Genetic Variation and Stability Analysis of an Artificially Synthesized Allohexaploid Brassica for Breeding Innovations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
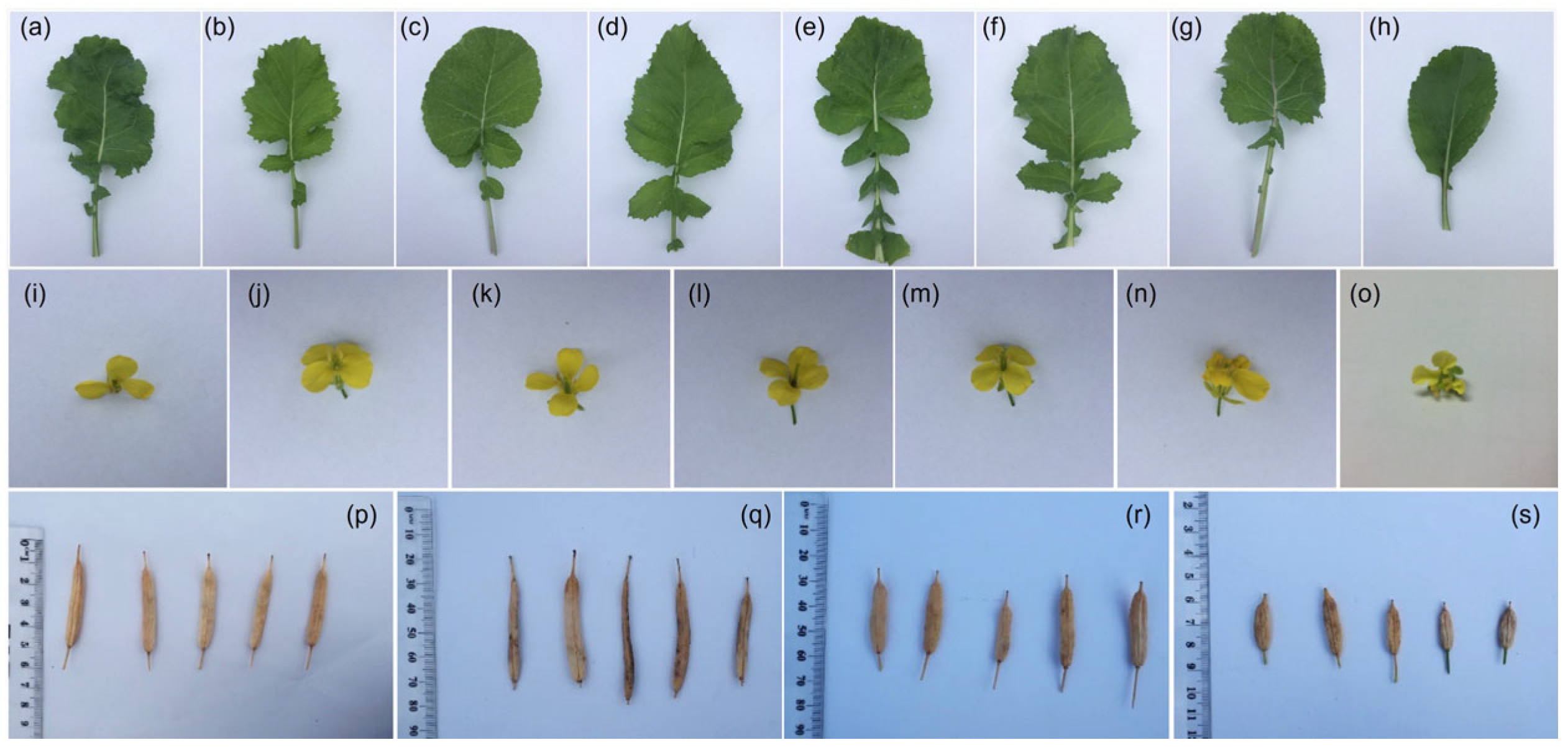
2.2. Phenotype Characterization
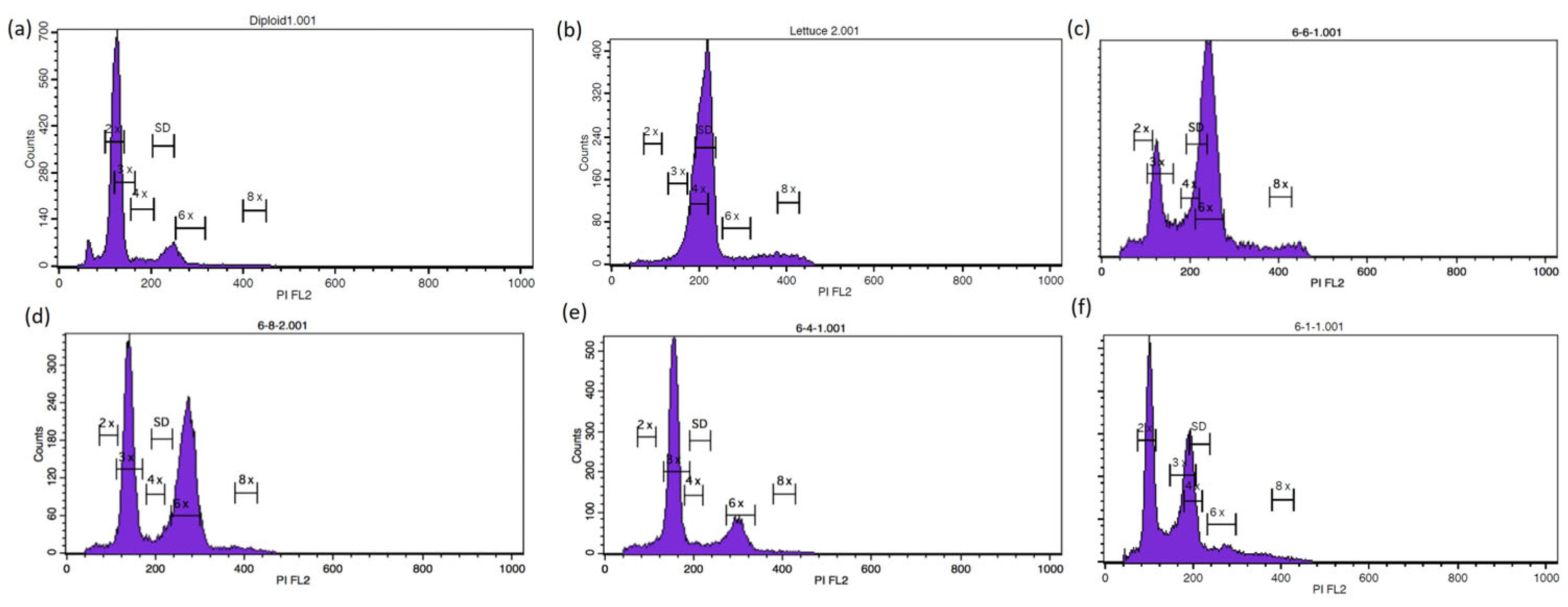
2.3. Flow Cytometry Analysis
2.4. Karyotyping by Multi-Color Fluorescence In Situ Hybridization (FISH)
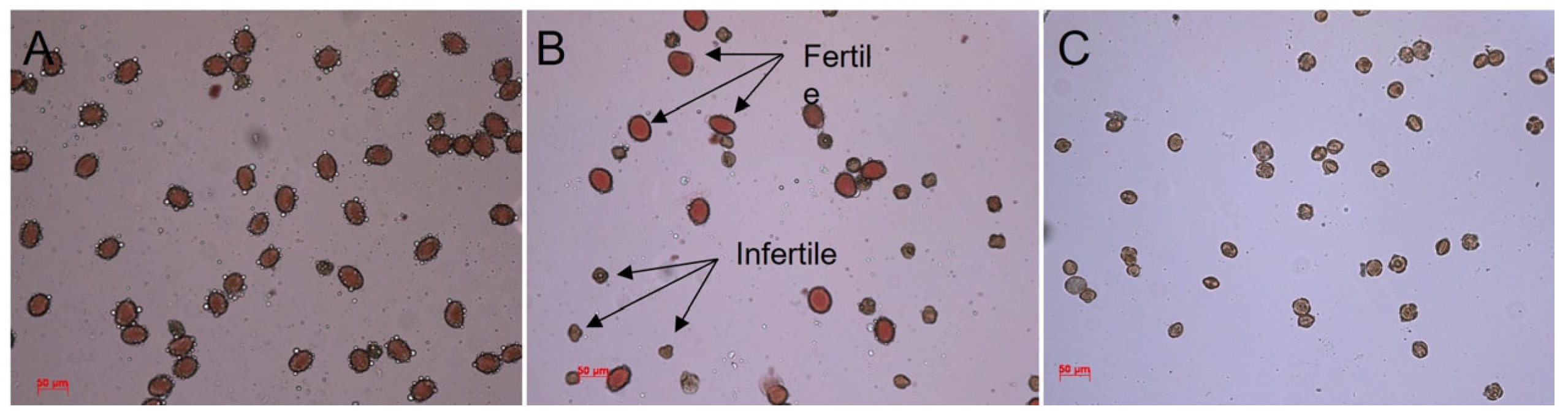
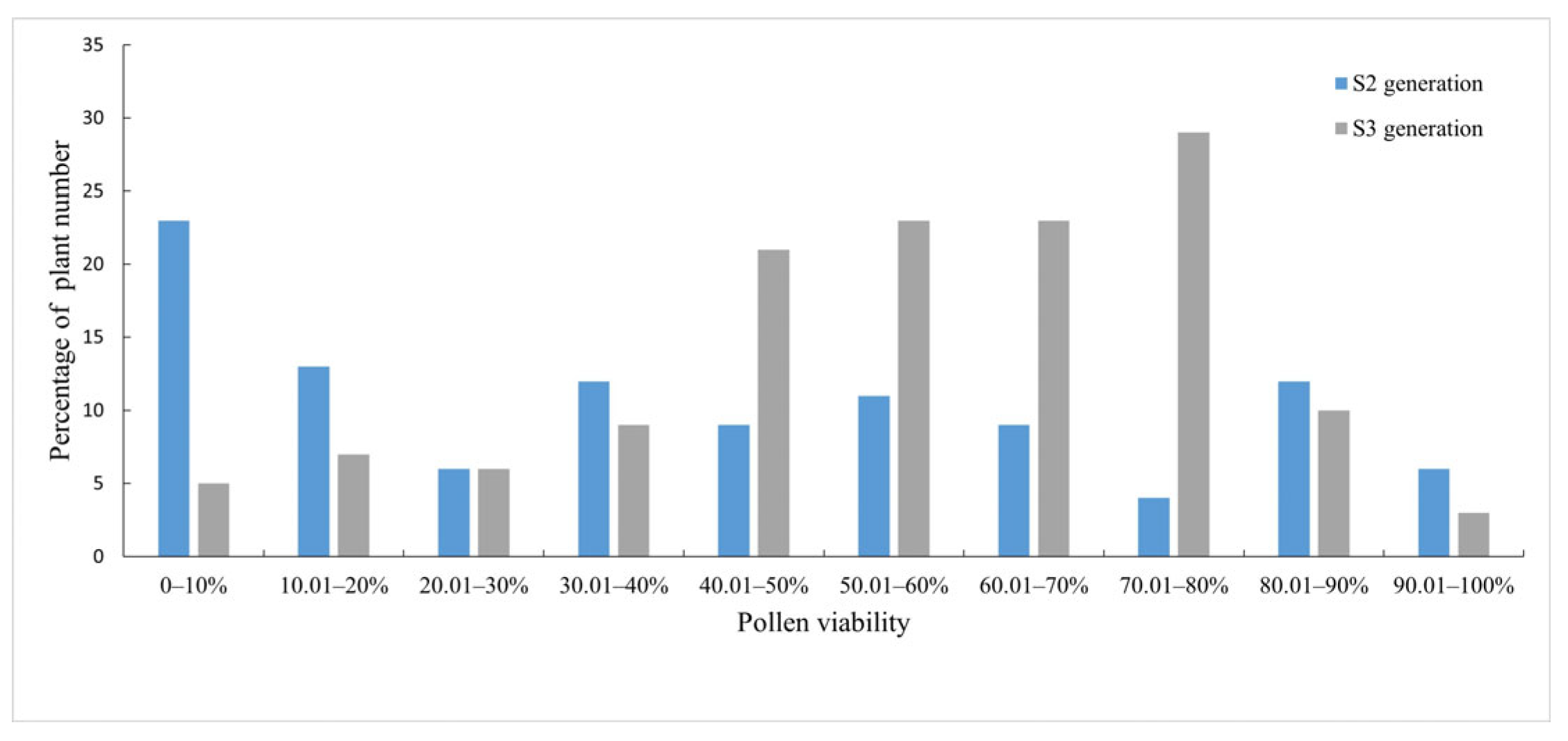
2.5. Pollen Viability Estimation
2.6. Chromosome Translocations Analysis
2.7. Statistical Analysis
3. Results
3.1. Morphological Variation
3.2. Ploidy Level Estimation
3.3. Karyotype Analysis
3.4. Pollen Vitality Evaluation
3.5. Agronomic Traits Analysis
3.6. Fixed Chromosome Translocations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, S.; Wu, X.M.; Bhat, S.R. History, evolution, and domestication of Brassica crops. Plant Breed. Rev. 2012, 35, 19–84. [Google Scholar] [CrossRef]
- Chen, S.; Nelson, M.N.; Chèvre, A.M.; Jenczewski, E.; Li, Z.; Mason, A.S.; Meng, J.; Plummer, J.A.; Pradhan, A.; Siddique, H.M.; et al. Trigenomic bridges for Brassica improvement. Crit. Rev. Plant Sci. 2011, 30, 524–547. [Google Scholar] [CrossRef]
- Tian, E.; Jiang, Y.; Chen, L.; Zou, J.; Liu, F.; Meng, J. Synthesis of a Brassica trigenomic allohexaploid (B. carinata × B. rapa) de novo and its stability in subsequent generations. Theor. Appl. Genet. 2010, 121, 1431–1440. [Google Scholar] [CrossRef]
- NAGARU, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Yang, S.; Chen, S.; Zhang, K.; Li, L.; Yin, Y.; Gill, R.A.; Yan, G.; Meng, J.; Cowling, W.A.; Zhou, W. A High-Density Genetic Map of an Allohexaploid Brassica Doubled Haploid Population Reveals Quantitative Trait Loci for Pollen Viability and Fertility. Front. Plant Sci. 2018, 9, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beversdorf, W.D.; Weiss-Lerman, J.; Erickson, L.R.; Machado, V.S. Transfer of cytoplasmically-inherited triazine resistance from bird’s rape to cultivated oilseed rape (Brassica campestris and B. napus). Can. J. Genet. Cytol. 1980, 22, 167–172. [Google Scholar] [CrossRef]
