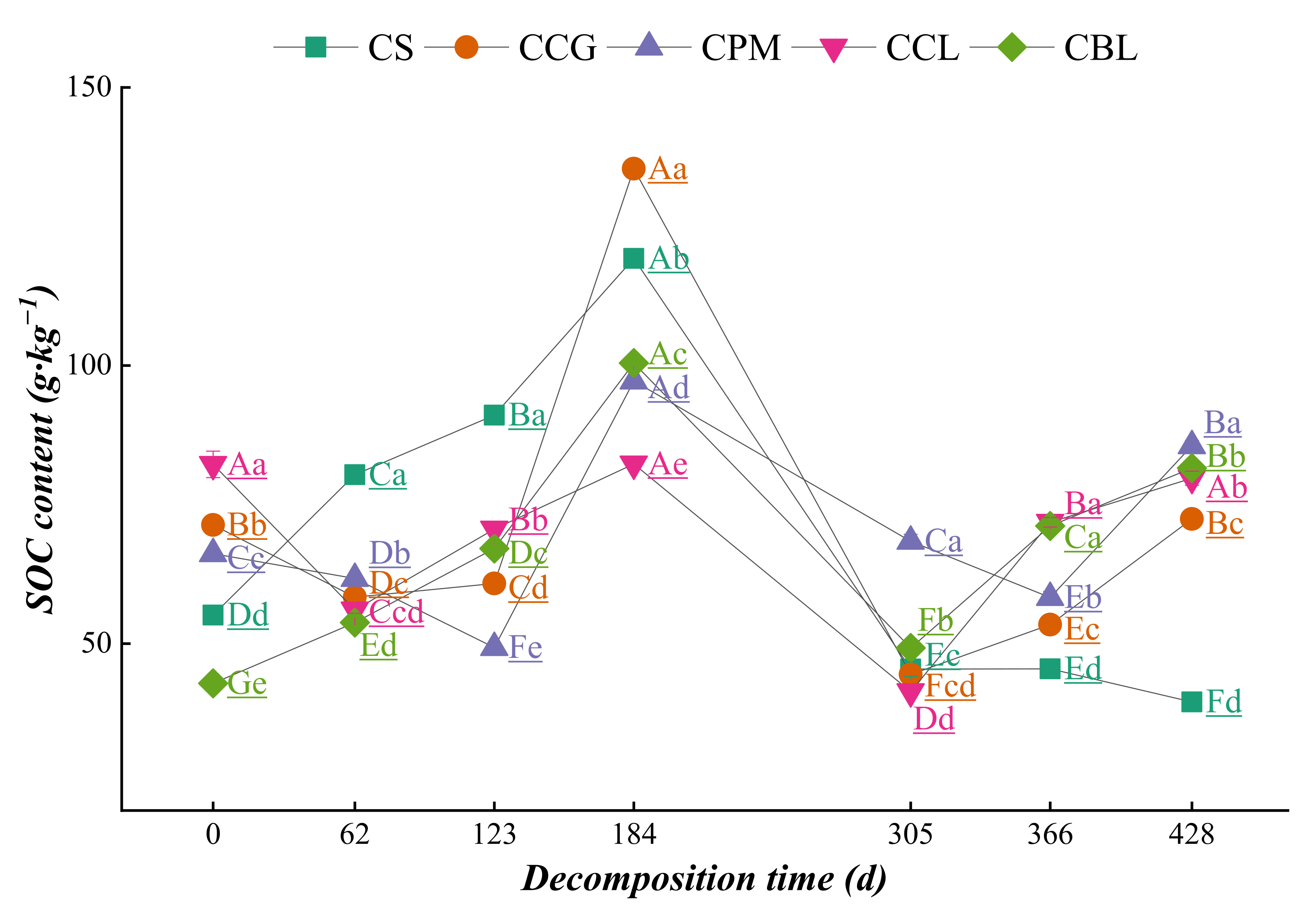
3.3. Dynamics of Element Changes in Topsoil DDuring Plant Residue Decomposition
3.4. Mixed Effect
3.4.1. Decomposition Rate Mixing Effect
3.4.2. Mixed Effect of Nutrient Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Zhang, C.; Duan, W. Temporal variations in phosphorus fractions and phosphatase activities in rhizosphere and bulk soil during the development of Larix olgensis plantations. J. Plant Nutr. Soil Sci. 2015, 179, 67–77. [Google Scholar] [CrossRef]
- Baietto, A.; Hernández, J.; del Pino, A. Comparative Dynamics of Above-Ground Litter Production and Decomposition from Eucalyptus grandis Hill ex Maiden and Pinus taeda L., and Their Contribution to Soil Organic Carbon. Forests 2021, 12, 349. [Google Scholar] [CrossRef]
- Bernhard-Reversat, F. The leaching of Eucalyptus hybrids and Acacia auriculiformis leaf litter: Laboratory experiments on early decomposition and ecological implications in congolese tree plantations. Appl. Soil Ecol. 1999, 12, 251–261. [Google Scholar] [CrossRef]
- Lin, K.M.; Zhang, Z.Q.; Cao, G.Q.; He, Z.M.; Ma, X.Q. Decomposition characteristics and its nutrient dynamics of leaf litter mixtures of both Chinese fir and Phoeba bournei. Acta Ecol. Sin. 2006, 26, 2732–2738. [Google Scholar] [CrossRef]
- He, H.; Ma, C.; Mu, H.; Shan, Y. The Decomposition Characteristics and Soil Nutrient Dynamics of Leaf Litter Mixture of Larix principis-rupprechtii and Betula platyphylla. For. Resour. Manag. 2018, 47, 93–100. [Google Scholar] [CrossRef]
- He, H.; Mu, H.; Ma, C.; Shan, Y. The decomposition characteristics and their effect on soil nutrients of leaf litter mixtures of Larix principis-rupprechtii and Lespedeza bicolor. J. Hebei Agric. Univ. 2018, 41, 55–61. [Google Scholar] [CrossRef]
- Mi, C.H.; Liu, Z.W.; Li, Q.; Mohamad, O.A. Effect of Litter Decomposition on Soil Polarization in Three Typical Planted Pure Coniferous Forests in Loess Plateau, China. Int. J. Agric. Biol. 2013, 15, 687–693. [Google Scholar]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Hättenschwiler, S. Effects of Tree Species Diversity on Litter Quality and Decomposition. Ecol. Stud. 2005, 176, 149–164. [Google Scholar] [CrossRef]
- Ge, L.; He, Z.; Meng, Q.; Lin, Y. Mixed Decomposition Process of Litterfall of Acacia aulacocarpa and Casuarina equisetifolia Plantations in Southeast Coastal Area. J. Northeast. For. Univ. 2019, 47, 35–40. [Google Scholar] [CrossRef]
