Aromatic Plants Metabolic Engineering: A Review
Abstract
:1. Introduction
2. The Specifics of Biotechnology Application in Improving the Quality of Aromatic Plants
2.1. Specialized Metabolites of EOs and Their Biosynthesis
2.2. Diseases of Aromatic Plants
2.3. The Biotransformation of Aromatic Plants
2.4. Prospects for the Development of Biotechnological Approaches for Large-Scale Cultivation of Aromatic Plants
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, S.; El Bethel Lalthavel Hmar, J.H.; Zothantluanga, H.K.S. Essential oils: A review on their salient biological activities and major delivery strategies. J. Mizo Acad. Sci. 2020, 20, 54–71. [Google Scholar]
- Gounaris, Y. Biotechnology for the production of essential oils, flavours and volatile isolates. A Review. Flav. Fragr. J. 2010, 25, 367–386. [Google Scholar] [CrossRef]
- Inoue, M.; Craker, L.E. Medicinal and aromatic plants-Uses and functions. In Horticulture: Plants for People and Places; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 645–669. [Google Scholar]
- Patel, D.K. Medicinal and Aromatic Plants: Role in Human Society. Med. Aromat. Plants 2016, 5, 3. [Google Scholar]
- Gutensohn, M.; Nagegowda, D.A.; Dudareva, N. Involvement of compartmentalization in monoterpene and sesquiterpene biosynthesis in plants. In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Bach, T.J., Rohmer, M., Eds.; Springer: New York, NY, USA, 2013; pp. 155–169. [Google Scholar]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Saxena, M.J.; Maurya, P. Distribution of aromatic plants in the world and their properties. In Feed Additives; Academic Press: Cambridge, MA, US, 2020; pp. 89–114. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 125–160. [Google Scholar]
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential-a review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Campêlo, M.C.S.; Medeiros, J.M.S.; Silva, J.B.A. Natural products in food preservation. Int. Food Res. J. 2019, 26, 130. [Google Scholar]
- Di Gioia, D.; Luziatelli, F.; Negroni, A.; Ficca, A.G.; Fava, F.; Ruzzi, M. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J. Biotechnol. 2011, 156, 309–316. [Google Scholar] [CrossRef]
- Lubbers, R.J.; Dilokpimol, A.; Visser, J.; Mäkelä, M.R.; Hildén, K.S.; de Vries, R.P. A comparison between the homocyclic aromatic metabolic pathways from plant-derived compounds by bacteria and fungi. Biotechnol. Adv. 2019, 37, 107396. [Google Scholar]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative approaches for recovery of phytoconstituents from medicinal/aromatic plants and biotechnological production. Molecules 2020, 25, 309. [Google Scholar]
- Kuluev, B.R.; Safiullina, M.G.; Knyazev, A.V.; Chemeris, A.V. Effect of ectopic expression of NtEXPA5 gene on cell size and growth of organs of transgenic tobacco plants. Russ. J. Dev. Biol. 2013, 44, 28–34. [Google Scholar] [CrossRef]
- Barone, R.P.; Knittel, D.K.; Ooka, J.K.; Porter, L.N.; Smith, N.T.; Owens, D.K. The production of plant natural products beneficial to humanity by metabolic engineering. Curr. Plant Biol. 2020, 24, 100121. [Google Scholar] [CrossRef]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, M.C.T.; Duarte, R.M.T.; Rodrigues, R.A.F.; Rodrigues, M.V.N. Essential oils and their characteristics. Essent. Oils Food Process. Chem. Saf. Appl. 2018, 1, 1–19. [Google Scholar]
- Belide, S.; Hac, L.; Singh, S.P.; Green, A.G.; Wood, C.C. Agrobacterium-mediated transformation of safflower and the efficient recovery of transgenic plants via grafting. Plant Methods 2011, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, I.; Hussain, T.; Ashraf, I.; Nafees, M.; Maryam, R.M.; Iqbal, M. Lethal effects of secondary metabolites on plant tissue culture. Am. Eurasian J. Agric. Environ. Sci. 2013, 13, 539–547. [Google Scholar]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [Green Version]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Liao, P.; Wang, H.; Hemmerlin, A.; Nagegowda, D.A.; Bach, T.J.; Wang, M.; Chye, M.L. Past achievements, current status and future perspectives of studies on 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS) in the mevalonate (MVA) pathway. Plant Cell Rep. 2014, 33, 1005–1022. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP Pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Dudareva, N. Orthologs of the archaeal isopentenyl phosphate kinase regulate terpenoid production in plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10050–10055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.P.; Yu, X.D.; Fan, J.; Wang, C.S.; Xia, L.Q. Expressing an (E)-β-farnesene synthase in the chloroplast of tobacco affects the preference of green peach aphid and its parasitoid. J. Integr. Plant Biol. 2015, 57, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Endo, T.; Rodríguez, A.; Fujii, H.; Goto, S.; Matsuura, T.; Hojo, Y.; Ikeda, Y.; Mori, I.C.; Fujikawa, T.; et al. Ectopic accumulation of linalool confers resistance to Xanthomonas citri subsp. citri in transgenic sweet orange plants. Tree Physiol. 2017, 37, 654–664. [Google Scholar]