- Chèvre, A.M.; Barret, P.; Eber, F.; Dupuy, P.; Brun, H.; Tanguy, X.; Renard, M. Selection of stable Brassica napus-B. juncea recombinant lines resistant to blackleg (Leptosphaeria maculans). Theor. Appl. Genet. 1997, 95, 1104–1111. [Google Scholar] [CrossRef]
- Bradshaw, J.E.; Gemmell, D.J.; Wilson, R.N. Transfer of resistance to clubroot (Plasmodiophora brassicae) to swedes (Brassica napus L. var. napobrassica Peterm) from B. rapa. Ann. Appl. Biol. 1997, 130, 337–348. [Google Scholar] [CrossRef]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M. Relationships between growth and gas exchange characteristics in some salt-tolerant amphidiploid Brassica species in relation to their diploid parents. Environ. Exp. Bot. 2001, 45, 155–163. [Google Scholar] [CrossRef]
- Rahman, M.H. Production of yellow-seeded Brassica napus through interspecific crosses. Plant Breed. 2001, 120, 463–472. [Google Scholar] [CrossRef]
- Saal, B.; Brun, H.; Glais, I.; Struss, D. Identification of a Brassica juncea-derived recessive gene conferring resistance to Leptosphaeria maculans in oilseed rape. Plant Breed. 2004, 123, 505–511. [Google Scholar] [CrossRef]
- Rygulla, W.; Friedt, W.; Seyis, F.; Lühs, W.; Snowdon, R.J. Combination of resistance to Verticillium longisporum from zero erucic acid Brassica oleracea and oilseed Brassica rapa genotypes in resynthesized rapeseed (Brassica napus) lines. Plant Breed. 2007, 126, 596–602. [Google Scholar] [CrossRef]
- Leitch, A.R.; Leitch, I.J. Genomic plasticity and the diversity of polyploid plants. Science 2008, 5875, 481–483. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.M.; Quiros, C.F. Generation of alien chromosome addition lines from synthetic Brassica napus: Morphology, cytology, fertility, and chromosome transmission. Genome 1990, 33, 374–383. [Google Scholar] [CrossRef]
- Masterson, J. Stomatal size in fossil plants-evidence for polyploidy in majority of angiosperms. Science 1994, 264, 421–424. [Google Scholar] [CrossRef]
- Mason, A.S.; Zhang, J.; Tollenaere, R.; Teuber, P.V.; Dalton-Morgan, J.; Hu, L.; Yan, G.; Edwards, D.; Redden, R.; Batley, J. High-throughput genotyping for species identification and diversity assessment in germplasm collections. Mol. Ecol. Resour. 2015, 15, 1091–1101. [Google Scholar] [CrossRef] [Green Version]
