Mitigating against Sclerotinia Diseases in Legume Crops: A Comprehensive Review
Abstract
:1. Introduction
2. Sclerotinia sclerotiorum Development and Infection Process
2.1. Sclerotia, Its Development and Survival
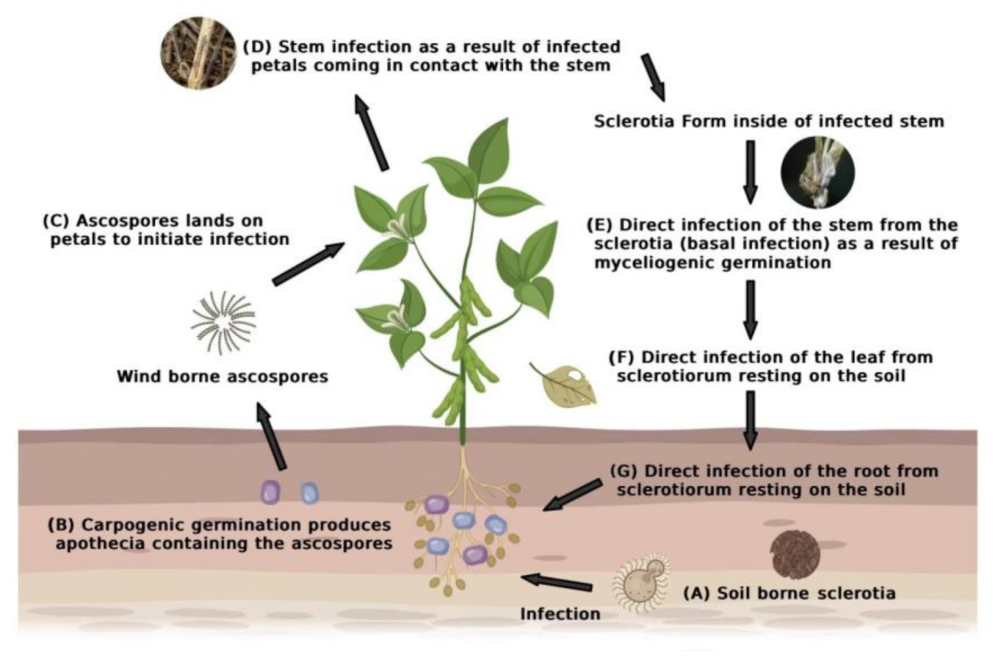
2.2. Sclerotinia sclerotiorum Infection Process
3. Sclerotinia Disease Occurrence and Yield Losses Caused in Legume Crops
3.1. Pathogens and Host Species
3.2. Yield Losses and Other Negative Effects by Sclerotinia spp.
| Crop (Species) Name | Sclerotinia spp. | Disease Name | Yield Loss (%) | Reference |
|---|---|---|---|---|
| Alfalfa (Medicago sativa L.) | Sclerotinia sclerotiorum | Blossom blight | Up to 100% | [83] |
| Sclerotinia trifoliorum Erikss | Sclerotinia crown and stem rot (SCSR) | 2–30% | [84] | |
| Chickpea (Cicer arietinum) | Sclerotinia sclerotiorum | Stem rot | up to 100% | [85,86] |
| Common bean (Phaseolus vulgaris L.) | Sclerotinia sclerotiorum (Lib.) de Bary | Stem rot/White mold | 30–100% | [73,87,88] |
| Faba bean (Vicia faba L.) | Sclerotinia trifoliorum Eriks | Stem rot | Up to 100% | [70] |
| Sclerotinia sclerotiorum | White mold | - | [89] | |
| Groundnut (Arachis hypogaea L.) | Sclerotinia minor Jagger/S. sclerotiorum/Sclerotium rolfsii Sacc | Sclerotinia blight | Over 50% yield losses | [68,90] |
| Lupin (Lupinus angustifolius L.) Lentil (Lens culinaris) | Sclerotinia sclerotiorum Sclerotinia sclerotiorum | Stem rot Sclerotinia white mold | 16 and 35% | [91] |
| Pea (Pisum sativum L.) | Sclerotinia sclerotiorum | White mold | - | [92] |
| Red clover (Trifolium pratense L.) | Sclerotinia trifoliorum | Sclerotinia crown and stem rot | Huge loss to foliage and seeds | [71] |
| Soybean (Glycine max L.) | Sclerotinia sclerotiorum (Lib.) de Bary | Sclerotinia stem rot | >60% yield losses | [72] |
| Sword bean (Canavalia gladiate L.) | Sclerotinia sclerotiorum | Sclerotinia rot | [93] |
4. Response of Legume Crops to Sclerotinia sclerotiorum Infection
4.1. Plant Symptoms
4.2. Physio-Biochemical Performance to Sclerotinia sclerotiorum Infection
5. Control Strategies for Managing Sclerotinia sclerotiorum Infection
5.1. Biological Control of Sclerotinia Diseases in Legumes
| Species | Environment | Effects | Tested Crop/Pathogens | Reference |
|---|---|---|---|---|
| Streptomyces albulus CK-15 | In vitro | Inhibits germination and formation of sclerotia and the growth of mycelia | Sclerotinia sclerotiorum | [127] |
| Streptomyces species (S. griseus, S. rochei & S. sampsonii) | In vitro & In vivo | Controls the disease by reducing the viability and germination of sclerotia | Green bean | [128] |
| Bacillus sp. FSQ1 | In vivo | Inhibits the growth and infection | Common bean | [129] |
| Trichoderma harzianum ESALQ-1306 & Trichoderma asperellum BRM-29104 | Field | Controls S. sclerotiorum | Common bean | [123] |
| Trichoderma hamatum & T. koningii | Improves grain yield by 50–100% by controlling Fusarium wilt | Chickpea | [124] | |
| Bacillus velezensis | Greenhouse | Inhibit disease growth | Lettuce | [130] |
| Arthrobacter FP15 | Diminishes disease symptoms | Lettuce | [131] | |
| Bacillus amyloliquefaciens | In vitro & Greenhouse | Impedes mycelium growth and limits lesion size | Tomatoes | [132] |
| Bacillus sp. B19 & Bacillus sp. P12 | Growth chamber | Improves crop germination potential by 15% and increases root and stem length | Common bean | [118] |
| Pseudomonas cholororaphis PA-23 | Greenhouse & In vitro | Suppresses S. sclerotiorum | Lettuce | [133] |
| Coniothyrium minitans | Growth chamber | Reduce disease incidence by 90% | Common bean | [114] |
| Pseudomonas aeruginosa; Bacillus subtilis; & Trichoderma harzianum | Greenhouse | Induced systematic resistance, and suppression of oxalic acid production | Pea | [134] |
| Bacillus amyloliquefaciencs | In vitro | Limits the effects of pathogens | Fungal pathogens | [135] |
| Trichoderma asperellum | Field | Reduction of S. sclerotiorum apothecia number and disease severity | Common bean | [120] |
| Bacillus subtilis | Growth chamber | Limit formation of apothecia by 91% and sclerotia by 30% | Soybean | [112] |
| Coniothyrium minitans | Growth chamber | Lower apothecia and sclerotia by 81% and 50% respectively | Soybean | [112] |
| Streptomyces lydicus | Growth chamber | Decrease apothecia by 100% and sclerotia by 30% | Soybean | [112] |
| Trichoderma harzianum T-22 | Field | Decrease the disease severity index (DSI) by 38.5% | Soybean | [112] |
| Pseudomonas brassicacearum DF41 | Greenhouse & In vitro | Suppresses S. sclerotiorum | Canola | [136] |
| Pseudomonas cholororaphis sp. PA-23 | Greenhouse | Suppresses S. sclerotiorum | Canola | [113] |
| Trichoderma asperellum & Clonostachys rosea | Greenhouse | Reduction in apothecium counts | Common bean | [121] |
| Mycotoxins (roridin A & roridin D) | In vitro | Inhibitors of S. sclerotiorum | Sclerotinia sclerotiorum | [137] |