- Steffensen, J.P.; Andersen, K.K.; Bigler, M.; Clausen, H.B.; Dahl-Jensen, D.; Fischer, H.; Goto-Azuma, K.; Hansson, M.; Johnsen, S.J.; Jouzel, J.; et al. High-Resolution Greenland Ice Core Data Show Abrupt Climate Change Happens in Few Years. Science 2008, 321, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y.; Hong, M.; Huo, L.X.; Ye, H.; Zhao, B.Y.; Shan, H. Effects of exogenous nitrogen input and water change on litter decomposition in a desert grassland. Chin. J. Appl. Ecol. 2018, 29, 3167–3174. [Google Scholar] [CrossRef]
- Hoorens, B.; Coomes, D.; Aerts, R. Neighbour identity hardly affects litter-mixture effects on decomposition rates of New Zealand forest species. Oecologia 2009, 162, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Chomel, M.; Guittonnylarcheveque, M.; DesRochers, A.; Baldy, V. Effect of mixing herbaceous litter with tree litters on decomposition and N release in boreal plantations. Plant Soil 2015, 398, 229–241. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Zhao, H.; Qiao, X.; Wang, S.; Zhang, L.; Cai, X. Mixing litter from deciduous and evergreen trees enhances decomposition in a subtropical karst forest in southwestern China. Soil Biol. Biochem. 2016, 101, 44–54. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Zhang, N.; Ma, K. The research of mixed litter effects on litter decomposition in terrestrial ecosystems. Acta Ecol. Sin. 2016, 36, 4977–4987. [Google Scholar] [CrossRef]
- Leroy, C.J.; Marks, J.C. Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw. Biol. 2006, 51, 605–617. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Site of leaf origin affects how mixed litter decomposes. Soil Biol. Biochem. 2006, 38, 2307–2317. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Gasser, P. Soil animals alter plant litter diversity effects on decomposition. Proc. Natl. Acad. Sci. USA 2005, 102, 1519–1524. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Nicholson, K.S. Biodiversity and Plant Litter: Experimental Evidence Which Does Not Support the View That Enhanced Species Richness Improves Ecosystem Function. Wiley Behalf Nord. Soc. Oikos 1997, 79, 247–258. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, D.; Zhang, Y.; Li, X.; Chen, Y.; Qin, Y.; Zhang, J. Enzyme activities in the early stage of mixed leaf litter decomposition from Pinus massoniana and broad-leaved tree species. Chin. J. Appl. Environ. Biol. 2018, 24, 508–517. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, D.J.; Li, X.; Zhang, Y.; Yuan, Y.L.; Wang, L.F.; Pang, Z.H.; Zhang, J. Changes of total phenols and condensed tannins during the decomposition of mixed leaf litter of Pinus massoniana and broad-leaved trees. Chin. J. Appl. Ecol. 2018, 29, 2224–2232. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Hou, C.; Ma, J.; Guo, J. Decomposition Characteristics of Lignin and Cellulose in Different Litters of Ecological Tea Gardens in Mountainous Areas of Guizhou. J. Tea Sci. 2021, 41, 654–668. [Google Scholar] [CrossRef]