- Bing, D.J.; Downey, R.K.; Rakow, G.F.W. Hybridizations among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the field. Plant Breed. 1996, 115, 470–473. [Google Scholar] [CrossRef]
- Zhang, K.; Mason, A.S.; Farooq, M.A.; Islam, F.; Quezada-Martinez, D.; Hu, D.; Yang, S.; Zou, J.; Zhou, W. Challenges and prospects for a potential allohexaploid brassica crop. Theor. Appl. Genet. 2021, 134, 2711–2726. [Google Scholar] [CrossRef]
- Howard, H.W. The effect of polyploidy and hybridity on seed size in crosses between Brassica chinensis, B. carinata, amphidiploid B. chinensis-carinata and autotetraploid B. chinensis. J. Genet. 1942, 43, 105–119. [Google Scholar] [CrossRef]
- Iwasa, S. Cytogenetic studies on the artificially raised trigenomic hexaploid hybrid forms in the genus Brassica. J. Fac. Agric. Kyushu Univ. 1964, 13, 309–349. [Google Scholar] [CrossRef]
- Mason, A.S.; Huteau, V.; Eber, F.; Coriton, O.; Yan, G.; Nelson, M.N.; Cowling, W.A.; Chèvre, A.M. Genome structure affects the rate of autosyndesis and allosyndesis in AABC, BBAC and CCAB Brassica interspecific hybrids. Chromosom. Res. 2010, 18, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.S.; Nelson, M.N.; Castello, M.C.; Yan, G.; Cowling, W.A. Genotypic effects on the frequency of homoeologous and homologous recombination in Brassica napus × B. carinata hybrids. Theor. Appl. Genet. 2011, 122, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.S.; Yan, G.; Cowling, W.A.; Nelson, M.N. A new method for producing allohexaploid Brassica through unreduced gametes. Euphytica 2012, 186, 277–287. [Google Scholar] [CrossRef]
- Comai, L.; Tyagi, A.P.; Winter, K.; Holmes-Davis, R.; Reynolds, S.H.; Stevens, Y.; Byers, B. Phenotypic instability and rapid gene silencing in newly formed Arabidopsis allotetraploids. Plant Cell 2000, 12, 1551–1567. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, H.; Gao, A.; Yang, X.; Liu, W.; Li, X.; Li, L. Intergenomic rearrangements after polyploidization of Kengyilia thoroldiana (Poaceae: Triticeae) affected by environmental factors. PLoS ONE 2012, 7, e31033. [Google Scholar] [CrossRef] [Green Version]