- Gilani, S.A.; Kikuchi, A.; Yu, X.; Ahmad, M.Z.; Sugano, M.; Fujii, Y.; Watanabe, K.N. Difference between non-transgenic and salt tolerant transgenic Eucalyptus camaldulensis for diversity and allelopathic effects of essential oils. Pak. J. Bot. 2017, 49, 345–351. [Google Scholar]
- Adal, A.M.; Binson, E.; Remedios, L.; Mahmoud, S.S. Expression of lavender AGAMOUS-like and SEPALLATA3-like genes promote early flowering and alter leaf morphology in Arabidopsis thaliana. Planta 2021, 254, 1–12. [Google Scholar] [CrossRef]
- Su, S.; Liu, X.; Pan, G.; Hou, X.; Zhang, H.; Yuan, Y. In vitro characterization of a (E)-β-farnesene synthase from Matricaria recutita L. and its up-regulation by methyl jasmonate. Gene 2015, 571, 58–64. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Li, G.; Guo, K.; Harvey, P.; Chen, Q.; Zhao, Z.; Wei, Y.; Li, J.; Yang, H. MAPK-mediated regulation of growth and essential oil composition in a salt-tolerant peppermint (Mentha piperita L.) under NaCl stress. Protoplasma 2016, 253, 1541–1556. [Google Scholar] [CrossRef]
- Navet, N.; Tian, M. Efficient targeted mutagenesis in allotetraploid sweet basil by CRISPR/Cas9. Plant Direct 2020, 4, e00233. [Google Scholar] [CrossRef]
- Khakdan, F.; Shirazi, Z.; Ranjbar, M. Identification and functional characterization of the CVOMTs and EOMTs genes promoters from Ocimum basilicum L. Plant Cell Tissue Organ Cult. 2022, 148, 387–402. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, S.S.; Dubey, B.K. Salt and drought stress tolerance with increased biomass in transgenic Pelargonium graveolens through heterologous expression of ACC deaminase gene from Achromobacter xylosoxidans. Plant Cell Tissue Organ Cult. 2021, 147, 297–311. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Deng, Z.; Liu, T. Strategies for terpenoid overproduction and new terpenoid discovery. Curr. Opin. Biotechnol. 2017, 48, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Park, S.; Kim, J.A.; Lee, S.I.; Lee, K. Metabolic perturbation and synthetic biology strategies for plant terpenoid production—An updated overview. Plants 2021, 10, 2179. [Google Scholar] [CrossRef]
- Fuchs, L.K.; Holland, A.H.; Ludlow, R.A.; Coates, R.J.; Armstrong, H.; Pickett, J.A.; Harwood, J.L.; Scofield, S. Genetic manipulation of biosynthetic pathways in mint. Front. Plant Sci. 2022, 13, 928178. [Google Scholar] [CrossRef]
- Lange, B.M.; Mahmoud, S.S.; Wildung, M.R.; Turner, G.W.; Davis, E.M.; Lange, I.; Baker, R.C.; Boydston, R.A. Improving peppermint essential oil yield and composition by metabolic engineering, Proc. Natl. Acad. Sci. USA 2011, 108, 16944–16949. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, K.; Subramaniyan, M.; Rawat, K.; Kalamuddin, M.; Qureshi, M.I.; Malhotra, P.; Mohmmed, A.; Cornish, K.; Daniell, H.; Kumar, S. Compartmentalized metabolic engineering for artemisinin biosynthesis and effective malaria treatment by oral delivery of plant cells. Mol. Plant 2016, 9, 1464–1477. [Google Scholar] [CrossRef] [Green Version]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Lisei-de-Sá, M.E.; Rodrigues-Silva, P.L.; Morgante, C.V.; de Melo, B.P.; Lourenço-Tessutti, I.T.; Arraes, F.B.M.; Sousa, J.P.A.; Galbieri, R.; Amorim, R.M.S.; de Lins, C.B.J.; et al. Pyramiding dsRNAs increases phytonematode tolerance in cotton plants. Planta 2021, 254, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R. Diseases of medicinal and aromatic plants: Insights in nematode biomanagement. Indian Phytopathol. 2017, 70, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Saikia, S.K.; Tiwari, S.; Pandey, R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita. Biol. Control 2013, 65, 225–234. [Google Scholar] [CrossRef]
- Gupta, R.; Tiwari, S.; Saikia, S.K.; Shukla, V.; Singh, R.; Singh, S.P.; Ajay Kumar, P.V.; Pandey, R. Exploitation of microbes for enhancing bacoside content and reduction of Meloidogyne incognita infestation in Bacopa monnieri L. Protoplasma 2015, 252, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, R.; Saikia, S.; Pant, A.; Pandey, R. Diseases of medicinal and aromatic plants, their biological impact and management. Plant Genet. Res. 2016, 14, 370–383. [Google Scholar] [CrossRef]
- Saeed, S.T.; Samad, A. Emerging threats of begomoviruses to the cultivation of medicinal and aromatic crops and their management strategies. Virus Dis. 2017, 28, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, S.; Harsh, N.S.K.; Sharma, A.K.; Mao, L.P.; Thakur, S. A database of diseases of medicinal plants in Uttarakhand. Ind. Forester 2014, 140, 518–527. [Google Scholar]