- Blair, J.M.; Parmelee, R.W.; Beare, M.H. Decay Rates, Nitrogen Fluxes, and Decomposer Communiies of Single- and Mixed-Species Foliar Litter. Ecology 1990, 71, 1976–1985. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Zhang, Y.; Fu, X.; Tsang, Y.; Wu, J.; Le, Y. Variable decomposition of two plant litters and their effects on the carbon sequestration ability of wetland soil in the Yangtze River estuary. Geoderma 2018, 319, 230–238. [Google Scholar] [CrossRef]
- Sun, Z.; Mou, X.; Zhang, D.; Sun, W.; Hu, X.; Tian, L. Impacts of burial by sediment on decomposition and heavy metal concentrations of Suaeda salsa in intertidal zone of the Yellow River estuary, China. Mar. Pollut. Bull. 2017, 116, 103–112. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Antignani, V.; Capodilupo, M.; Mazzoleni, S. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 2010, 331, 481–496. [Google Scholar] [CrossRef]
- Yang, L.; Deng, C.C.; Ya-mei, C.; He, R.L.; Zhang, J.; Liu, Y. Relationships between decomposition rate of leaf litter and initial quality across the alpine timberline ecotone in Western Sichuan, China. Chin. J. Appl. Ecol. 2015, 26, 3602–3610. [Google Scholar] [CrossRef]
- Xie, T.; Li, L.; Liu, M.; Chen, X.; Chen, C.; Yang, Z.; Li, C. Characteristics of litter decomposition and nutrient release of several dominant annual herbaceous species in the riparian zone of the Three Gorges Reservoir Area. Pratacultural Sci. 2020, 37, 226–236. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, D.; Ren, B. Effects of nitrogen and phosphorus availability on the decomposition of aquatic plants. Aquat. Bot. 2004, 80, 29–37. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Zhao, H.; Meng, F.; Liu, G. Decomposition characteristics and its nutrient dynamics of the leaf litter mixtures of Larix principis-rupprechtii and Betula platyphylla. For. Ecol. Sci. 2018, 33, 29–36. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Hui, Y.; Wang, S.J.; Chen, P. Leaf litter decomposition and nutrient return characteristics of Pinus yunnanensis at different forest ages. Ecol. Environ. Sci. 2018, 27, 1981–1986. [Google Scholar] [CrossRef]
- Guo, P.; Jiang, H.; Yu, S.; Ma, Y.; Dou, R.; Song, X. Comparason of Litter Decomposition of Six Species of Coniferous and Broad-leaved Trees in Subtropical China. Chin. J. Apppl. Environ. Biol. 2009, 2009, 655–659. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, X.; Jiang, T.; Xu, Z.Q. Review on studies of forest litter decomposition. Hebei J. For. Orchard. Res. 2012, 27, 396–401. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Q.; Wu, Y.; He, H. Advances in the studies of forest litter. Chin. J. Ecol. 2004, 23, 60–64. [Google Scholar]