- Gaeta, R.T.; Pires, J.C.; Iniguez-Luy, F.; Osborn, L.T.C. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 2007, 19, 3403–3417. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.L.; Percifield, R.; Wendel, J.F. Organ-specific silencing of duplicated genes in a newly synthesized cotton allotetraploid. Genetics 2004, 168, 2217–2226. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, R.; Taylor, J.M.; Sasaki, T.; Kawanabe, T.; Dennis, E.S. Genome wide gene expression in artificially synthesized amphidiploids of Arabidopsis. Plant Mol. Biol. 2011, 77, 419. [Google Scholar] [CrossRef]
- Yang, S.; Chen, S.; Geng, X.; Yan, G.; Li, Z.; Meng, J.; Cowling, W.A.; Zhou, W. The first genetic map of a synthesized allohexaploid Brassica with A, B and C genomes based on simple sequence repeat markers. Theor. Appl. Genet. 2016, 129, 689–701. [Google Scholar] [CrossRef]
- Madlung, A.; Tyagi, A.P.; Watson, B.; Jiang, H.; Kagochi, T.; Doerge, R.W.; Martienssen, R.; Comai, L. Genomic changes in synthetic Arabidopsis polyploids. Plant J. 2005, 41, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, C.; Cui, C.; Ge, X.; Li, Z. Distinct subgenome stabilities in synthesized Brassica allohexaploids. Theor. Appl. Genet. 2016, 129, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Mwathi, M.W.; Gupta, M.; Atri, C.; Banga, S.S.; Batley, J.; Mason, A.S. Segregation for fertility and meiotic stability in novel Brassica allohexaploids. Theor. Appl. Genet. 2017, 130, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Chen, S.; Astarini, A.; Yan, G.; Tian, E.; Meng, J.; Li, Z.; Ge, X.; Nelson, M.N.; Mason, A.S.; et al. Doubled haploids of novel trigenomic Brassica derived from various interspecific crosses. Plant Cell Tissue Organ Cult. 2013, 113, 501–511. [Google Scholar] [CrossRef]
- Yang, S.; Gill, R.A.; Zaman, Q.U.; Ulhassan, Z.; Zhou, W. Insights on SNP types, detection methods and their utilization in Brassica species: Recent progress and future perspectives. J. Biotechnol. 2020, 20, 11–20. [Google Scholar] [CrossRef]
- Pradhan, A.; Plummer, J.A.; Nelson, M.N.; Cowling, W.A.; Yan, G. Successful induction of trigenomic hexaploid Brassica from a triploid hybrid of B. napus L. and B. nigra (L.) Koch. Euphytica 2010, 176, 87–98. [Google Scholar] [CrossRef]