- Gatak, S.; Polley, S.K.; Ghosh, S.K.; Chakrabarty, N. Biological Control (In Vitro) of the pathogen causing leaf blight disease of mint (Mentha arvensis L.). Plant Cell Biotechnol. Mol. Biol. 2020, 21, 57–67. [Google Scholar]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.E.; Mesarich, C.H.; Thomma, B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015, 53, 541–563. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 2011, 91, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Qi, D.; Ashfield, T.; Helm, M.; Innes, R.W. Using decoys to expand the recognition specificity of a plant disease resistance protein. Science 2016, 351, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA interference mechanisms and applications in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M. Pyramiding resistance-conferring gene sequences in crops. Curr. Opin. Virol. 2017, 26, 36–42. [Google Scholar] [CrossRef]
- Bertaccini, A.; Duduk, B. Phytoplasma and phytoplasma diseases: A review of recent research. Phytopathol. Mediter. 2010, 48, 355–378. [Google Scholar]
- Marcone, C.; Bellardi, M.G.; Bertaccini, A. Phytoplasma diseases of medicinal and aromatic plants. J. Plant Pathol. 2016, 379–404. [Google Scholar]
- Lakhanpaul, S.; Singh, V.; Kumar, S.; Singh, A. Molecular Mechanism Underlying Symptom Development in Phytoplasma Associated Diseases-The Key Players and their Role. Indian J. Entomol. 2022, 1–10. [Google Scholar] [CrossRef]
- Sallaud, C.; Giacalone, C.; Topfer, R.; Goepfert, S.; Bakaher, N.; Rosti, S.; Tissier, A. Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J. 2012, 72, 1–17. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, G.; Xian, M.; Xie, C. Improved cis-abienol production through increasing precursor supply in Escherichia coli. Sci. Rep. 2020, 10, 16791. [Google Scholar] [CrossRef]
- Chacón, M.G.; Marriott, A.; Kendrick, E.G.; Styles, M.Q.; Leak, D.J. Esterification of geraniol as a strategy for increasing product titre and specificity in engineered Escherichia coli. Microb. Cell Fact. 2019, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Zhang, J.; Zhou, Y.; Zhang, Y.; Wang, F.; Li, X. Engineering Escherichia coli for production of geraniol by systematic synthetic biology approaches and laboratory-evolved fusion tags. Metabol. Eng. 2021, 66, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Zhang, J.; Xiao, L.; Zhou, Y.; Zhang, Y.; Wang, F.; Li, X. Genetic and bioprocess engineering for the selective and high-level production of geranyl acetate in Escherichia coli. ACS Sustain. Chem. Eng. 2022, 10, 2881–2889. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mirjalili, M.H.; Hadian, J. Chemical profiles of the essential oil of wild and in vitro regenerated Zataria multiflora Boiss (Lamiaceae). Bulg. Chem. Commun. 2014, 46, 362–367. [Google Scholar]
- Máthé, A.; Hassan, F.; Abdul Kader, A. In vitro micropropagation of medicinal and aromatic plants. In Medicinal and Aromatic Plants of the World; Springer: Dordrecht, The Netherlands, 2015; pp. 305–336. [Google Scholar]
- Chandra, S.; Chandra, R. Engineering secondary metabolite production in hairy roots. Phytochem. Rev. 2011, 10, 371–395. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Oksman-Caldentey, K.M. Progress and prospects of hairy root research. Hairy Roots 2018, 3–19. [Google Scholar] [CrossRef]
- Figlan, S.; Makunga, N.P. Genetic transformation of the medicinal plant Salvia runcinata L. f. using Agrobacterium rhizogenes. S. Afr. J. Bot. 2017, 112, 193–202. [Google Scholar] [CrossRef]
- Pedreño, M.A.; Almagro, L. Carrot hairy roots: Factories for secondary metabolite production. J. Exp. Bot. 2020, 71, 6861–6864. [Google Scholar] [CrossRef]
- Shi, M.; Liao, P.; Nile, S.H.; Georgiev, M.I.; Kai, G. Biotechnological exploration of transformed root culture for value-added products. Trends Biotechnol. 2021, 39, 137–149. [Google Scholar] [CrossRef]
- Yousefian, S.; Lohrasebi, T.; Farhadpour, M.; Haghbeen, K. Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tissue Organ Cult. 2020, 142, 285–297. [Google Scholar] [CrossRef]