| Coniothyrium minitans | Field | Suppress pod rot from 42–72% to 29–38% | Alfalfa | [115] |
5.2. Genetic Improvement of Host Resistance to S. sclerotiorum
5.3. Chemical Control of Sclerotinia Diseases in Legumes
6. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewis, G.P.; Schrire, B.; Mackinder, B.; Lock, M. (Eds.) Legumes of the World; Royal Botanic Gardens Kew: London, UK, 2005. [Google Scholar]
- Lemke, R.L.; Zhong, Z.; Campbell, C.A.; Zentner, R. Can pulse crops play a role in mitigating greenhouse gases from North American agriculture? J. Agron. 2007, 99, 1719–1725. [Google Scholar] [CrossRef]
- Peoples, M.B.; Hauggaard-Nielsen, H.; Jensen, E.S. The potential environmental benefits and risks derived from legumes in rotations. In Nitrogen Fixation in Crop Production; Emerich, D.W., Krishnan, H.B., Eds.; Agronomy Monograph, no. 52; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2009; Volume 13, pp. 349–385. [Google Scholar]
- Voisin, A.S.; Guéguen, J.; Huyghe, C.; Jeuffroy, M.H.; Magrini, M.B.; Meynard, J.M.; Mougel, C.; Pellerin, S.; Pelzer, E. Legumes for feed, food, biomaterials and bioenergy in Europe: A review. Agron. Sustain. Dev. 2014, 34, 361–380. [Google Scholar] [CrossRef]
- Kissinger, G. Pulse Crops and Sustainability: A Framework to Evaluate Multiple Benefits; FAO: Rome, Italy, 2016. [Google Scholar]
- Considine, M.J.; Siddique, K.H.M.; Foyer, C.H. Nature’ s pulse power: Legumes, food security and climate change. J. Exp. Bot. 2017, 68, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Wang, X.; Fu, S.; Zhao, J. Legume plants enhance the resistance of soil to ecosystem disturbance. Front. Plant Sci. 2017, 8, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akpapunam, M.A.; Sefa-Dedeh, S. Jack bean (Canavalia ensiformis) nutrition-related aspects and needed research. Plant Food Hum. Nutr. 1997, 10, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Mooney, H.; Drake, J. Ecology of Biological Invasions of North America and Hawaii; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bressani, R. Nutritive value of cowpea. In Cowpea Research, Production, and Utilization; Singh, S.R., Rachie, K.O., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1985. [Google Scholar]
- Suri, D.J.; Tano-Debrah, K.; Ghosh, S.A. Optimization of the nutrient content and protein quality of cereal—Legume blends for use as complementary foods in Ghana. Food Nutr. Bull. 2014, 35, 372–381. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, G. Biofortification of pulses and legumes to enhance nutrition. Heliyon 2020, 6, e03682. [Google Scholar] [CrossRef]
- Desire, M.F.; Blessing, M.; Elijah, N.; Ronald, M.; Agather, K.; Tapiwa, Z.; Florence, M.R.; George, N. Exploring food fortification potential of neglected legume and oil seed crops for improving food and nutrition security among smallholder farming communities: A systematic review. J. Agric. Sci. Food Res. 2021, 3, 100117. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; Nilsson, A.; Johansson, M.; Bjorck, I. Combining functional features of whole-grain barley and legumes for dietary reduction of cardiometabolic risk: A randomized cross-over intervention in mature women. Br. J. Nutr. 2014, 111, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didinger, C.; Foster, M.T.; Bunning, M.; Thompson, H.J. Nutrition and human health benefits of dry beans and other pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 481–504. [Google Scholar] [CrossRef]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.Y.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Abdullah, M.M.H.; Hughes, J.; Grafenauer, S. Legume intake is associated with potential savings in coronary heart disease-related health care costs in Australia. Nutrients 2022, 14, 2912. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.; Fyfe, C.L.; Wareham, N.J.; Khaw, K.T.; Johnstone, A.M.; Myint, P.K. Association between legume consumption and risk of hypertension in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Cohort. Nutrients 2022, 14, 3363. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Mundus, S.; Jensen, E.S. Nitrogen dynamics following grain legumes and subsequent catch crops and the effects on succeeding cereal crops. Nutr. Cycl. Agroecosyst. 2009, 84, 281–291. [Google Scholar] [CrossRef]
- Watson, C.A.; Reckling, M.; Preissel, S.; Bachinger, J.; Bergkvist, G.; Kuhlman, T.; Lindström, K.; Nemecek, T.; Topp, C.F.E.; Vanhatalo, A.O.; et al. Grain legume production and use in European agricultural systems. Adv. Agron. 2017, 144, 235–303. [Google Scholar] [CrossRef] [Green Version]
- Reckling, M.; Schläfke, N.; Hecker, J.M.; Bachinger, J.; Zander, P.; Bergkvist, G.; Frankow-Lindberg, B.; Båth, B.; Pristeri, A.; Monti, M.; et al. Legume Futures Report 4.2 Generation and Evaluation of Legume-Supported Crop Rotations in Five Case Study Regions Across Europe. 2014. Available online: www.legumefutures.de (accessed on 29 September 2022).