- Kato, A.; Lamb, J.C.; Birchler, J.A. Chromosome painting using repetitive DNA sequence as probes for somatic chromosome identification in maize. Proc. Natl. Acad. Sci. USA 2004, 101, 13554–13559. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Xie, B.; Wu, T.; Xin, X.; Mang, L.; Tan, G.; Xiong, Z. Karyotyping and identifying all of the chromosomes of allopolyploid Brassica juncea using multicolor FISH. Crop J. 2016, 4, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 396, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Bayer, M.M.; Rapazote-Flores, P.; Ganal, M.; Hedley, P.E.; Macaulay, M.; Plieske, J.; Ramsay, L.; Russell, J.; Shaw, P.D.; Thomas, W.; et al. Development and evaluation of a barley 50k iSelect SNP array. Front. Plant Sci. 2017, 8, 1792. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Lv, D.; Ge, Y.; Shi, J.; Weijers, D.; Yu, G.; Chen, J. RIdeogram: Drawing SVG graphics to visualize and map genome-wide data on the idiograms. PeerJ Comput. Sci. 2020, 6, e251. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef] [Green Version]
- Comai, L. Genetic and epigenetic interactions in allopolyploid plants. In Plant Gene Silencing; Springer: Dordrecht, The Netherlands, 2000; pp. 387–399. [Google Scholar] [CrossRef]
- Song, K.; Lu, P.; Tang, K.; Osborn, T.C. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 7719–7723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, D.; Yan, Z.; Zhou, Y. Rapid changes of microsatellite flanking sequence in the allopolyploidization of new synthesized hexaploid wheat. Sci. China Ser. C Life Sci. 2004, 47, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Pelé, A.; Rousseau-Gueutin, M.; Chèvre, A.M. Speciation success of polyploid plants closely relates to the regulation of meiotic recombination. Front. Plant Sci. 2018, 9, 907. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, J.; Schemske, D.W. Neopolyploidy in flowering plants. Annu. Rev. Ecol. Syst. 2002, 33, 589–639. [Google Scholar] [CrossRef] [Green Version]
- Udall, J.A.; Quijada, P.A.; Osborn, T.C. Detection of chromosomal rearrangements derived from homeologous recombination in four mapping populations of Brassica napus L. Genetics 2005, 169, 967–979. [Google Scholar] [CrossRef] [Green Version]