- Wu, S.J.; Xie, X.G.; Feng, K.M.; Zhai, X.; Ming, Q.L.; Qin, L.P.; Rahman, K.; Zhang, Z.Z.; Han, T. Transcriptome sequencing and signal transduction for the enhanced tanshinone production in Salvia miltiorrhiza hairy roots induced by Trichoderma atroviride D16 polysaccharide fraction. Biosci. Biotechnol. Biochem. 2020, 86, zbac088. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.B.; Han, J.Y.; Ahn, C.H.; Choi, Y.E. Lipid transfer proteins (AaLTP3 and AaLTP4) are involved in sesquiterpene lactone secretion from glandular trichomes in Artemisia annua. Plant Cell Physiol. 2019, 60, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, J.; Qiu, S.; Qi, F.; Su, H.; Bu, Q.; Jiang, R.; Tang, K.; Zhang, L.; Chen, W. The transcription factors TLR1 and TLR2 negatively regulate trichome density and artemisinin levels in Artemisia annua. J. Integr. Plant Biol. 2022, 64, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Inthima, P.; Nakano, M.; Otani, M.; Niki, T.; Nishijima, T.; Koshioka, M.; Supaibulwatana, K. Overexpression of the gibberellin 20-oxidase gene from Torenia fournieri resulted in modified trichome formation and terpenoid metabolities of Artemisia annua L. Plant Cell Tissue Organ Cult. 2017, 129, 223–236. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Kanagarajan, S.; Lundgren, A.; Brodelius, P.E. Studies on the expression of sesquiterpene synthases using promoter-β-glucuronidase fusions in transgenic Artemisia annua L. PLoS ONE 2013, 8, e80643. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Fu, X.; Pan, Q.; Tang, Y.; Shen, Q.; Lv, Z.; Yan, T.; Shi, P.; Li, L.; Zhang, L.; et al. Overexpression of AaWRKY1 leads to an enhanced content of artemisinin in Artemisia annua. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kanagarajan, S.; Han, J.; Hao, M.; Yang, Y.; Lundgren, A.; Brodelius, P.E. Studies on the expression of linalool synthase using a promoter-β-glucuronidase fusion in transgenic Artemisia annua. J. Plant Physiol. 2014, 171, 85–96. [Google Scholar] [CrossRef]
- Shen, Q.; Chen, Y.F.; Wang, T.; Wu, S.Y.; Lu, X.; Zhang, L.; Zhang, F.Y.; Jiang, W.M.; Wang, G.F.; Tang, K.X. Overexpression of the cytochrome P450 monooxygenase (cyp71av1) and cytochrome P450 reductase (cpr) genes increased artemisinin content in Artemisia annua (Asteraceae). Genet. Mol. Res. 2012, 11, 3298–3309. [Google Scholar] [CrossRef]
- Gou, Y.; Zhang, F.; Tang, Y.; Jiang, C.; Bai, G.; Xie, H.; Liao, Z. Engineering nootkatone biosynthesis in Artemisia annua. ACS Synth. Biol. 2021, 10, 957–963. [Google Scholar] [CrossRef]
- Hsu, K.H.; Huang, W.K.; Lin, Y.L.; Chang, S.T.; Chu, F.H. A genetic marker of 4-coumarate: Coenzyme A ligase gene in the cinnamaldehyde-chemotype Cinnamomum osmophloeum. Holzforschung 2012, 66, 897–904. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, A.; Patel, M.K.; Jha, B. An efficient method for Agrobacterium–mediated genetic transformation and plant regeneration in cumin (Cuminum cyminum L.). Appl. Biochem. Biotechnol. 2013, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.M.; Pasquali, G.; Astarita, L.V.; Cassel, E. Comparison of genetically engineered (GE) and non-GE Eucalyptus trees using secondary metabolites obtained by steam distillation. J. Essent. Oil Res. 2017, 29, 22–31. [Google Scholar] [CrossRef]
- Fernando, S.C.; Goodger, J.Q.; Gutierrez, S.S.; Johnson, A.A.; Woodrow, I.E. Plant regeneration through indirect organogenesis and genetic transformation of Eucalyptus polybractea R.T. Baker. Ind. Crops Prod. 2016, 86, 73–78. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Muñoz-Bertomeu, J.; Navarro, A.; Arrillaga, I.; Segura, J. Enhanced levels of S-linalool by metabolic engineering of the terpenoid pathway in spike lavender leaves. Metabol. Engin. 2014, 23, 136–144. [Google Scholar] [CrossRef]
- Adal, A.M.; Mahmoud, S.S. Short-chain isoprenyl diphosphate synthases of lavender (Lavandula). Plant Mol. Biol. 2020, 102, 517–535. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Arrillaga, I.; Eisenreich, W. Dynamics of monoterpene formation in spike lavender plants. Metabolites 2017, 7, 65. [Google Scholar] [CrossRef]
- Zagorcheva, T.; Stefanova, K.; Mourdjeva, M.; Atanassov, I. Fusion of the transit peptide region of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) gene from Lavandula angustifolia directs GFP reporter protein into the chloroplasts. Comptes Rendus De L’académie Bulg. Des Sci. 2017, 70, 387–395. [Google Scholar]
- Tsuro, M.; Asada, S. Differential expression of limonene synthase gene affects production and composition of essential oils in leaf and floret of transgenic lavandin (Lavandula× intermedia Emeric ex Loisel.). Plant Biotechnol. Rep. 2014, 8, 193–201. [Google Scholar] [CrossRef]
- Tsuro, M.; Tomomatsu, K.; Inukai, C.; Tujii, S.; Asada, S. RNAi targeting the gene for 1,8-cineole synthase induces recomposition of leaf essential oil in lavandin (Lavandula × intermedia Emeric). In Vitro Cell. Dev. Biol.-Plant 2019, 55, 165–171. [Google Scholar] [CrossRef]
- Rahman, E.; Maroufi, A. In vitro regeneration and Agrobacterium-mediated genetic transformation of Dragon’s Head plant (Lallemantia iberica). ” Sci. Rep. 2022, 12, 1784. [Google Scholar]
- Hwang, H.S.; Adhikari, P.B.; Jo, H.J.; Han, J.Y.; Choi, Y.E. Enhanced monoterpene emission in transgenic orange mint (Mentha × piperita f. citrata) overexpressing a tobacco lipid transfer protein (NtLTP1). Planta 2020, 252, 1–12. [Google Scholar]