- Kebede, E. Grain legumes production in Ethiopia: A review of adoption, opportunities, constraints and emphases for future interventions. Turk. J. Agric. Food Sci. Technol. 2020, 8, 977–989. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crop. Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Luce, M.S.; Grant, C.A.; Zebarth, B.J.; Ziadi, N.; O’Donovan, J.T.; Blackshaw, R.E.; Harker, K.N.; Johnson, E.N.; Gan, Y.; Lafond, G.P.; et al. Legumes can reduce economic optimum nitrogen rates and increase yields in a wheat–canola cropping sequence in western Canada. Field Crop. Res. 2015, 179, 1225. [Google Scholar]
- Angus, J.F.; Kirkegaard, J.A.; Hunt, J.R.; Ryan, M.H.; Ohlander, L.; Peoples, M.B. Break crops and rotations for wheat. Crop Pasture Sci. 2015, 66, 523–552. [Google Scholar] [CrossRef]
- Uzoh, I.M.; Igwe, C.A.; Okebalama, C.B.; Babalola, O.O. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci. Rep. 2019, 9, 8539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larney, F.J.; Pearson, D.C.; Li, L.; Blackshaw, R.E.; Lupwayi, N.Z. Conservation management practices and rotations for irrigated dry bean production in southern Alberta. J. Agron. 2015, 107, 2281–2293. [Google Scholar] [CrossRef]
- Erental, A.; Dickman, M.B.; Yarden, O. Sclerotial development in Sclerotinia sclerotiorum: Awakening molecular analysis of a “Dormant” structure. Fungal Biol. Rev. 2008, 22, 6–16. [Google Scholar] [CrossRef]
- Li, M.; Rollins, J.A. The development-specific protein (Ssp1) from Sclerotinia sclerotiorum is encoded by a novel gene expressed exclusively in sclerotium tissues. Mycologia 2009, 101, 34–43. [Google Scholar] [CrossRef]
- Ordóñez-Valencia, C.; Ferrera-Cerrato, R.; Quintanar-Zúñiga, R.E.; Flores-Ortiz, C.M.; Guzmán, G.J.M.; Alarcón, A.; Larsen, J.; García-Barradas, O. Morphological development of sclerotia by Sclerotinia sclerotiorum: A view from light and scanning electron microscopy. Ann. Microbiol. 2015, 65, 765–770. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Liang, Y.; Xiong, W.; Steinkellner, S.; Feng, J. Deficiency of the melanin biosynthesis genes SCD1 and THR1 affects sclerotial development and vegetative growth, but not pathogenicity, in Sclerotinia sclerotiorum. Mol. Plant Pathol. 2018, 19, 1444–1453. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.; Denton-Giles, M.; Derbyshire, M.; Kamphuis, L.G. Abiotic conditions governing the myceliogenic germination of Sclerotinia sclerotiorum allowing the basal infection of Brassica napus. Australas. Plant Pathol. 2019, 48, 85–91. [Google Scholar] [CrossRef]
- Shahoveisi, F.; del Río Mendoza, L.E. Effect of wetness duration and incubation temperature on development of ascosporic infections by Sclerotinia sclerotiorum. Plant Dis. 2020, 104, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Shahoveisi, F.; Manesh, M.R.; del Río Mendoza, L.E. Modeling risk of Sclerotinia sclerotiorum-induced disease development on canola and dry bean using machine learning algorithms. Sci. Rep. 2022, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Strelkov, S.E.; Kav, N.N.V. The proteome of liquid Sclerotial exudates from Sclerotinia sclerotiorum. J. Proteome Res. 2010, 9, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Willetts, H.J.; Bullock, S. Developmental biology of sclerotia. Mycol Res. 1992, 96, 801–816. [Google Scholar] [CrossRef]
- Pandey, M.K.; Sarma, B.K.; Singh, D.P.; Singh, U.P. Biochemical investigations of sclerotial exudates of Sclerotium rolfsii and their antifungal activity. J. Phytopathol. 2007, 155, 84–89. [Google Scholar] [CrossRef]
- Rahman, M.M.E.; Suzuki, K.; Islam, M.M.; Dey, T.K.; Harada, N.; Hossain, D.M. Molecular characterization, mycelial compatibility grouping, and aggressiveness of a newly emerging phytopathogen, Sclerotinia sclerotiorum, causing white mold disease in new host crops in Bangladesh. J. Plant Pathol. 2020, 102, 775–785. [Google Scholar] [CrossRef]
- Wu, B.M.; Subbarao, K.V.; Liu, Y.-B. Comparative survival of sclerotia of Sclerotinia minor and S. sclerotiorum. Phytopathology 2008, 98, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Matheron, M.E.; Porchas, M. Impact of summer flooding on viability of Sclerotinia minor and S. sclerotiorum sclerotia in soil. Plant Health Prog. 2018, 19, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Matheron, M.E.; Porchas, M. Influence of soil temperature and moisture on eruptive germination and viability of sclerotia of Sclerotinia minor and S. sclerotiorum. Plant Dis. 2005, 89, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Cosic, J.; Jurkowic, D.; Vrandecic, K.; Kaucic, D. Survival of buried Sclerotinia sclerotiorum sclerotia in undisturbed soil. Helia 2012, 35, 73–78. [Google Scholar] [CrossRef]
- Duncan, R.W.; Dilantha Fernando, W.G.; Rashid, K.Y. Time and burial depth influencing the viability and bacterial colonization of sclerotia of Sclerotinia sclerotiorum. Soil Biol. Biochem. 2006, 38, 275–284. [Google Scholar] [CrossRef]