- Quezada-Martinez, D.; Zou, J.; Zhang, W.; Meng, J.; Batley, J.; Mason, A.S. Allele segregation analysis of F1 hybrids between independent Brassica allohexaploid lineages. Chromosoma 2022, 131, 147–161. [Google Scholar] [CrossRef]
- Li, M.T.; Li, Z.Y.; Zhang, C.Y.; Qian, W.; Meng, J. Reproduction and cytogenetic characterization of interspecific hybrids derived from crosses between Brassica carinata and B. rapa. Theor. Appl. Genet. 2005, 110, 1284–1289. [Google Scholar] [CrossRef]
- Sundberg, E.; Landgren, M.; Glimelius, K. Fertility and chromosome stability in Brassica napus resynthesised by protoplast fusion. Theor. Appl. Genet. 1987, 75, 96–104. [Google Scholar] [CrossRef]
- Peng, J.; Wei, Y.R.; Xiong, X.H. Polyploid induction of plant research summary. Chin. Agric. Sci. Bull. 2010, 26, 45–49. [Google Scholar]
- Chiang, B.Y.; Grant, W.F.; Chiang, M.S. Transfer of resistance to race 2 of Plasmodiophora brassicae from Brassica napus to cabbage (B. oleracea var. capitata). II. Meiosis in the interspecific hybrids between B. napus and 2x and 4x cabbage. Euphytica 1978, 27, 81–93. [Google Scholar] [CrossRef]
- Gaebelein, R.; Schiessl, S.V.; Samans, B.; Batley, J.; Mason, A.S. Inherited allelic variants and novel karyotype changes influence fertility and genome stability in Brassica allohexaploids. New Phytol. 2019, 223, 965–978. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Mason, A.S. Plant speciation through chromosome instability and ploidy change: Cellular mechanisms, molecular factors and evolutionary relevance. Curr. Plant Biol. 2014, 1, 10–33. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Wang, X.; Wang, H.; Tian, J.; Li, B.; Chen, L.; Chao, H.; Long, Y.; Xiang, J.; Gan, J.; et al. Genome-wide identification of QTL for seed yield and yield-related traits and construction of a high-density consensus map for QTL comparison in Brassica napus. Front. Plant Sci. 2016, 7, 17. [Google Scholar] [CrossRef]


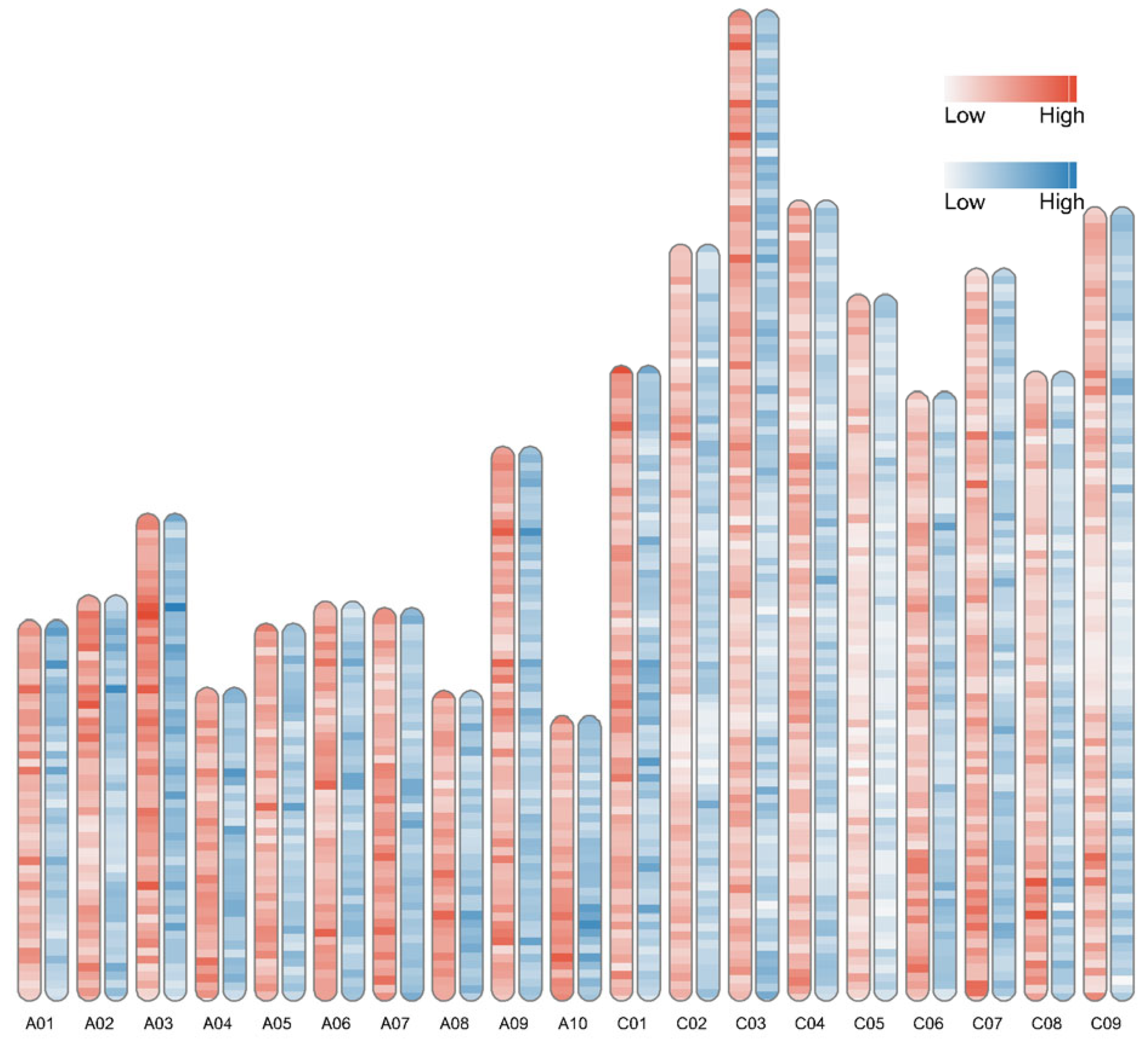
| S1 Generation | Seed Yield (g/Plant) | Selection Category | S2 Generation | Germination Rate (%) | Mean Hexaploid Percentage (%) | Standard Deviation | Coefficient of Variation (%) |
|---|---|---|---|---|---|---|---|
| 7-6-088 | 2.216 | C1 | 6-1 | 92.86 | 15.98 | 0.03 | 18.78 |