- Mahmoud, S.S.; Maddock, S.; Adal, A.M. Isoprenoid metabolism and engineering in glandular trichomes of Lamiaceae. Front. Plant Sci. 2021, 12, 699157. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Ahkami, A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes-current status and future opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Reddy, V.A.; Panicker, D.; Mao, H.Z.; Kumar, N.; Rajan, C.; Venkatesh, P.N.; Chua, N.H.; Sarojam, R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MsYABBY5 from spearmint (Mentha spicata). Plant Biotechnol. J. 2016, 14, 1619–1632. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.A.; Wang, Q.; Dhar, N.; Kumar, N.; Venkatesh, P.N.; Rajan, C.; Panicker, D.; Sridhar, V.; Mao, H.Z.; Sarojam, R. Spearmint R2R3-MYB transcription factor MsMYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (MsGPPS.LSU). Plant Biotechnol. J. 2017, 15, 1105–1119. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.M.; Park, D.J.; Lee, D.G.; Song, H.J.; Kang, S.M.; Min, J.Y.; Choi, M.S. Over-expression of IPP isomerase and limonene synthase enzymes in Mentha spicata and their influence on the terpenoid metabolism. Rom. Biotechnol. Lett. 2015, 20, 10358–10368. [Google Scholar]
- Dhar, N.; Sarangapani, S.; Reddy, V.A.; Kumar, N.; Panicker, D.; Jin, J.; Chua, N.H.; Sarojam, R. Characterization of a sweet basil acyltransferase involved in eugenol biosynthesis, J. Exp. Bot. 2020, 71, 3638–3652. [Google Scholar] [CrossRef]
- Prudente, D.O.D.; Paiva, R.; Carpentier, S.; Swennen, R.; Crlota Nery, F.; Cautinho Silva, L.; Panis, B. Characterization of the formation of somatic embryos from mature zygotic embryos of Passiflora ligularis Juss. Plant Cell Tissue Organ Cult. 2017, 131, 95–107. [Google Scholar]
- Blerot, B.; Baudino, S.; Prunier, C. Botany, agronomy and biotechnology of Pelargonium used for essential oil production. Phytochem Rev. 2016, 15, 935–960. [Google Scholar] [CrossRef]
- Benazir, J.F.; Suganthi, R.; Chandrika, P. In vitro regeneration and transformation studies on Pelargonium graveolens (geranium)—An important medicinal and aromatic plant. J. Med. Plants Res. 2013, 7, 2815–2822. [Google Scholar]
- Singh, P.; Khan, S.; Kumar, S. Establishment of an efficient Agrobacterium-mediated genetic transformation system in Pelargonium graveolens: An important aromatic plant. Plant Cell Tiss Organ Cult. 2017, 129, 35–44. [Google Scholar] [CrossRef]
- Paul, A.; Bakshi, S.; Sahoo, D.P.; Kalita, M.C.; Sahoo, L. Agrobacterium-mediated genetic transformation of Pogostemon cablin (Blanco) Benth. using leaf explants: Bactericidal effect of leaf extracts and counteracting strategies. Appl. Biochem. Biotechnol. 2012, 166, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zeng, Y.; Wei, T.; Zhu, M.; Fang, X.; Yuan, X.; Luo, Y.; Feng, L. Cloning and functional verification of genes related to 2-phenylethanol biosynthesis in Rosa rugosa. Genes 2018, 9, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, L.; Zang, S.; Wang, J.; Wei, T.; Xu, Y.; Feng, L. Overexpression of a Rosa rugosa Thunb. NUDX gene enhances biosynthesis of scent volatiles in petunia. PeerJ 2021, 9, e11098. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, F.; Makris, A.; Argiriou, A.; Degenhardt, J.; Kanellis, A. EST analysis and annotation of transcripts derived from a trichome-specific cDNA library from Salvia fruticosa. Plant Cell Rep. 2010, 29, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Hussain, R.M.; Rehman, N.U.; She, G.; Li, P.; Wan, X.; Guo, L.; Zhao, J. De novo transcriptome sequencing and metabolite profiling analyses reveal the complex metabolic genes involved in the terpenoid biosynthesis in Blue Anise Sage (Salvia guaranitica L.). DNA Res. 2018, 25, 597–617. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yan, H.; Li, Y.; Xiong, Y.; Niu, M.; Zhang, X.; Teixeira da Silva, J.A.; Ma, G. Molecular cloning and functional analysis of 1-deoxy-D-xylulose 5-phosphate reductoisomerase from Santalum album. Genes 2021, 12, 626. [Google Scholar] [CrossRef]
- Yan, H.; Xiong, Y.; Teixeira da Silva, J.A.; Pang, J.; Zhang, T.; Yu, X.; Zhang, X.; Niu, M.; Ma, G. Molecular cloning and functional characterization of bisabolene synthetase (SaBS) promoter from Santalum album. Forests 2020, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Nomani, M.; Tohidfar, M. Plant regeneration and transformation of Trachyspermum ammi using Agrobacterium tumefaciens and zygotic embryos. J. Gen. Engin. Biotechnol. 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Vamenani, R.; Pakdin-Parizi, A.; Mortazavi, M.; Gholami, Z. Establishment of hairy root cultures by Agrobacterium rhizogenes mediated transformation of Trachyspermum ammi L. for the efficient production of thymol. Biotechnol. Appl. Biochem. 2019, 67, 389–395. [Google Scholar] [CrossRef]