- Mueller, D.S.; Hartman, G.L.; Pedersen, W.L. Effect of crop rotation and tillage system on sclerotinia stem rot on soybean. Can. J. Plant Pathol. 2002, 24, 450–456. [Google Scholar] [CrossRef]
- Kurle, J.E.; Grau, C.R.; Oplinger, E.S.; Mengistu, A. Tillage, crop sequence, and cultivar effects of sclerotinia stem rot incidence and yield in soybean. Agron. J. 2001, 93, 973–982. [Google Scholar] [CrossRef]
- McLean, D.M. Some experiments concerned with the formation and inhibition of apothecia of Sclerotinia sclerotiorum (Lib.) D. By. Plant Dis. Report. 1958, 42, 409–412. [Google Scholar]
- Schwartz, H.F.; Steadman, J.R. Factors affecting sclerotium populations of, and apothecium production by, Sclerotinia sclerotiorum. Phytopathology 1978, 68, 383. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.B.; Ayers, W.A. Ecology of Sclerotinia species. Phytopathology 1979, 69, 896–899. [Google Scholar] [CrossRef]
- Kamal, M.M.; Savocchia, S.; Lindbeck, K.D.; Ash, G.J. Biology and biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary in oilseed Brassicas. Aust. Plant Pathol. 2016, 45, 1–14. [Google Scholar] [CrossRef]
- Smolińska, U.; Kowalska, B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum—A review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, C.A.; Belt, K.; Thatcher, L.F. Tackling control of a cosmopolitan phytopathogen: Sclerotinia. Front. Plant Sci. 2021, 12, 707509. [Google Scholar] [CrossRef]
- Willbur, J.F.; Fall, M.L.; Byrne, A.M.; Chapman, S.A.; McCaghey, M.M.; Mueller, B.D.; Schmidt, R.; Chilvers, M.I.; Muller, D.S.; Kabbage, M.; et al. Validating Sclerotinia sclerotiorum apothecial models to predict sclerotinia stem rot in soybean (Glycine max) fields. Plant Dis. 2018, 102, 2592–2601. [Google Scholar] [CrossRef] [Green Version]
- Grau, C.R.; Dorrance, A.E.; Bond, J.; Russin, J.S. Fungal diseases. In Soybeans: Improvement, Production, and Uses, 3rd ed.; Boerma, H.R., Specht, J.E., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2004; Volume 16, pp. 679–763. [Google Scholar]
- Willbur, J.; Mccaghey, M.; Kabbage, M.; Smith, D.L. An overview of the Sclerotinia sclerotiorum pathosystem in soybean: Impact, fungal biology, and current management strategies. Trop. Plant Pathol. 2019, 44, 3–11. [Google Scholar] [CrossRef]
- Peltier, A.J.; Bradley, C.A.; Chilvers, M.I.; Malvick, D.K.; Mueller, D.S.; Wise, K.A.; Esker, P.D. Biology, yield loss and control of Sclerotinia stem rot of soybean. J. Integr. Pest Manag. 2012, 3, B1–B7. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.F.; Rollins, J.A. Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1128–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharan, G.S.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Njambere, E.N.; Peever, T.L.; Vandemark, G.; Chen, W. Genotypic variation and population structure of Sclerotinia trifoliorum infecting chickpea in California. Plant Pathol. 2014, 63, 994–1004. [Google Scholar] [CrossRef]
- Kohn, L.M. A monographic revision of the genus Sclerotinia. Mycotaxon 1979, 9, 365–444. [Google Scholar]
- Willetts, H.J.; Wong, A.L. The biology of Sclerotinia sclerotiorum, S. trifoliorum, and S. minor with emphasis on specific nomenclature. Bot. Rev. 1980, 46, 101–165. [Google Scholar] [CrossRef]
- Dong, D.; Sun, S.L.; Du, C.Z.; Xiang, C.; Long, J.C.; Chen, W.D.; Zhu, Z.D. Three Sclerotinia species as the cause of white mold on pea in Chongqing and Sichuan of China. J. Integr. Agric. 2021, 20, 2957–2965. [Google Scholar] [CrossRef]
- Melzer, M.S.; Smith, E.A.; Boland, G.J. Index of plant hosts of Sclerotinia minor. Can. J. Plant Pathol. 1997, 19, 272–280. [Google Scholar] [CrossRef]
- Huang, H.C.; Kokko, E.G.; Erickson, R.S. Infection of Alfalfa Pollen by Sclerotinia sclerotiorum. Phytoparasitica 1997, 25, 17–24. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Tzavella-Klonari, K.; Roupakias, D.G. The causal fungus of stem rot disease of faba beans in Greece. J. Phytopathol. 2003, 151, 631–635. [Google Scholar] [CrossRef]
- Vilčinskas, E.; Dabkevičienė, G. Qualitative and quantitative characteristics of clover (Trifolium spp.) species in the first year of growing. Zemdirb. Agric. 2009, 96, 170–180. (In Lithuanian) [Google Scholar]
- Cunha, W.G.; Tinoco, M.L.P.; Pancoti, H.L.; Ribeiro, R.E.; Aragão, F.J.L. High resistance to Sclerotinia sclerotiorum in transgenic soybean plants transformed to express an oxalate decarboxylase gene. Plant Pathol. 2010, 59, 654–660. [Google Scholar] [CrossRef]
- Abán, C.L.; Taboada, G.M.; Casalderrey, N.B.; Maggio, M.E.; Chocobar, M.O.; Spedaletti, Y.A.; Gonzalez, M.A.A.; Vizgarra, O.V.; Galván, M.Z. Screening common bean germplasm for resistance to genetically diverse Sclerotinia sclerotiorum isolates from Argentina. Acta Sci. Agron. 2020, 42, e42786. [Google Scholar] [CrossRef] [Green Version]