| 7-6-088 | 2.485 | C1 | 6-2 | 92.86 | 18.27 | 0.04 | 21.89 |
| 7-6-203 | 3.485 | C1 | 6-3 | 92.86 | 26.53 | 0.15 | 55.40 |
| 7-6-053 | 2.273 | C1 | 6-4 | 78.57 | 19.73 | 0.06 | 27.88 |
| 7-6-107 | 2.109 | C1 | 6-5 | 71.43 | 35.75 | 0.09 | 24.05 |
| 7-6-107 | 6.355 | C1 | 6-6 | 100.00 | 59.09 | 0.13 | 21.32 |
| 7-6-204 | 5.076 | C1 | 6-7 | 42.86 | 42.56 | 0.26 | 59.92 |
| 7-6-204 | 3.091 | C1 | 6-8 | 78.57 | 47.45 | 0.23 | 48.68 |
| 7-6-279 | 5.663 | C1 | 6-9 | 64.29 | 34.99 | 0.13 | 36.87 |
| 7-6-050 | 3.063 | C1 | 6-10 | 92.86 | 40.79 | 0.15 | 35.55 |
| 7-6-050 | 5.993 | C1 | 6-11 | 71.43 | 50.31 | 0.13 | 25.84 |
| 7-6-323 | 14.802 | C1 | 6-12 | 100.00 | 48.61 | 0.05 | 11.11 |
| 7-2-003 | 2.452 | C1 | 6-13 | 78.57 | 37.73 | 0.14 | 37.64 |
| 7-6-103 | 2.349 | C1 | 6-14 | 50.00 | 40.41 | 0.07 | 16.08 |
| 7-6-267 | 3.684 | C1 | 6-15 | 60.00 | 27.63 | 0.15 | 54.28 |
| 7-6-267 | 4.855 | C1 | 6-16 | 64.29 | 22.71 | 0.09 | 37.87 |
| 7-6-267 | 2.126 | C1 | 6-17 | 71.43 | 22.22 | 0.03 | 13.95 |
| 7-6-30 | 6.031 | C1 | 6-18 | 64.29 | 41.91 | 0.20 | 46.76 |
| 7-6-30 | 4.956 | C1 | 6-19 | 85.71 | 36.91 | 0.19 | 52.56 |
| 7-6-30 | 4.849 | C1 | 6-20 | 100.00 | 29.43 | 0.11 | 38.39 |
| 7-6-30 | 3.985 | C1 | 6-21 | 100.00 | 25.89 | 0.08 | 31.29 |
| 7-6-30 | 2.975 | C1 | 6-22 | 57.14 | 30.45 | 0.06 | 20.36 |
| 7-6-30 | 33.965 | C1 | 6-23 | 92.86 | 13.70 | 0.02 | 13.14 |
| 7-6-30 | 0.171 | C2 | 6-24 | 50.00 | 37.51 | 0.20 | 54.12 |
| 7-6-053 | 0.07 | C2 | 6-25 | 78.57 | 38.83 | 0.09 | 23.18 |
| 7-6-204 | 0.162 | C2 | 6-26 | 85.71 | 21.51 | 0.03 | 13.48 |
| 7-6-050 | 0.109 | C2 | 6-27 | 85.71 | 27.03 | 0.05 | 18.50 |
| 7-2-003 | 0.104 | C2 | 6-28 | 78.57 | 41.26 | 0.15 | 35.14 |
| 7-6-267 | 0.331 | C2 | 6-29 | 78.57 | 25.87 | 0.14 | 52.57 |
| 7-4-001 | 0.056 | C3 | 6-30 | 85.71 | 52.04 | 0.15 | 29.21 |
| 7-4-001 | 0.111 | C3 | 6-31 | 85.71 | 51.82 | 0.19 | 36.66 |
| 7-4-001 | 0.039 | C3 | 6-32 | 100.00 | 55.55 | 0.17 | 31.14 |
| 7-4-001 | 0.043 | C3 | 6-33 | 50.00 | 52.36 | 0.03 | 5.92 |
| 7-6-080 | 0.094 | C3 | 6-34 | 64.29 | 53.31 | 0.19 | 35.27 |
| 7-6-080 | 0.027 | C3 | 6-35 | 85.71 | 51.23 | 0.01 | 2.73 |
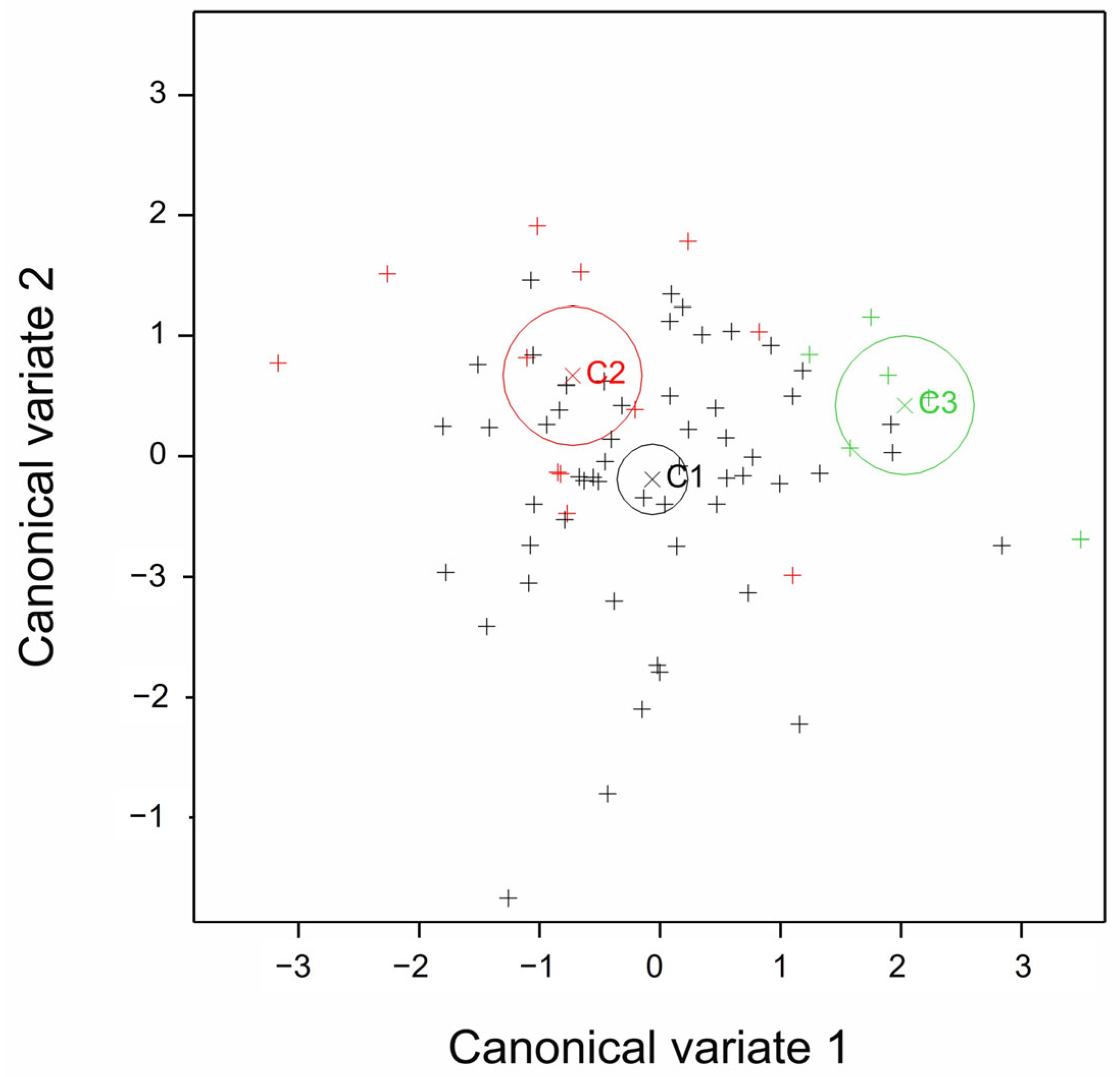
| Phenotypic Traits | C1 Group | C2 Group | C3 Group |
