Humic Acid Alleviates the Toxicity of Nanoplastics towards Solanum lycopersicum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Experimental Design
2.3. Characterization Study
2.3.1. Particle Size Determination
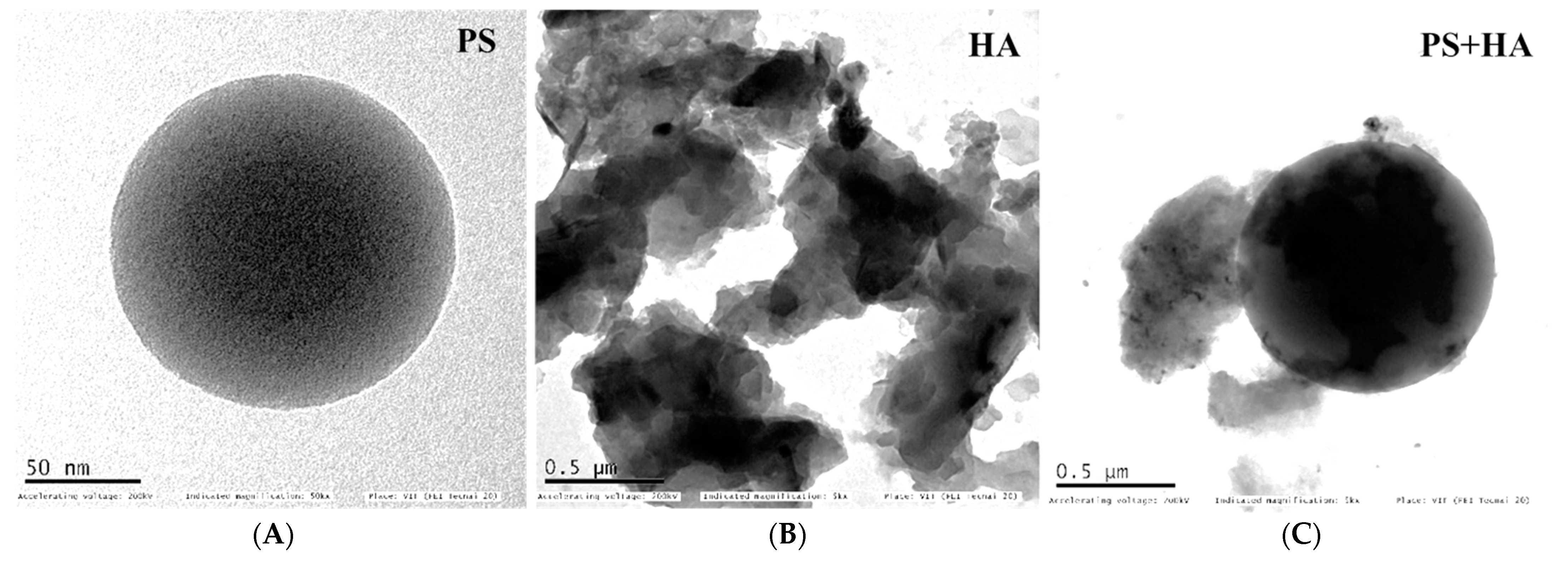
2.3.2. Transmission Electron Microscopy
2.3.3. FTIR—Fourier Transform Infrared Spectroscopy Analysis
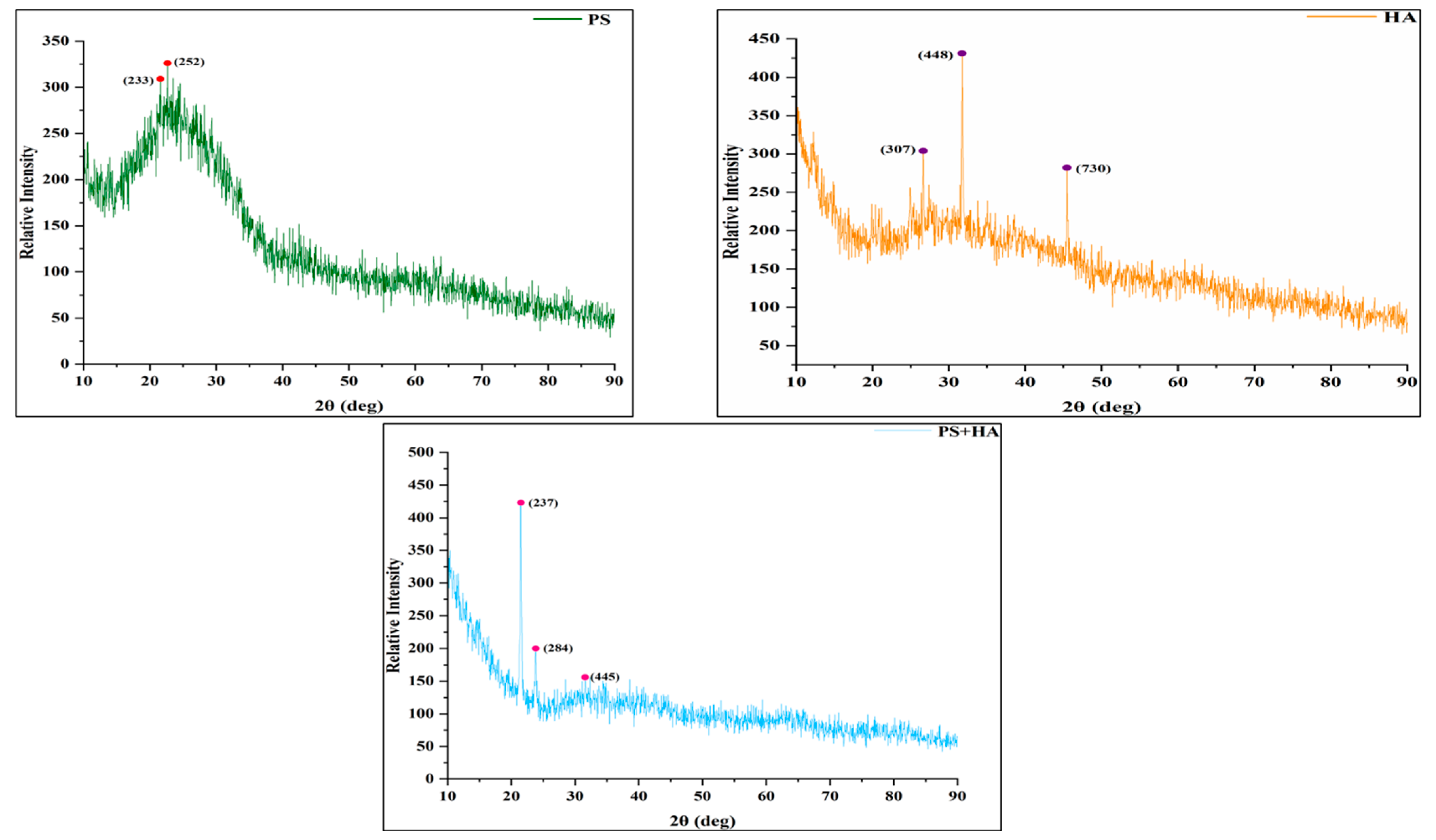
2.3.4. XRD—X-ray Diffraction Analysis
2.4. Seeds and Plants Growth
2.5. Plant Physiology
Photosynthetic Pigment Measurement
2.6. Oxidative Stress Analysis
2.6.1. Sample Preparation for Oxidative Stress Analysis
2.6.2. Overall Reactive Oxygen Species (ROS)
2.6.3. Superoxide Dismutase (SOD)
2.6.4. Catalase
2.6.5. Statistics
3. Results
3.1. Characterization Study
3.1.1. Particle Size
3.1.2. Transmission Electron Microscope (TEM)
3.1.3. Fourier Transform Infrared (FTIR)
3.1.4. X-ray Diffraction (XRD)
3.2. Effect of PS, HA, and PS + HA on Solanum lycopersicum (Seeds)
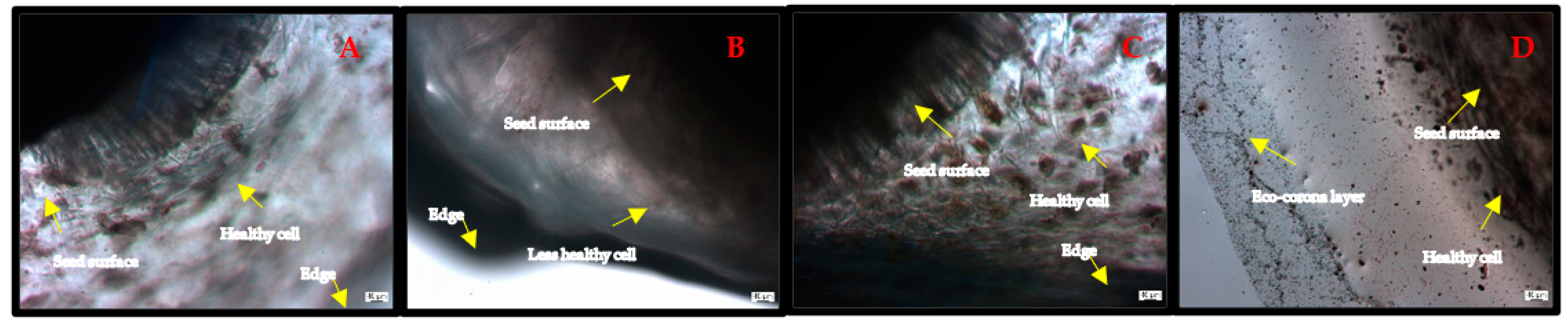
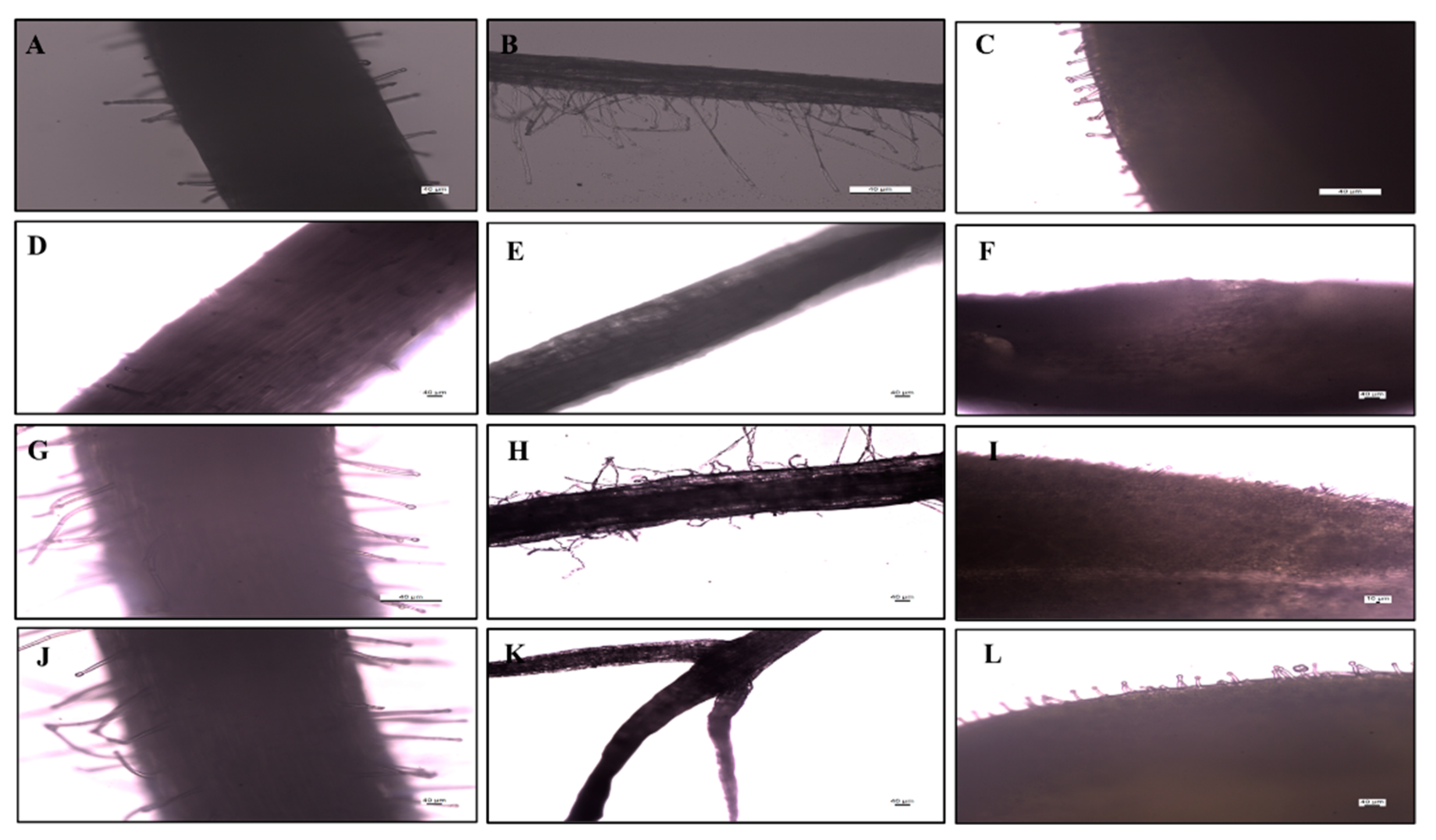
3.2.1. Seed Imaging by Optical Microscopy
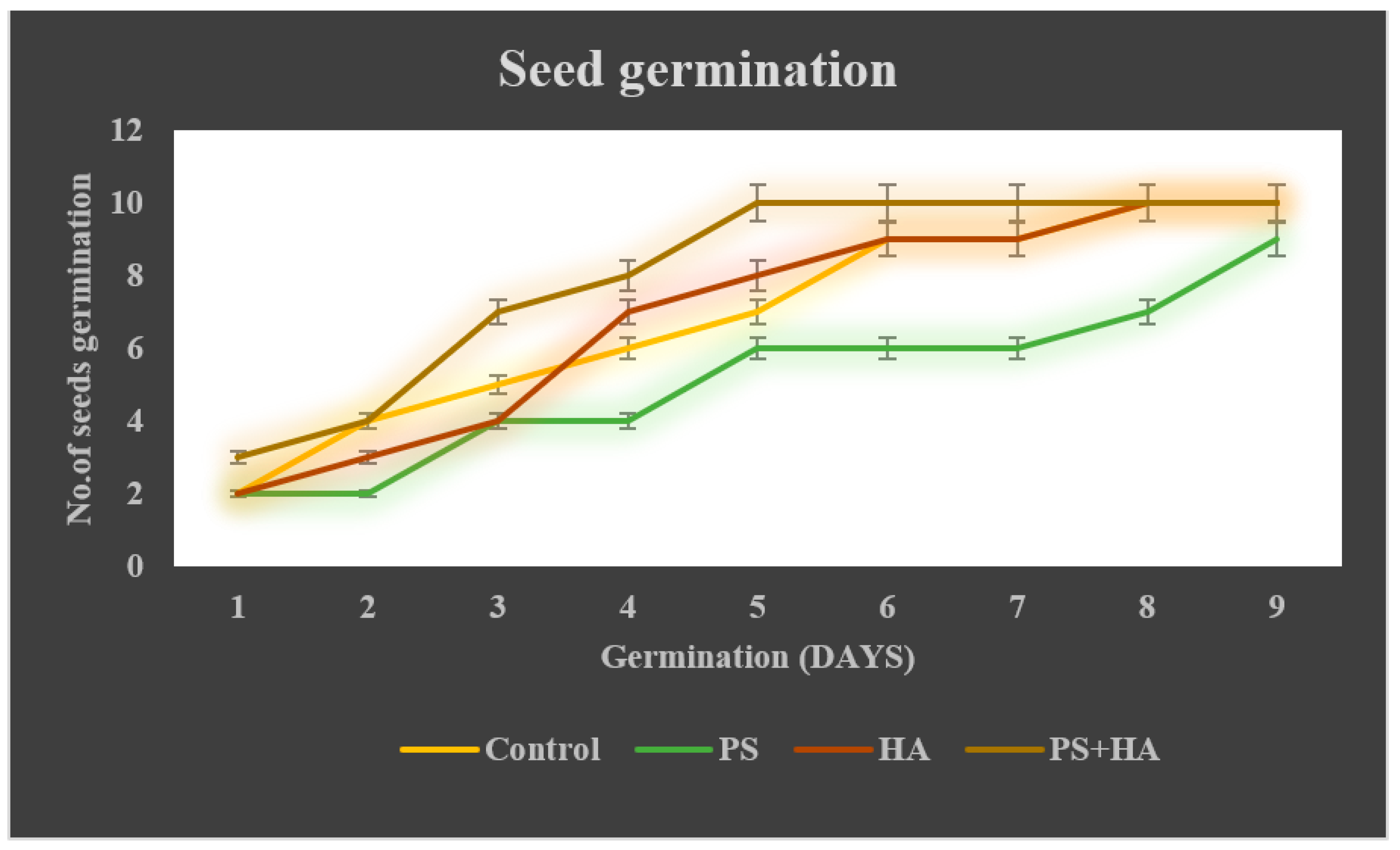
3.2.2. Seed Germination
3.3. Effect of PS, HA, and PS + HA on Solanum lycopersicum (Plants)
3.3.1. Shoot and Root Length
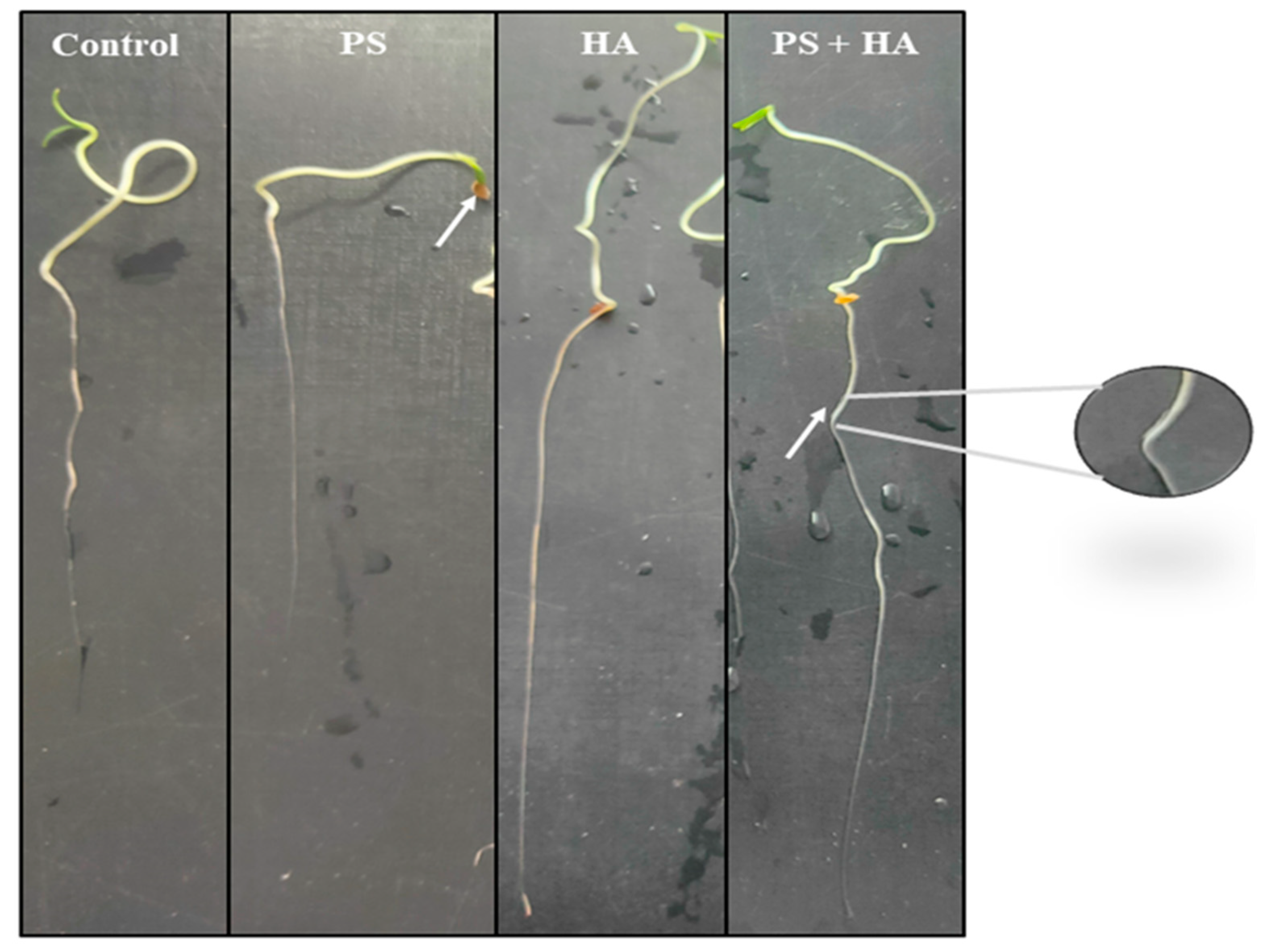
3.3.2. Plant Imaging by Optical Microscopy
3.3.3. Photosynthetic Pigment Estimation
3.4. Oxidative Stress Analysis
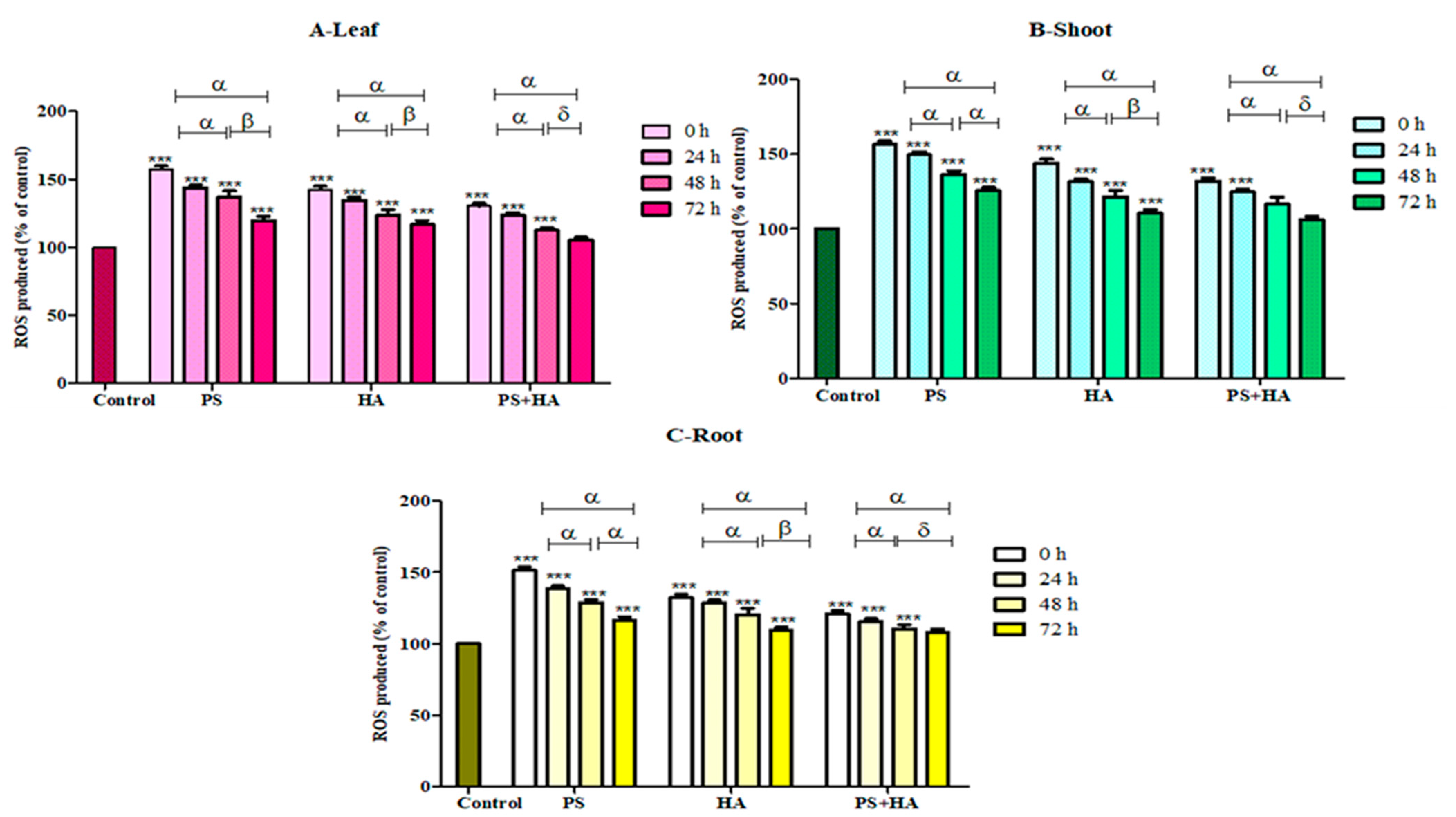
3.4.1. ROS Production
3.4.2. Effects on Superoxide Dismutase Activity
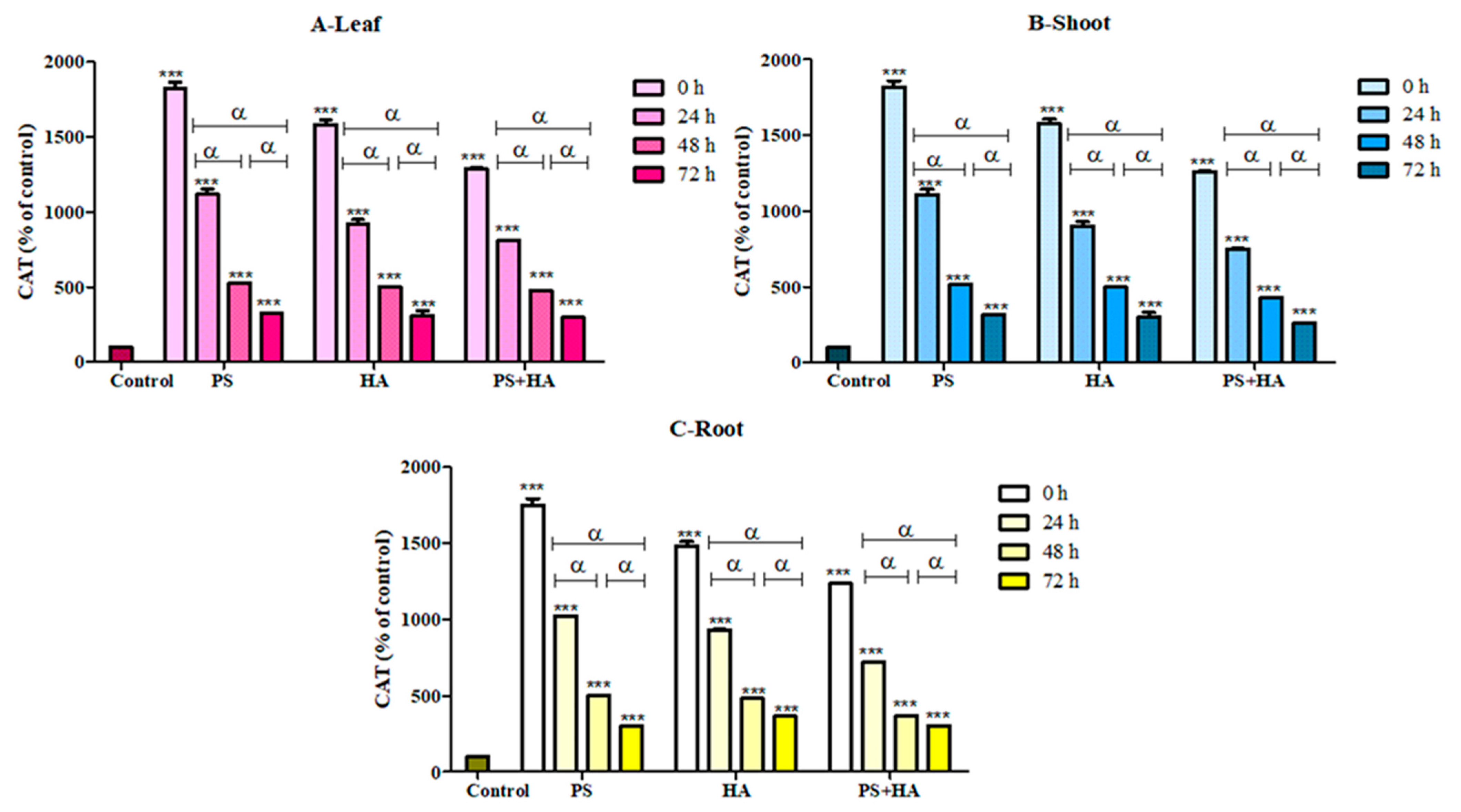