- Antwi-Boasiako, A.; Zheng, L.; Begum, N.; Amoah, S.; Zhao, T. Progress towards germplasm evaluation and genetic improvement for resistance to Sclerotinia white mold in soybean. Euphytica 2021, 217, 178. [Google Scholar] [CrossRef]
- Yorgancilar, M.; Bilgiçli, N. Chemical and nutritional changes in bitter and sweet lupin seeds (Lupinus albus L.) during bulgur production. J. Food Sci. Technol. 2014, 51, 1384–1389. [Google Scholar] [CrossRef] [Green Version]
- Derbyshire, M.C.; Denton-Giles, M. The control of sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, K.A.; Sisson, A.J.; Kandel, Y.R.; Ortiz, V.; Chilvers, M.I.; Smith, D.L.; Mueller, D.S. Effects of Mowing, Seeding Rate, and Foliar Fungicide on Soybean Sclerotinia Stem Rot and Yield. Plant Health Prog. 2021, 22, 129–135. [Google Scholar] [CrossRef]
- Danielson, G.A.; Nelson, B.D.; Helms, T.C. Effect of Sclerotinia Stem Rot on Yield of Soybean Inoculated at Different Growth Stages. Plant Dis. 2004, 88, 297–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perveen, K.; Haseeb, A.; Shukla, P.K. Effect of Sclerotinia sclerotiorum on the disease development, growth, oil yield and biochemical changes in plants of Mentha arvensis. Saudi J. Biol. Sci. 2010, 17, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlin, K.D.; Bennett, R.S.; Damicone, J.P. Registration of ‘VENUS’ peanut. J. Plant Regist. 2017, 11, 33–37. [Google Scholar] [CrossRef]
- Chamberlin, K.D.; Bennett, R.S.; Damicone, J.P. Registration of ‘Lariat’ Peanut. J. Plant Regist. 2018, 12, 36–42. [Google Scholar] [CrossRef]
- Young, C.S.; Smith, J.A.; Watling, M.; Clarkson, J.P.; Whipps, J.M. Environmental Conditions Influencing Apothecial Production and Lettuce Infection by Sclerotinia sclerotiorum in field conditions. In Proceedings of the 6th international Sclerotinia Workshop, York, UK, 8–12 July 2001; pp. 181–182. [Google Scholar]
- Gossen, B.D. Blossom blight, a new constraint to alfalfa seed production in Western Canada. In Proceedings of the 12th Eucarpia Meeting of the Group Medicago, Brno, Czech Republic, 2–5 July 1996; Academia, Publishing House of the Czechoslovak Academy of Sciences: Prague, Czech Republic, 1997; pp. 111–113. [Google Scholar]
- Welty, R.E.; Busbice, T.H. Field Tolerance in Alfalfa to Sclerotinia Crown and Stem Rot. Crop Sci. 1978, 13, 508–509. [Google Scholar] [CrossRef]
- Pulse Australia. APB Chickpea IDM Strategies. 2020. Available online: https://www.pulseaus.com.au/growing-pulses/bmp/chickpea/idm-strategies (accessed on 20 July 2022).
- Mwape, V.W.; Khoo, K.H.; Chen, K.; Khentry, Y.; Newman, T.E.; Derbyshire, M.C.; Mather, D.E.; Kamphuis, L.G. Identification of Sclerotinia stem rot resistance quantitative trait loci in a chickpea (Cicer arietinum) recombinant inbred line population. Funct. Plant Biol. 2022, 49, 634–646. [Google Scholar] [CrossRef]
- Singh, S.P.; Schwartz, H.F. Breeding common bean for resistance to diseases: A review. Crop Sci. 2010, 50, 2199–2223. [Google Scholar] [CrossRef]
- Vasconcellos, R.C.; Oraguzie, O.B.; Soler, A.; Arkwazee, H.; Myers, J.R.; Ferreira, J.J.; Song, Q.; McClean, P.; Miklas, P.N. Meta-QTL for resistance to white mold in common bean. PLoS ONE 2017, 12, e0171685. [Google Scholar] [CrossRef] [Green Version]
- Chapara, V.; Chittem, K.; Mendoza, L.D.R. First report of white mold caused by Sclerotinia sclerotiorum on faba beans in North Dakota. Plant Dis. 2018, 102, 1669. [Google Scholar] [CrossRef]
- Porter, D.M.; Melouk, H.A. Sclerotinia blight. In Compendium of Peanut Diseases; Kokkalis-Burelle, N., Porter, D.M., Rodriguez-Kabana, R., Smith, D.H., Subrahmanyam, P., Eds.; APS Press: St. Paul, MI, USA, 1997; pp. 34–36. [Google Scholar]
- Simpfendorfer, S.; Heenan, D.P.; Kirkegaard, J.A.; Lindbeck, K.D.; Murray, G.M. Impact of tillage on lupin growth and the incidence of pathogenic fungi in southern New South Wales. Aust. J. Exp. Agric. 2004, 44, 53. [Google Scholar] [CrossRef]
- Islam, M.; Prova, A.; Akanda, A.M.; Hossain, M. First report of white mould caused by Sclerotinia sclerotiorum on pea in Bangladesh. J. Plant Pathol. 2020, 102, 941. [Google Scholar] [CrossRef] [Green Version]
- Han, I.; Park, K.; Lee, H.; Lee, S.M.; Shin, J.; Choi, S.L. First report of Sclerotinia rot in sword bean caused by Sclerotinia sclerotiorum in South Korea. Plant Dis. 2020, 104, 988. [Google Scholar] [CrossRef]
- McLaren, D.L.; Conner, R.L.; Platford, R.G.; Lamb, J.L.; Lamey, H.A.; Kutcher, H.R. Predicting diseases caused by Sclerotinia sclerotiorum on canola and bean—A western Canadian perspective. Can. J. Plant Pathol. 2004, 26, 489–497. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Uawisetwathana, U.; Graham, S.F.; Kamolsukyunyong, W.; Sukhaket, W.; Klanchui, A.; Toojinda, T.; Vanavichit, A.; Karoonuthaisiri, N.; Elliott, C.T. Quantitative 1H NMR metabolome profiling of Thai Jasmine rice (Oryza sativa) reveals primary metabolic response during brown planthopper infestation. Metabolomics 2015, 11, 1640–1655. [Google Scholar] [CrossRef]