- Nomani, M.; Noori, S.A.S.; Tohidfar, M.; Ramshini, H. Overexpression of TPS2 gene to increase thymol content using Agrobacterium tumefaciens-mediated transformation in Trachyspermum ammi (Qom ecotype). Ind. Crops Prod. 2019, 130, 63–70. [Google Scholar] [CrossRef]
- Zeng, X.F.; Zhao, D.G. Expression of IPT in Asakura-sanshoo (Zanthoxylum piperitum (L.) DC. f. inerme Makino) alters tree architecture, delays leaf senescence, and changes leaf essential oil composition. Plant Mol. Biol. Rep. 2016, 34, 649–658. [Google Scholar]
- Wu, H.; Acanda, Y.; Canton, M.; Zale, J. Efficient biolistic transformation of immature citrus rootstocks using phosphomannose-isomerase selection. Plants 2019, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Citrus cell suspension culture establishment, maintenance, efficient transformation and regeneration to complete transgenic plant. Plants 2021, 10, 664. [Google Scholar] [CrossRef]
- Zeng, R.F.; Zhou, H.; Fu, L.M.; Yan, Z.; Ye, L.X.; Hu, S.F.; Gan, Z.M.; Ai, X.Y.; Hu, C.G.; Zhang, J.Z. Two citrus KNAT-like genes, CsKN1 and CsKN2, are involved in the regulation of spring shoot development in sweet orange. J. Exp. Bot. 2021, 72, 7002–7019. [Google Scholar] [CrossRef]
- Sun, L.; Ke, F.; Nie, Z.; Wang, P.; Xu, J. Citrus genetic engineering for disease resistance: Past, present and future. Int. J. Mol. Sci. 2019, 20, 5256. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Orbović, V.; Wang, N. CRISPR-LbCas12a-mediated modification of citrus. Plant Biotechnol. J. 2019, 17, 1928–1937. [Google Scholar] [CrossRef] [Green Version]
- Conti, G.; Gardella, V.; Vandecaveye, M.A.; Gomez, C.A.; Joris, G.; Hauteville, C.; Burdyn, L.; Almasia, N.I.; Nahirnak, V.; Vazquez-Rovere, C.; et al. Transgenic Citrange troyer rootstocks overexpressing antimicrobial potato Snakin-1 show reduced citrus canker disease symptoms. J. Biotechnol. 2020, 324, 99–102. [Google Scholar] [CrossRef]
- Orbović, V.; Ravanfar, S.A.; Acanda, Y.; Narvaez, J.; Merritt, B.A.; Levy, A.; Lovatt, C.J. Stress-inducible Arabidopsis thaliana RD29A promoter constitutively drives Citrus sinensis APETALA1 and LEAFY expression and precocious flowering in transgenic Citrus spp. Transgen. Res. 2021, 30, 687–699. [Google Scholar] [CrossRef]
- Segura, J.; Muñoz-Bertomeu, J.; Mendoza-Poudereux, I.; Arrillaga, I. Biotechnological approaches to increase essential oil yield and quality in aromatic plants: The Lavandula latifolia (spike lavender) example. In Past and Recommendations for the Future BT—Essential Oil Research: Trends in Biosynthesis, Analytics, Industri; Malik, S., Ed.; Springer International: Cham, Switzerland, 2019; pp. 301–325. [Google Scholar]
- Wells, R.S.; Adal, A.M.; Bauer, L.; Najafianashrafi, E.; Mahmoud, S.S. Cloning and functional characterization of a floral repressor gene from Lavandula angustifolia. Planta 2020, 251, 1–11. [Google Scholar] [CrossRef]
- Gandhi, S.G.; Mahajan, V.; Bedi, Y.S. Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta 2015, 241, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Roberts, A.C.; Carmody, M.E.; Pogson, B.J. Transcriptional control of SET domain group 8 and carotenoid isomerase during Arabidopsis development. Mol. Plant 2010, 3, 174–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, V.; Mahajan, A.; Pagoch, S.S.; Bedi, Y.; Gandhi, S. MicroRNA mediated regulation of plant secondary metabolism: An in silico analysis. J. Nat. Sci. Biol. Med. 2011, 2, 44. [Google Scholar]
- Ludwig-Müller, J.; Jahn, L.; Lippert, A.; Püschel, J.; Walter, A. Improvement of hairy root cultures and plants by changing biosynthetic pathways leading to pharmaceutical metabolites: Strategies and applications. Biotechnol. Adv. 2014, 32, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, F.; Ma, Y.; Hassani, D.; Peng, B.; Pan, Q.; Zhang, Y.; Deng, Z.; Liu, W.; Zhang, J.; et al. High-Level Patchoulol Biosynthesis in Artemisia annua L. Front. Bioeng. Biotechnol. 2021, 8, 621127. [Google Scholar] [CrossRef]
- Bindu, K.H.; Mythili, J.B.; Radhika, R.M. Genetic engineering in medicinal and aromatic plants. In Genetic Engineering of Horticultural Crops; Academic Press: Cambridge, MA, USA, 2018; pp. 249–271. [Google Scholar]
- Marco, F.; Bitrián, M.; Carrasco, P.; Rajam, M.V.; Alcázar, R.; Tiburcio, A.F. Genetic engineering strategies for abiotic stress tolerance in plants, In Plant Biology and Biotechnology; Springer: New Delhi, India, 2015; pp. 579–609. [Google Scholar]
- Shinwari, Z.K.; Jan, S.A.; Nakashima, K.; Yamaguchi-Shinozaki, K. Genetic engineering approaches to understanding drought tolerance in plants. Plant Biotechnol. Rep. 2020, 14, 151–162. [Google Scholar] [CrossRef]