- Wang, C.; Dong, X.-T.; DU, R.-P.; Zhang, Z.-D.; Huang, X.-R. Changes of nutrient release and enzyme activity during the decomposition of mixed leaf litter of Larix principis-rupprechtii and broadleaved tree species. Ying yong sheng tai xue bao = J. Appl. Ecol. 2021, 32, 1709–1716. [Google Scholar] [CrossRef]
- Lin, K.; Hong, W.; Yu, X.; Huang, B. Decomposition interaction of mixed litter between Chinese fir and various accompanying plant species. Chin. J. Appl. Ecol. 2001, 12, 321–325. [Google Scholar] [CrossRef]
- Hu, N.; Ma, Z.; Lan, J.; Wu, Y.; Fu, W.; Yuan, H.; Lou, L. Leaf litter decomposition characters and impact on soil organic carbon/nitrogen in different vegetation restorations of karst rocky desertification: An example of the Zhongliang mountain in Chongqing. Carsologica Sin. 2016, 35, 539–549. [Google Scholar] [CrossRef]
- Liao, L.; Ma, Y.; Wang, S.; Gao, H.; Yu, X. Decomposition of leaf litter of chinese fir in mixture with major associated broad-leaved plantation species. Acta Phytoecol. Sin. 2000, 24, 27–33. [Google Scholar]



| Plant Residue Type | TC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | Lignin (mg·g−1) | Cellulose (mg·g−1) | C/N Ratio | Lignin/N Ratio |
|---|---|---|---|---|---|---|---|---|
| Camellia sinensis | 450.01 ± 19.24 b | 17.85 ± 1.40 ab | 1.88 ± 0.10 a | 1.26 ± 0.04 c | 171.40 ± 34.70 a | 16.37 ± 0.54 a | 25.35 ± 2.86 bc | 10.49 ± 1.41 b |
| Cunninghamia lanceolata | 452.12 ± 14.05 b | 15.28 ± 1.07 c | 1.27 ± 0.09 b | 0.72 ± 0.01 d | 184.74 ± 11.31 a | 12.64 ± 0.95 c | 29.7 ± 2.74 b | 12.16 ± 1.50 b |
| Pinus massoniana | 530.34 ± 16.94 a | 11.78 ± 1.62 d | 0.87 ± 0.05 c | 0.26 ± 0.01 e | 195.06 ± 25.34 a | 14.79 ± 0.49 b | 45.49 ± 5.48 a | 18.01 ± 2.40 a |
| Cinnamomum glanduliferum | 509.17 ± 8.5 a | 16.38 ± 0.74 bc | 0.91 ± 0.03 c | 2.14 ± 0.01 a | 117.20 ± 15.57 b | 16.51 ± 0.55 a | 31.11 ± 0.96 b | 7.19 ± 1.30 c |
| Betula luminifera | 432.22 ± 13.73 b | 19.37 ± 0.70 a | 1.82 ± 0.12 a | 1.49 ± 0.01 b | 159.05 ± 25.38 ab | 10.31 ± 0.14 d | 22.33 ± 0.62 d | 7.46 ± 0.47 c |
| Plant Residue Type | Regression Equation | k | R2 | Half-Life (a) | Turnover Period (a) |
|---|---|---|---|---|---|
| Camellia sinensis (CS) | y = 78.305e−0.045t | 0.045 ab | 0.8697 ** | 1.31 ± 0.20 bc | 5.68 ± 0.88 bc |
| Camellia sinensis + Cinnamomum glanduliferum (CCG) | y = 73.903e−0.047t | 0.047 a | 0.9579 ** | 1.23 ± 0.05 c | 5.32 ± 0.12 c |
| Camellia sinensis + Pinus massoniana (CPM) | y = 80.114e−0.038t | 0.038 b | 0.9585 ** | 1.51 ± 0.10 b | 6.53 ± 0.42 b |