- Malenčić, D.; Kiprovski, B.; Popović, M.; Prvulović, D.; Miladinović, J.; Djordjević, V. Changes in antioxidant systems in soybean as affected by Sclerotinia sclerotiorum (Lib.) de Bary. Plant Physiol. Biochem. 2010, 48, 903–908. [Google Scholar] [CrossRef]
- Nováková, M.; Šašek, V.; Dobrev, P.I.; Valentová, O.; Burketová, L. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum–Reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiol. Biochem. 2014, 80, 308–317. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Gupta, Y.P. Anti-nutritional and toxic factors in food legumes: A review. Plant Foods Hum. Nutr. 1987, 37, 201–228. [Google Scholar] [CrossRef]
- Böttger, A.; Vothknecht, U.; Bolle, C.; Wolf, A. Plant secondary metabolites and their general function in plants. In Lessons on Caffeine, Cannabis & Co: Plant Derived Drugs and Their Interaction with Human Receptors; Springer Nature: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Liang, X.; Liberti, D.; Li, M.; Kim, Y.T.; Hutchens, A.; Wilson, R.; Rollins, J.A. Oxaloacetate acetylhydrolase gene mutants of S clerotinia sclerotiorum do not accumulate oxalic acid, but do produce limited lesions on host plants. Mol. Plant Pathol. 2015, 16, 559–571. [Google Scholar] [CrossRef]
- Leite, M.E.; Santos, J.B.; Ribeiro, P.M.; Souza, D.A.; Lara, M.L.V.; Resende, M.L.V. Biochemical responses associated with common bean defence against Sclerotinia sclerotiorum. Eur. Plant Pathol. 2014, 138, 391–404. [Google Scholar] [CrossRef]
- Abdalla, S.A.; Algam, S.A.A.; Ibrahim, E.A.; El Naim, A.M. In vitro screening of Bacillus isolates for biological control of early blight disease of tomato in Shambat soil. World J. Agric. Res. 2014, 2, 47–50. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Umesha, S.; Singh, P.K.; Singh, R.P. Microbial biotechnology and sustainable agriculture. In Biotechnology for Sustainable Agriculture; Woodhead Publishing: London, UK, 2018; pp. 185–205. [Google Scholar]
- Gracia-Garza, J.A.; Reeleder, R.D.; Paulitz, T.C. Degradation of sclerotia Sclerotinia sclerotiorum by fungus gnats (Bradysia coprophila) and the biocontrol fungi Trichoderma spp. Soil Biol. Biochem. 1997, 29, 123–129. [Google Scholar] [CrossRef]
- Figueirêdo, G.S.D.; Figueirêdo, L.C.D.; Cavalcanti, F.C.N.; Santos, A.C.D.; Costa, A.F.D.; Oliveira, N.T.D. Biological and chemical control of Sclerotinia sclerotiorum using Trichoderma spp. and Ulocladium atrum and pathogenicity to bean plants. Braz. Arch. Biol. Technol. 2010, 53, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Wang, D.; Kirk, W.; Hao, J. Use of Coniothyrium minitans and other microorganisms for reducing Sclerotinia sclerotiorum. Biol. Control 2012, 60, 225–232. [Google Scholar] [CrossRef]
- Selin, C.; Habibian, R.; Poritsanos, N.; Athukorala, S.N.; Fernando, D.; de Kievit, T.R. Phenazines are not essential for Pseudomonas chlororaphis PA23 biocontrol of Sclerotinia sclerotiorum, but do play a role in biofilm formation. FEMS Microbiol. Ecol. 2010, 71, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Elsheshtawi, M.; Elkhaky, M.T.; Sayed, S.R.; Bahkali, A.H.; Mohammed, A.A.; Gambhir, D.; Mansour, A.S.; Elgorban, A.M. Integrated control of white rot disease on beans caused by Sclerotinia sclerotiorum using Contans® and reduced fungicides application. Saudi J. Biol. Sci. 2017, 24, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Q.; Huang, H.C.; Acharya, S.N.; Ericson, R.S. Effectiveness of Coniothyrium minitans and Trichoderma atroviride in suppression of sclerotinia blossom blight of alfalfa. Plant Pathol. 2005, 54, 204–211. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Mihasan, M. Application of plant growth-promoting Rhizobacteria to runner bean increases seed Carbohydrate and protein yield. J. Exp. Mol. Biol. 2013, 14, 29–39. [Google Scholar]
- Pandey, C.; Bajpai, V.K.; Negi, Y.K.; Rather, I.A.; Maheshwari, D.K. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi J. Biol. Sci. 2018, 25, 1066–1071. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Brandan, C.P.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary on common bean by native lipopeptide-producer Bacillus strains. Microbiol. Res. 2018, 211, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Geraldine, A.M.; Lopes, F.A.C.; Carvalho, D.D.C.; Barbosa, E.T.; Rodrigues, A.F.; Brandao, R.S.; Ulhoa, C.J.; Junior, M.L. Cell wall-degrading enzymes and parasitism of sclerotia are key factors on field biocontrol of white mold by Trichoderma spp. Biol. Control 2013, 67, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Morandi, M.A.; Costa, L.B. Biological control of Sclerotinia sclerotiorum on beans in field by Trichoderma asperellum and Clonostachys rosea. Biol. Control. Fungal Bact. Plant Pathog. 2009, 43, 243–246. [Google Scholar]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Mangaiello, G.; Lorito, M. Trichoderma-based products and their wdespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- De Azevedo, S.F.; de Oliveira, V.V.; Carrenho, R.; Rodrigues, V.B.; Junior, M.L.; da Silva, G.F.; Soares, M.A. Influence of the biocontrol agents Trichoderma spp. on the structure and functionality of the edaphic microbial community in common bean cultivars (Phaseolus vulgaris L.) inoculated with Sclerotinia sclerotiorum (Lib.) de Bary. Appl. Soil Ecol. 2021, 168, 104190. [Google Scholar] [CrossRef]