- Mackelprang, R.; Lemaux, P.G. Genetic engineering and editing of plants: An analysis of new and persisting questions. Ann. Rev. Plant Biol. 2020, 71, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Rastogi, A.; Shukla, A.; Srivastava, S.; Yadav, S. Prospects of genetic engineering utilizing potential genes for regulating arsenic accumulation in plants. Chemosphere 2018, 211, 397–406. [Google Scholar] [CrossRef]
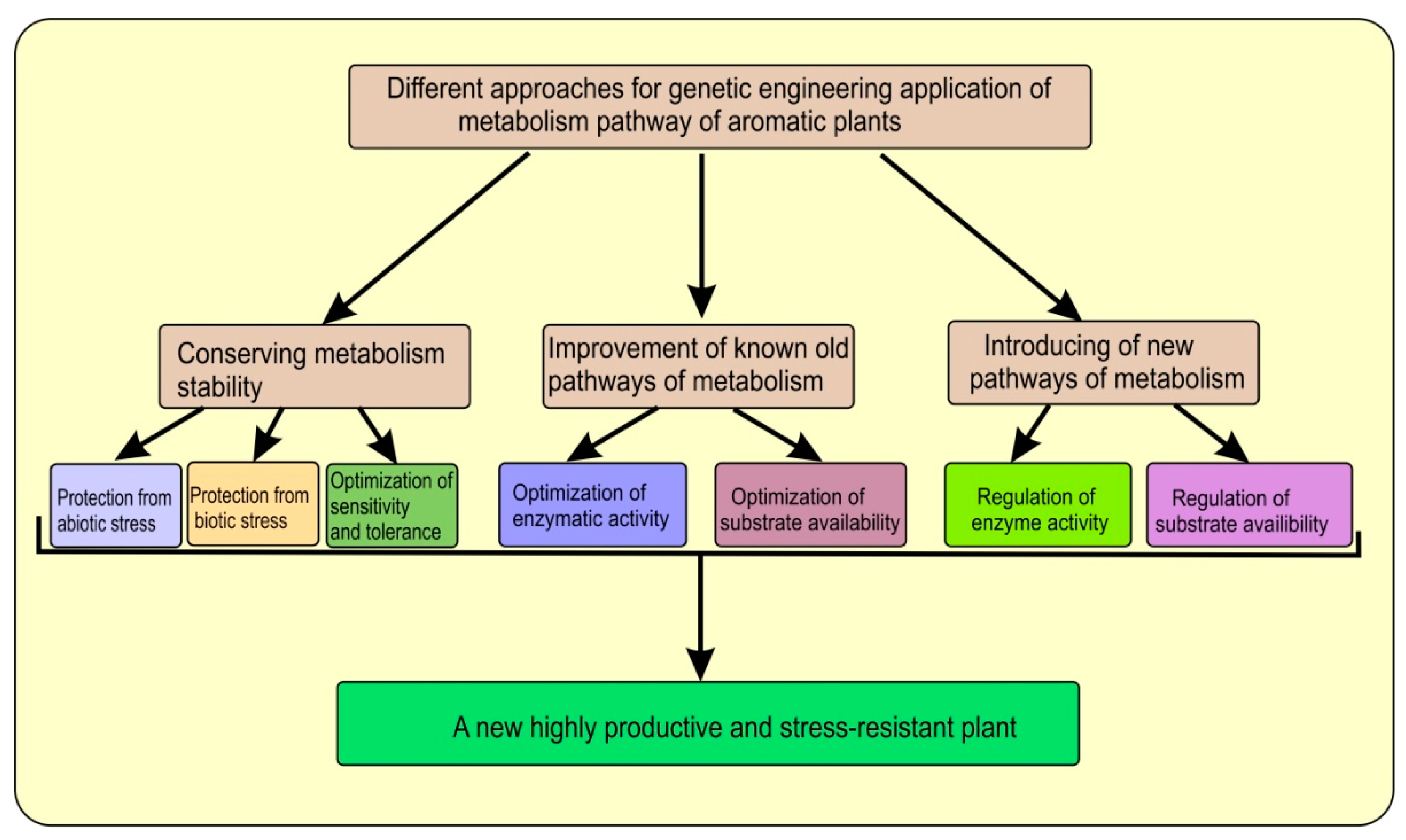
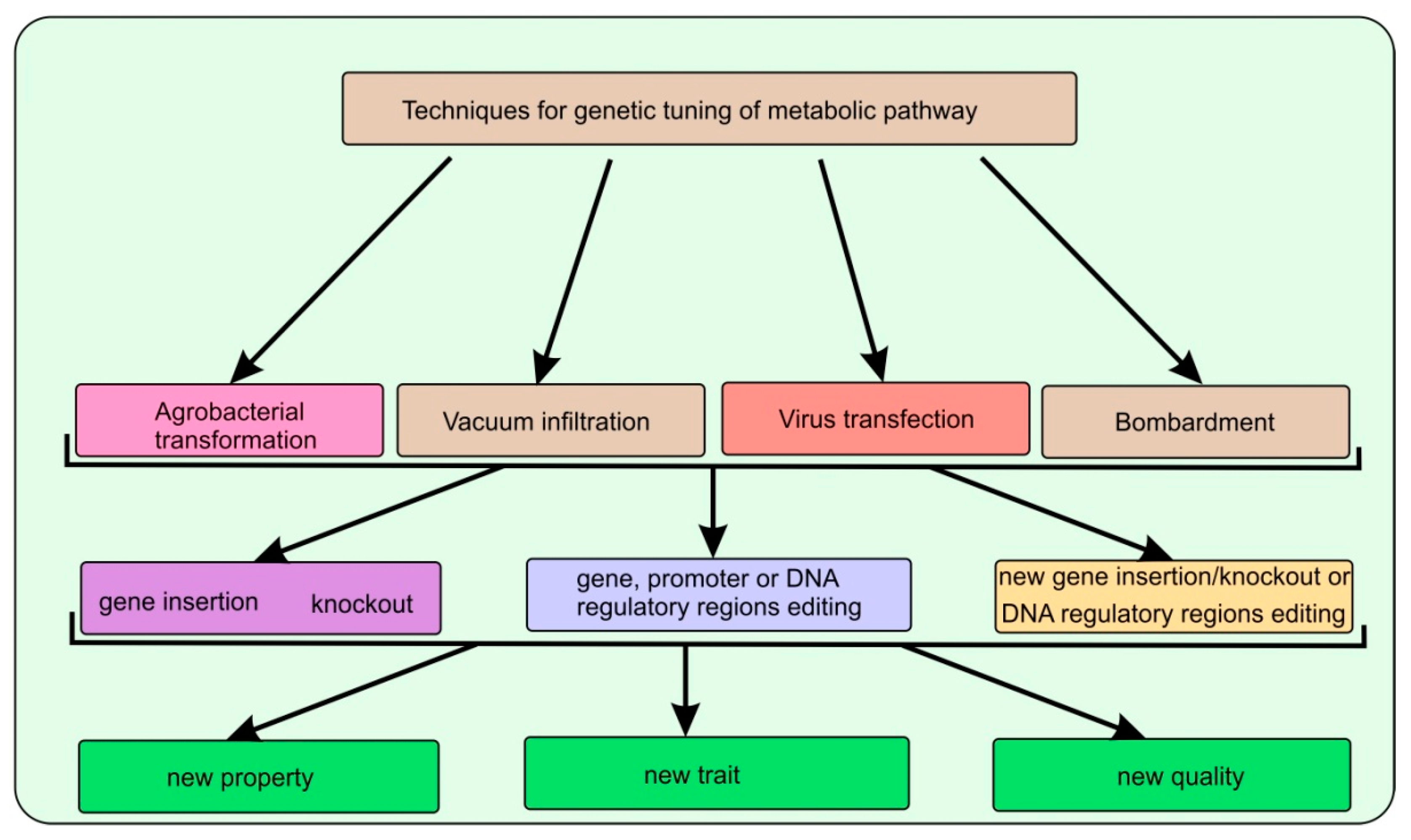
| Artemisia annua L. | AaβFS1 (an EβF synthase gene) | The CTP + AaβFS1 transgenic tobacco plants could emit EβF what enhanced repellence to green peach aphid (Myzus persicae) | [27] |
| Citrus sinensis L. Osbeck | Linalool synthase (CuSTS3-1) | Transgenic sweet orange plants showing the highest linalool content, demonstrated strong resistance to cancer in citrus (Xanthomonas citri subsp. citri) | [28] |
| Eucalyptus Camaldulensis Dehnh. | “Mangrin” gene-homolog of the allene oxide cyclase (AOC) gene | The mangrin gene is one approach to safely enhance salt tolerance in Eucalyptus camaldulensis. Salt-tolerant transgenic eucalyptus plants had somewhat less α-pinene in their essential oil and in the case of 1,8-cineole no differences were observed between transgenic and non-transgenic genotypes. | [29] |
| Lavandula spp. | AG-like and SEP3-like genes | Study of genes regulating flowering time in commercial lavender species | [30] |
| Matricaria recutita | (E)-β-farnesene synthase gene | The expression pattern of the gene encoding βFS, which is involved in chemical communication, has been studied, which provides the basis for the subsequent increase in crop resistance to aphids. | [31] |
| Mentha piperita L. | Mitogen-activated protein kinase (MAPK) | Data demonstrated the MAPK-dependent regulation mechanism of EOs biosynthesis in the salt-tolerant peppermint | [32] |
| Ocimum basilicum L. | ObDMR1 | Editing of the ObDMR1 gene was tested, the mutation of which gives resistance to the causative agent of downy mildew | [33] |
| β-glucuronidase (GUS) | The GUS expression is induced and up-regulated by increasing of water deficit stress. | [34] | |
| Pelargonium graveolens cv. Hemanti | ACC deaminase | Transgenic P. graveolens expressing ACC deaminase showed immense tolerance to salinity and drought stress. Additionally, expression of ACC deaminase enhanced the total biomass under normal conditions, important in increasing the productivity of the rose-scented geranium oil. | [35] |
| Species | Gene | Result of Transgenesis | Reference |
|---|---|---|---|
| Artemisia annua L. | trichome-specific LTP genes (AaLTP3 and AaLTP4) | Overexpression of AaLTP3 or AaLTP4 in transgenic A. annua plants resulted in enhanced production of sesquiterpene lactones (arteannuin B, artemisinin, dihydroartemisinic acid and artemisinic acid) | [77] |
| TLR1 and TLR2 | TLR1 and TLR2 negatively regulate trichome density and reduces production of sesquiterpene (artemisinin) | [78] | |
| TfGA20ox2 | enhances production of essential oil yields and sesquiterpene (artemisinin) | [79] | |
| Five sesquiterpene synthases (ADS, GAS, CPS, ECS and FS | GAS, ECS or CPS genes not improve artemisinin production; ADS and FS genes have an effect on the yield of artemisinin. | [80] | |
| AaWRKY1 (expression of ADS) | The regulation (increase) of artemisinin production | [81] | |
| Monoterpene synthase linalool synthase (LIS) | The expression of LIS not influence artemisinin production | [82] | |
| cyp71av1 and cpr genes | Overexpressing cyp71av1 and cpr is an effective means for increasing artemisinin content | [83] | |
| valencene synthase (VS) valencene oxidase (VO) | Transgenic Artemisia annua coexpressing VS and VO in the cytosol ans farnesyl diphosphate synthase (FPS), VS, and VO in plastids produced a valuable sesquiterpene noocatone | [84] | |
| Cinnamomum osmophloeum Kaneh | CoPAL, Co4CL1, Co4CL4 and CoCCR | Identification of four genes (CoPAL, Co4CL1, Co4CL4 and CoCCR) involved in the cinnamaldehyde biosynthesis pathway. | [85] |
| Cuminum cyminum L. | GUS | The first report on Agrobacterium-mediated genetic transformation in cumin. | [86] |
| Eucalyptus grandis × E. urophylla | GFP and GUS | There were no significant differences in leaf essential oil content or chemistry between transgenic (to improve wood production, wood quality and disease resistance) and non-transgenic eucalyptus trees. | [87] |
| Eucalyptus polybractea R.T. Baker | mgfp6 and hpt genes | Developed a system that can be used as an efficient protocol for the genetic transformation of E. polybractea. | [88] |
| Lavandula spp. | Linalool synthase (LIS) | Increased linalool synthesis and EO yield | [89] |
| LiGPPS, LiGGPPS, LiFPPS | The work functionally characterized cDNAs encoding the main short-chain trans-IDS genes of Lavandula x intermedia. | [90] | |