3.4.3. Effects on Catalase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, V.P. Integrated Plastic Waste Management: Environmental and Improved Health Approaches. Procedia. Environ. Sci. 2016, 35, 692–700. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Huang, Y.; Zhao, Y. Polystyrene Nanoplastic Exposure Induces Immobilization, Reproduction, and Stress Defense in the Freshwater Cladoceran Daphnia Pulex. Chemosphere 2019, 215, 74–81. [Google Scholar] [CrossRef]
- Kumari, A.; Chaudhary, D.R. Engineered Microbes and Evolving Plastic Bioremediation Technology. Bioremediation Pollut. 2020, 417–443. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Das, S.; Sharma, P.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Assessment of Agricultural Plastics and Their Sustainability: A Call for Action; FAO: Rome, Italy, 2021.
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage Sludge for Sustainable Agriculture: Contaminants’ Contents and Potential Use as Fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Rout, P.R.; Mohanty, A.; Sharma, A.; Miglani, M.; Liu, D.; Varjani, S. Micro- and Nanoplastics Removal Mechanisms in Wastewater Treatment Plants: A Review. J. Hazard. Mater. Adv. 2022, 6, 100070. [Google Scholar] [CrossRef]
- Scalenghe, R. Resource or Waste? A Perspective of Plastics Degradation in Soil with a Focus on End-of-Life Options. Heliyon 2018, 4, 941. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effects of Microplastic on Arsenic Accumulation in Chlamydomonas Reinhardtii in a Freshwater Environment. J. Hazard. Mater 2021, 405, 124232. [Google Scholar] [CrossRef]