- Poveda, J. Biological control of Fusarium oxysporum f. sp. ciceri and Ascochyta rabiei infecting protected geographical indication Fuentesaúco-Chickpea by Trichoderma species. Eur. J. Plant Pathol. 2021, 160, 825–840. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, M.; Rani, A. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar] [CrossRef]
- Cibanal, I.L.; Fernández, L.A.; Rodriguez, S.A.; Pellegrini, C.N.; Gallez, L.M. Propolis extract combined with oregano essential oil applied to lima bean seeds against Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2022, 164, 33–43. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Lv, Z.; Shi, L.; Zhang, K.; Ge, B. Evaluation of the inhibitory effects of wuyiencin, a secondary metabolite of streptomyces albulus CK-15, against Sclerotinia sclerotiorum In vitro. Plant Dis. 2022, 106, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Gebily, D.A.S.; Ghanem, G.A.M.; Ragab, M.M.; Ali, A.M.; Soliman, N.E.K.; El-Moity, T.H.A. Characterization and potential antifungal activities of three Streptomyces spp. as biocontrol agents against Sclerotinia sclerotiorum (Lib.) de Bary infecting green bean. Egypt. J. Biol. Pest Control 2021, 31, 33. [Google Scholar] [CrossRef]
- Villarreal-Delgado, M.F.; Parra-Cota, F.I.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; De los Santos-Villalobos, S. Bacillus sp. FSQ1: A promising biological control agent against Sclerotinia sclerotiorum, the causal agent of white mold in common bean (Phaseolus vulgaris L.). Biol. Bull. 2021, 48, 729–739. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, S.E.; Lee, W.J.; Fumei, Z.; Cho, M.S.; Moon, J.S.; Oh, H.; Park, H.; Kim, S.U. Isolation and characterization of a high iturin yielding Bacillus velezensis UV mutant with improved antifungal activity. PLoS ONE 2020, 15, e0234177. [Google Scholar] [CrossRef]
- Aggeli, F.; Ziogas, I.; Gkizi, D.; Fragkogeorgi, G.A.; Tjamos, S.E. Novel biocontrol agents against Rhizoctonia solani and Sclerotinia sclerotiorum in lettuce. BioControl 2020, 65, 763–773. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the induction of systemic resistance and regulation of antioxidant pathways in tomato using fengycin produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Nandi, M.; Selin, C.; Brawerman, G.; Fernando, W.D.; de Kievit, T. Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol. Control 2017, 108, 47–54. [Google Scholar] [CrossRef]
- Jain, A.; Singh, A.; Singh, S.; Singh, V.; Singh, H.B. Comparative proteomic analysis in pea treated with microbial consortia of beneficial microbes reveals changes in the protein network to enhance resistance against Sclerotinia sclerotiorum. J. Plant Physiol. 2015, 182, 79–94. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014, 5, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, C.; Fernando, W.D.; Loewen, P.C.; De Kievit, T.R. Lipopeptides are essential for Pseudomonas sp. DF41 biocontrol of Sclerotinia sclerotiorum. Biol. Control 2010, 55, 211–218. [Google Scholar] [CrossRef]
- Xie, L.W.; Jiang, S.M.; Zhu, H.H.; Sun, W.; Ouyang, Y.C.; Dai, S.K.; Li, X. Potential inhibitors against Sclerotinia sclerotiorum, produced by the fungus Myrothecium sp. associated with the marine sponge Axinella sp. Eur. J. Plant Pathol. 2008, 122, 571–578. [Google Scholar] [CrossRef]
- Botha, C.; McLaren, N.W.; Swart, W.J. Evaluation of greenhouse inoculation techniques used to screen for Sclerotinia stem rot resistance in soybeans. S. Afr. J. Plant Soil. 2009, 26, 48–50. [Google Scholar] [CrossRef]
- Kandel, R.; Chen, C.Y.; Grau, C.R.; Dorrance, A.E.; Liu, J.Q.; Wang, Y.; Wang, D. Soybean resistance to white mold: Evaluation of soybean germplasm under different conditions and validation of QTL. Front. Plant Sci. 2018, 9, 505. [Google Scholar] [CrossRef]
- Chauhan, S.; Katoch, S.; Sharma, S.K.; Sharma, P.N.; Rana, J.C.; Singh, K.; Singh, M. Screening and identification of resistant sources against Sclerotinia sclerotiorum causing white mold disease in common bean. Crop Sci. 2020, 60, 1986–1996. [Google Scholar] [CrossRef]
- Bakhshi, S.; Zadeh, H.R.Z.; Atghia, O. Molecular identification of bean genotypes for partial resistance to Sclerotinia sclerotiorum based on SCAR markers. J. Plant Pathol. 2020, 102, 369–375. [Google Scholar] [CrossRef]