| HMGR | Overexpression of HMGR did not have significant impact upon the crosstalk between the MVA and MEP pathways for the synthesis of C5 monoterpene precursors in lavender. | [91] | |
| DXR | Characteristics of the lavender DXR gene and assessment of its effect on EO biosynthesis are presented | [92] | |
| CINS and LIMS | The composition of the EO of transgenic regenerants has been changed. | [93,94] | |
| GFP and GUS | Transformation protocol developed L. iberica | [95] | |
| Lallemantia iberica (M.Bieb.) Fisch.& C.A. Mey. | NtLTP1 | Overexpression of NtLTP1 gene in transgenic orange mint resulted in enhanced accumulation of monoterpenes in the glandular trichomes | [96] |
| Mentha citrata L.(Mentha × piperita f. citrata) | IPP, DMAPP | Data on the development of pathways for the biosynthesis of isoprenoids in glandular trichomes are presented | [97] |
| Mentha piperita L. | DXPS, IPPI, GPPS, MFS | The overexpression of DXR led to oil yield increases, the expression of MFS in transgenic peppermint plants (elite line MFS7A) resulted in desired decreases in the relative amounts of (+)-menthofuran and (+)-pulegone. | [98] |
| MsYABBY5 MsMYB | The reduced expression of MsYABBY5 led to increased levels of terpenes and that overexpression decreased terpene levels. MsMYB is a novel negative regulator of monoterpene biosynthesis. | [99,100] | |
| IPP isomerase, limonene synthase | It was found that overexpression of the IPP isomerase and limonene synthase genes can lead to the synthesis of more terpenoids in transgenic plants. | [101] | |
| Mentha spicata L. | ObCAAT1 | The BAHD ObCAAT1 acyltransferase gene has been isolated, which is involved in eugenol synthesis. | [102] |
| Ocimum basilicum L. | β-glucuronidase (GUS) | The GUS expression is induced and up-regulated by increasing of water deficit stress. | [34] |
| β-glucuronidase (GUS) | A protocol for obtaining a transgenic plant has been developed | [103] | |
| β-glucuronidase (GUS) | An effective protocol for the regeneration and transformation of P. gravolens was developed | [104,105,106] | |
| Pelargonium graveolens cv. Hemanti | GUS | The developed transformation method should provide new opportunities for the genetic improvement of patchouli according to the desired trait | [107] |
| RrAADC, RrAAAT, RrPPDC1, RrNUDX1 | The overexpression of genes responsible for the synthesis and accumulation of the main components of rose EOs has been studied | [108,109] | |
| Pogostemon cablin (Blanco) Benth | SfCinS1, SfCinS2 and SfBPPS | The analysis of gene expression in trichomes of transgenic Salvia fruticosa was carried out according to the glandular trichome library | [110] |
| Rosa rugosa Thunb. | terpene synthase (TPS) | The identification of genes encoding enzymes involved in the biosynthesis of terpenoids was carried out, a relationship was found between the levels of expression of TPS genes and end products. | [111] |
| Salvia fruticosa Mill. | SaDXR | The role of SaDXR in the biosynthesis of photosynthetic pigments has been studied. SaDXR expression has been shown to enhance the biosynthesis of sandalwood-specific sesquiterpenoids. | [112] |
| Salvia guaranitica A.St.-Hill ex Benth. | Bisabolene synthetase (SaBS) | The mechanism of transcription regulation of the SaBS gene, which is a key enzyme in the synthesis of bisabolene in the EOs of S. album, was studied. | [113] |
| Santalum album L. | Terpene synthase (TPS) | Increase in thymol content | [114,115,116] |
| IPT | The quality of the EOs has been modified by the introduction of the IPT gene. The amount of oxygenated sesquiterpenoid compounds in transgenic lines was 15–21% higher than in wild type plants. | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelepova, O.V.; Baranova, E.N.; Tkacheva, E.V.; Evdokimenkova, Y.B.; Ivanovskii, A.A.; Konovalova, L.N.; Gulevich, A.A. Aromatic Plants Metabolic Engineering: A Review. Agronomy 2022, 12, 3131. https://doi.org/10.3390/agronomy12123131
Shelepova OV, Baranova EN, Tkacheva EV, Evdokimenkova YB, Ivanovskii AA, Konovalova LN, Gulevich AA. Aromatic Plants Metabolic Engineering: A Review. Agronomy. 2022; 12(12):3131. https://doi.org/10.3390/agronomy12123131
Chicago/Turabian StyleShelepova, Olga V., Ekaterina N. Baranova, Ekaterina V. Tkacheva, Yulia B. Evdokimenkova, Aleksandr A. Ivanovskii, Ludmila N. Konovalova, and Alexander A. Gulevich. 2022. "Aromatic Plants Metabolic Engineering: A Review" Agronomy 12, no. 12: 3131. https://doi.org/10.3390/agronomy12123131
APA StyleShelepova, O. V., Baranova, E. N., Tkacheva, E. V., Evdokimenkova, Y. B., Ivanovskii, A. A., Konovalova, L. N., & Gulevich, A. A. (2022). Aromatic Plants Metabolic Engineering: A Review. Agronomy, 12(12), 3131. https://doi.org/10.3390/agronomy12123131