| Camellia sinensis + Cunninghamia lanceolata (CCL) | y = 78.713e−0.032t | 0.032 b | 0.9919 ** | 1.83 ± 0.12 a | 7.90 ± 0.51 a |
| Camellia sinensis + Betula luminifera (CBL) | y = 72.981e−0.042t | 0.042 ab | 0.8862 ** | 1.37 ± 0.13 bc | 5.93 ± 0.56 bc |
| Plant Residue Type | Value Type | Decomposition Time (d) | |||||
|---|---|---|---|---|---|---|---|
| 62 | 123 | 184 | 305 | 366 | 428 | ||
| CCG | Expected value | 38.67 ± 1.58 b | 46.28 ± 0.37 a | 50.04 ± 0.85 a | 54.90 ± 1.02 a | 55.50 ± 2.21 a | 57.44 ± 4.89 a |
| Measured value | 43.43 ± 1.28 a | 45.03 ± 2.47 a | 50.55 ± 2.31 a | 55.30 ± 3.42 a | 56.39 ± 2.00 a | 57.29 ± 1.42 a | |
| CPM | Expected value | 31.97 ± 1.26 b | 39.65 ± 0.30 a | 42.26 ± 1.28 a | 46.48 ± 1.67 a | 47.32 ± 2.30 a | 49.98 ± 2.90 a |
| Measured value | 34.96 ± 0.79 a | 35.89 ± 2.71 b | 42.16 ± 4.68 a | 46.03 ± 4.01 a | 48.62 ± 3.12 a | 49.13 ± 1.33 a | |
| CCL | Expected value | 36.86 ± 1.02 a | 42.73 ± 0.54 a | 45.54 ± 0.85 a | 49.05 ± 1.27 a | 50.19 ± 0.63 a | 51.86 ± 4.09 a |
| Measured value | 35.55 ± 1.03 a | 37.64 ± 1.46 b | 39.85 ± 2.21 b | 43.31 ± 2.00 b | 44.54 ± 2.19 b | 45.64 ± 1.46 b | |
| CBL | Expected value | 37.95 ± 1.96 b | 46.15 ± 1.28 a | 49.45 ± 1.00 a | 51.86 ± 1.80 a | 52.81 ± 0.33 a | 54.32 ± 3.60 a |
| Measured value | 41.53 ± 1.75 a | 47.79 ± 2.16 a | 50.70 ± 1.12 a | 53.49 ± 1.48 a | 54.34 ± 2.33 a | 55.10 ± 1.29 a | |
| Plant Residue Type | Decomposition Rate (k) | Mixed Effect | |
|---|---|---|---|
| Measured Value | Expected Value | ||
| CCG | 0.047 ± 0.002 a | 0.049 ± 0.005 a | n |
| CPM | 0.038 ± 0.003 a | 0.039 ± 0.004 a | n |
| CCL | 0.032 ± 0.002 b | 0.040 ± 0.004 a | - |
| CBL | 0.042 ± 0.004 a | 0.042 ± 0.004 a | n |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yang, R.; Hou, C.; Guo, J.; Ma, J. Effects of the Decomposition of Mixed Plant Residues in Ecological Tea Garden Soil. Agronomy 2022, 12, 2717. https://doi.org/10.3390/agronomy12112717
Liu S, Yang R, Hou C, Guo J, Ma J. Effects of the Decomposition of Mixed Plant Residues in Ecological Tea Garden Soil. Agronomy. 2022; 12(11):2717. https://doi.org/10.3390/agronomy12112717
Chicago/Turabian StyleLiu, Shaqian, Rui Yang, Chunlan Hou, Jiarui Guo, and Juebing Ma. 2022. "Effects of the Decomposition of Mixed Plant Residues in Ecological Tea Garden Soil" Agronomy 12, no. 11: 2717. https://doi.org/10.3390/agronomy12112717
APA StyleLiu, S., Yang, R., Hou, C., Guo, J., & Ma, J. (2022). Effects of the Decomposition of Mixed Plant Residues in Ecological Tea Garden Soil. Agronomy, 12(11), 2717. https://doi.org/10.3390/agronomy12112717