- Mohajerani, A.; Karabatak, B. Microplastics and Pollutants in Biosolids Have Contaminated Agricultural Soils: An Analytical Study and a Proposal to Cease the Use of Biosolids in Farmlands and Utilise Them in Sustainable Bricks. Waste Manag. 2020, 107, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Heinze, W.M. Nanoplastic Transport in Soils by Advection and Bioturbation; SLU, Department of Soil and Environment: Uppsala, Sweden, 2019. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Larue, C.; Sarret, G.; Castillo-Michel, H.; Pradas, A.E.; Real, D.A. A Critical Review on the Impacts of Nanoplastics and Microplastics on Aquatic and Terrestrial Photosynthetic Organisms. Open Sci. 2021, 17, 200584. [Google Scholar] [CrossRef] [PubMed]
- Liwarska-Bizukojc, E. Effect of (Bio)Plastics on Soil Environment: A Review. Sci. Total Environ. 2021, 795, 148889. [Google Scholar] [CrossRef]
- Jayalalitha, M.; Rajeswari, M.; Saravanapandian, P.; Lalitha, R. Effect of Plastic Mulches and Irrigation Levels on Yield Parameters of Tomato (Solanum Lycopersicum) in Madurai District of Tamil Nadu. Eco. Env. Cons. 2020, 26, 1184–1188. [Google Scholar]
- Pathan, S.I.; Arfaioli, P.; Bardelli, T.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G. Soil Pollution from Micro-and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability 2020, 12, 7255. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of Different Levels of Humic Acids on the Nutrient Content, Plant Growth, and Soil Properties under Conditions of Salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Türkmen, Ö.; Dursun, A.; Turan, M.; Erdinç, Ç. Calcium and Humic Acid Affect Seed Germination, Growth, and Nutrient Content of Tomato (Lycopersicon Esculentum L.) Seedlings under Saline Soil Conditions. Acta Agric Scand B Soil Plant Sci. 2004, 54, 168–174. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of Microplastics in Wastewater Treatment Plants and Their Environmental Dispersion with Effluent and Sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Rashid, A.; Khan, M.S. Impact of Humic Acid and Chemical Fertilizer Application on Growth and Grain Yield of Rainfed Wheat (Triticum Aestivum L.). Pak. J. Agric. Res. 2010, 23, 113–121. [Google Scholar]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Gao, J.; Liu, Y.; Gao, J.; Yao, L.; Yang, X.; Liu, X.; He, B.; Hu, L.; Shi, J.; et al. Role of Protein Corona in the Biological Effect of Nanomaterials: Investigating Methods. TrAC Trends Anal. Chem. 2019, 118, 303–314. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Secreted Protein Eco-Corona Mediates Uptake and Impacts of Polystyrene Nanoparticles on Daphnia Magna. J. Proteomics. 2016, 137, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Jiang, R.; You, J.; Muir, D.C.G.; Zeng, E.Y. Microplastic Impacts on Microalgae Growth: Effects of Size and Humic Acid. Environ. Sci. Technol. 2020, 54, 1782–1789. [Google Scholar] [CrossRef]
- Basheer, M.; Agrawal, O.P. Effect of Vermicompost on the Growth and Productivity of Tomato Plant (Solanum Lycopersicum) under Field Conditions. Int. J. Recent Sci. Res. 2013, 3, 247–249. [Google Scholar]
- Osman, A.S.; Rady, M.M. Effect of Humic Acid as an Additive to Growing Media to Enhance the Production of Eggplant and Tomato Transplants. J. Hortic. Sci. Biotechnol. 2014, 89, 237–244. [Google Scholar] [CrossRef]