|---|---|---|---|
| Hexaploid percentage (%) | 33.44% ± 0.16 b | 32.00% ± 0.13 b | 52.72% ± 0.12 a |
| Pollen viability (%) | 37.59% ± 0.32 a | 48.26% ± 0.31 a | 46.50% ± 0.22 a |
| Plant height (cm) | 145.62 ± 35.28 a | 153.28 ± 33.12 a | 118.06 ± 33.69 b |
| Flowering time (day) | 86.61 ± 14.60 a | 92.56 ± 16.37 a | 83.33 ± 7.24 a |
| Above-ground biomass (g) | 48.38 ± 21.36 a | 55.64 ± 24.53 a | 19.39 ± 11.98 b |
| Seed yield (g) | 2.61 ± 2.86 a | 1.33 ± 1.35 ab | 0.31 ± 0.42 b |
| 1000-seed weight (g) | 4.66 ± 0.90 a | 4.50 ± 1.68 a | 4.73 ± 2.04 a |
| Flowering Time | Chromosome Number | Pollen Viability | Seed Yield | 1000-Seed Weight (TSW) | Plant Height | Above-Ground Biomass | |
|---|---|---|---|---|---|---|---|
| Flowering time | 1 | ||||||
| Chromosome number | 0.290 ** | 1 | |||||
| Pollen viability | 0.036 | −0.211 * | 1 | ||||
| Seed yield | −0.076 | −0.228 * | 0.368 ** | 1 | |||
| 1000-seed yield | 0.233 * | 0.085 | 0.019 | −0.137 | 1 | ||
| Plant height | 0.210 * | 0.023 | −0.076 | 0.264 ** | 0.044 | 1 | |
| Above-ground biomass | 0.007 | −0.125 | 0.201 * | 0.453 ** | −0.079 | 0.653 ** | 1 |
| Canonical Variate | Characteristic Value | Contribution Rate (%) | Overall Contribution Rate (%) |
|---|---|---|---|
| CV1 | 0.4471 | 78.73 | 78.73 |
| CV2 | 0.1208 | 21.27 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, K.; Lu, C.; Chen, G.; Huang, Q.; Ulhassan, Z.; Wei, J.; Farooq, M.A.; Zhou, W. Genetic Variation and Stability Analysis of an Artificially Synthesized Allohexaploid Brassica for Breeding Innovations. Agronomy 2022, 12, 2843. https://doi.org/10.3390/agronomy12112843
Yang S, Zhang K, Lu C, Chen G, Huang Q, Ulhassan Z, Wei J, Farooq MA, Zhou W. Genetic Variation and Stability Analysis of an Artificially Synthesized Allohexaploid Brassica for Breeding Innovations. Agronomy. 2022; 12(11):2843. https://doi.org/10.3390/agronomy12112843
Chicago/Turabian StyleYang, Su, Kangni Zhang, Chenze Lu, Guangna Chen, Qian Huang, Zaid Ulhassan, Ji’an Wei, Muhammad Ahsan Farooq, and Weijun Zhou. 2022. "Genetic Variation and Stability Analysis of an Artificially Synthesized Allohexaploid Brassica for Breeding Innovations" Agronomy 12, no. 11: 2843. https://doi.org/10.3390/agronomy12112843