- Gogoi, M.; Basumatary, M. Estimation of the Chlorophyll Concentration in Seven Citrus Species of Kokrajhar District, BTAD, Assam, India. Trop. Plant Res. 2018, 5, 83–87. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free. Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Kono, Y. Generation of Superoxide Radical during Autoxidation of Hydroxylamine and an Assay for Superoxide Dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Yilancioglu, K.; Cokol, M.; Pastirmaci, I.; Erman, B.; Cetiner, S. Oxidative Stress Is a Mediator for Increased Lipid Accumulation in a Newly Isolated Dunaliella Salina Strain. PLoS ONE 2014, 9, e91957. [Google Scholar] [CrossRef] [Green Version]
- Calero, E.; West, S.; Hinson, K. Water Absorption of Soybean Seeds and Associated Causal Factors. Crop Sci. 1981, 21, 926–933. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics Accumulate on Pores in Seed Capsule and Delay Germination and Root Growth of the Terrestrial Vascular Plant Lepidium Sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.R.; Stretz, H.A.; Wells, M.J.M. Abiotic Reversible Self-Assembly of Fulvic and Humic Acid Aggregates in Low Electrolytic Conductivity Solutions by Dynamic Light Scattering and Zeta Potential Investigation. Sci. Total Environ. 2015, 537, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, U.D.; Marković, M.M.; Cupać, S.B.; Tomić, Z.P. Soil Humic Acid Aggregation by Dynamic Light Scattering and Laser Doppler Electrophoresis. J. Plant Nutr. Soil Sci. 2013, 176, 674–679. [Google Scholar] [CrossRef]
- Giri, S.; Mukherjee, A. Ageing with Algal EPS Reduces the Toxic Effects of Polystyrene Nanoplastics in Freshwater Microalgae Scenedesmus Obliquus. J. Environ. Chem. Eng. 2021, 9, 105978. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Liu, K.; Yang, Y.; Zhao, L.; Guo, L.H. Eco-Corona vs Protein Corona: Effects of Humic Substances on Corona Formation and Nanoplastic Particle Toxicity in Daphnia magna. Environ. Sci. Technol. 2020, 54, 8001–8009. [Google Scholar] [CrossRef]
- Giri, S.; Mukherjee, A. Eco-Corona Reduces the Phytotoxic Effects of Polystyrene Nanoplastics in Allium Cepa: Emphasizing the Role of ROS. Environ. Exp. Bot. 2022, 198, 104850. [Google Scholar] [CrossRef]
- Natarajan, L.; Omer, S.; Jetly, N.; Jenifer, M.A.; Chandrasekaran, N.; Suraishkumar, G.K.; Mukherjee, A. Eco-Corona Formation Lessens the Toxic Effects of Polystyrene Nanoplastics towards Marine Microalgae Chlorella sp. Environ. Res. 2020, 188, 109842. [Google Scholar] [CrossRef]
- Ding, Y.; Bai, X.; Ye, Z.; Gong, D.; Cao, J.; Hua, Z. Humic Acid Regulation of the Environmental Behavior and Phytotoxicity of Silver Nanoparticles to Lemna Minor. Environ. Sci. Nano 2019, 6, 3712–3722. [Google Scholar] [CrossRef]
- Umamaheswari, S. FTIR Spectroscopic Study of Fungal Degradation of Poly(Ethylene Terephthalate) and Polystyrene Foam. Elixir Salem India Chem. Engg. 2013, 64, 19159–19164. [Google Scholar]
- Ribeiro, J.S.; Ok, S.S.; Garrigues, S.; de la Guardia, M. FTIR Tentative Characterization of Humic Acids Extracted from Organic Materials. Spectrosc. Lett. 2001, 34, 179–190. [Google Scholar] [CrossRef]
- Niculăescu, C.; Olar, L.; Stefan, R.; Todica, M.; Pop, C.V. XRD and IR Investigations of Some Commercial Polystyrene Samples Thermally Degraded. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 63–70. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Bertoli, A.C.; Borges, A.C.C.P.; Gomes, R.A.B.; Garcia, J.S.; Trevisan, M.G. New Organomineral Complex from Humic Substances Extracted from Poultry Wastes: Synthesis, Characterization and Controlled Release Study. J. Braz. Chem. Soc. 2018, 29, 140–150. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of Salinity and Humic Acid on the Aggregation and Toxicity of Polystyrene Nanoplastics with Different Functional Groups and Charges. Environ. Pollut. 2019, 245, 836–843. [Google Scholar] [CrossRef]
- Abobatta, W.F.; Mbarki, R. Effect of Humate Compounds and Magnetic Iron on Growth and Fruiting of Valencia Orange Trees (Citrus Sinensis L.). Ph.D. Thesis, Horticulture Research Institute Giza, Giza, Egypt, 2014. [Google Scholar]
- da Silva, M.S.R.; dos Santos, B.D.M.S.; da Silva, C.S.R.D.A.; da Silva, C.S.R.D.A.; Antunes, L.F.D.S.; dos Santos, R.M.; Santos, C.H.B.; Rigobelo, E.C. Humic Substances in Combination With Plant Growth-Promoting Bacteria as an Alternative for Sustainable Agriculture. Front Microbiol 2021, 12, 719653. [Google Scholar] [CrossRef]
- Nunes, R.O.; Domiciano, G.A.; Alves, W.S.; Melo, A.C.A.; Nogueira, F.C.S.; Canellas, L.P.; Olivares, F.L.; Zingali, R.B.; Soares, M.R. Evaluation of the Effects of Humic Acids on Maize Root Architecture by Label-Free Proteomics Analysis. Sci. Rep. 2019, 9, 12019. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-Applied Polystyrene Nanoplastics (PSNPs) Reduce the Growth and Nutritional Quality of Lettuce (Lactuca Sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Meganid, A.S.; Al-Zahrani, H.S.; El-Metwally, M.S. Effect of Humic Acid Application on Growth and Chlorophyll Contents of Common Bean Plants (Phaseolus Vulgaris L.) Under Salinity Stress Conditions. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 2651–2660. [Google Scholar] [CrossRef]
- Hanachi, P.; Khoshnamvand, M.; Walker, T.R.; Hamidian, A.H. Nano-Sized Polystyrene Plastics Toxicity to Microalgae Chlorella Vulgaris: Toxicity Mitigation Using Humic Acid. Aquat. Toxicol. 2022, 245, 106123. [Google Scholar] [CrossRef]
- Kizhuveetil, U.; Omer, S.; Karunagaran, D.; Suraishkumar, G.K. Improved Redox Anti-Cancer Treatment Efficacy through Reactive Species Rhythm Manipulation. Sci. Rep. 2020, 10, 1588. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Alberti, M.; Folke, C.; Moran, E.; Pell, A.N.; Deadman, P.; Kratz, T.; Lubchenco, J.; et al. Complexity of Coupled Human and Natural Systems. Science 2007, 317, 1513–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on Lipid Production by Scenedesmus Obliquus Triggered by Low Dose Exposure to Nanoparticles. Sci. Rep. 2017, 7, 15526. [Google Scholar] [CrossRef] [PubMed]




| Contents | PS | PS + HA |
|---|---|---|
| DLS size | 149.5 nm | 168.3 nm |
| ZETA potential (mV) | 11.7 mV | −41.9 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshmikanthan, D.; Chandrasekaran, N. Humic Acid Alleviates the Toxicity of Nanoplastics towards Solanum lycopersicum. Agronomy 2022, 12, 2787. https://doi.org/10.3390/agronomy12112787
Lakshmikanthan D, Chandrasekaran N. Humic Acid Alleviates the Toxicity of Nanoplastics towards Solanum lycopersicum. Agronomy. 2022; 12(11):2787. https://doi.org/10.3390/agronomy12112787
Chicago/Turabian StyleLakshmikanthan, Dhivya, and Natarajan Chandrasekaran. 2022. "Humic Acid Alleviates the Toxicity of Nanoplastics towards Solanum lycopersicum" Agronomy 12, no. 11: 2787. https://doi.org/10.3390/agronomy12112787