- Robison, F.M.; Turner, M.F.; Jahn, C.E.; Schwartz, H.F.; Prenni, J.E.; Brick, M.A.; Heuberger, A.L. Common bean varieties demonstrate differential physiological and metabolic responses to the pathogenic fungus Sclerotinia sclerotiorum. Plant Cell Environ. 2018, 41, 2141–2154. [Google Scholar] [CrossRef]
- McCaghey, M.; Willbur, J.; Ranjan, A.; Grau, C.R.; Chapman, S.; Diers, B.; Groves, C.; Kabbage, M.; Smith, D.L. Development and evaluation of Glycine max germplasm lines with quantitative resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2017, 8, 1495. [Google Scholar] [CrossRef] [Green Version]
- Porto, A.; Cardon, C.H.; Vasconcellos, R.C.; Novaes, E.; Leite, M.E.; Chalfun-Junior, A.; Pereira, W.A.; Santos, J.B. Expression of candidate genes related to white mold resistance in common beans. Trop. Plant Pathol. 2019, 44, 483–493. [Google Scholar] [CrossRef]
- Campa, A.; García-Fernández, C.; Ferreira, J.J. Genome-wide association study (GWAS) for resistance to Sclerotinia sclerotiorum in common bean. Genes 2020, 11, 1496. [Google Scholar] [CrossRef]
- Liang, Y.; Cason, J.M.; Baring, M.R.; Septiningsih, E.M. Identification of QTLs associated with Sclerotinia blight resistance in peanut (Arachis hypogaea L.). Genet. Resour. Crop Evol. 2021, 68, 629–637. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Y.; Li, Y.; Liu, D.; Sun, M.; Zhao, Y.; Lv, C.; Li, D.; Yang, Z.; Huang, L.; et al. Loci and candidate gene identification for resistance to Sclerotinia sclerotiorum in soybean (Glycine max L. Merr.) via association and linkage maps. Plant J. 2015, 82, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Boudhrioua, C.; Bastien, M.; Torkamaneh, D.; Belzile, F. Genome-wide association mapping of Sclerotinia sclerotiorum resistance in soybean using whole-genome resequencing data. BMC Plant Biol. 2020, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Halimi, E.S.; Rowe, D.E.; Pratt, R.G. Responses of alfalfa to stem-tip inoculations with five isolates of Sclerotinia trifoliorum. Crop Sci. 1998, 38, 1179–1182. [Google Scholar] [CrossRef]
- Kanbe, M.; Mizukami, Y.; Fujimoto, F. Improvement of resistance to Sclerotinia crown and stem rot of alfalfa through phenotypic recurrent selection. Jpn. Agric. Res. Q. 2020, 36, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lithourgidis, A.S.; Roupakias, D.G.; Damalas, C.A. Inheritance of resistance to Sclerotinia stem rot (Sclerotinia trifoliorum) in faba beans (Vicia faba L.). Field Crop. Res. 2005, 91, 125–130. [Google Scholar] [CrossRef]
- Mikaliuniene, J.; Lemeziene, N.; Danyte, V.; Suproniene, S. Evaluation of red clover (Trifolium pratense L.) resistance to Sclerotinia crown and root rot (Sclerotinia trifoliorum) in the laboratory and field conditions. Zemdirb. Agric. 2015, 102, 167–176. [Google Scholar] [CrossRef] [Green Version]
- McCaghey, M.; Willbur, J.; Smith, D.L.; Kabbage, M. The complexity of the Sclerotinia sclerotiorum pathosystem in soybean: Virulence factors, resistance mechanisms, and their exploitation to control Sclerotinia stem rot. Trop. Plant Pathol. 2019, 44, 12–22. [Google Scholar] [CrossRef]
- Bennett, R.S.; Chamberlin, K.D.; Damicone, J.P. Sclerotinia blight resistance in the US peanut mini-core collection. Crop Sci. 2010, 58, 1306–1317. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Li, H.; Niu, L.; Xing, G.; Zhang, Y.; Xu, W.; Zhao, Q.; Li, Q.; Dong, Y. Overexpression of the chitinase gene CmCH1 from Coniothyrium minitans renders enhanced resistance to Sclerotinia sclerotiorum in soybean. Transgenic Res. 2020, 29, 187–198. [Google Scholar] [CrossRef]
- Zou, J.; Li, W.; Zhang, Y.; Song, W.; Jiang, H.; Zhao, J.; Zhan, Y.; Teng, W.; Qiu, L.; Zhao, X.; et al. Identification of glutathione transferase gene associated with partial resistance to Sclerotinia stem rot of soybean using genome-wide association and linkage mapping. Theor. Appl. Genet. 2021, 134, 2699–2709. [Google Scholar] [CrossRef]
- Wu, J.; Yin, S.; Lin, L.; Liu, D.; Ren, S.; Zhang, W.; Meng, W.; Chen, P.; Sun, Q.; Fang, Y.; et al. Host-induced gene silencing of multiple pathogenic factors of Sclerotinia sclerotiorum confers resistance to Sclerotinia rot in Brassica napus. Crop J. 2021, 10, 661–671. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, A.; Mamidi, S.; Miklas, P.N.; Lee, R.; McClean, P. Linked Candidate Genes of Different Functions for White Mold Resistance in Common Bean (Phaseolus vulgaris L) are Identified by QTL-Based Pooled Sequencing. 2019. Preprint. Available online https://www.researchgate.net/publication/340203599_Linked_candidate_genes_of_different_functions_for_white_mold_resistance_in_common_bean_Phaseolus_vulgaris_L_are_identified_by_QTL-based_pooled_sequencing (accessed on 1 June 2022).
- Huzar-Novakowiski, J.; Paul, P.A.; Dorrance, A.E. Host resistance and chemical control for management of Sclerotinia stem rot of soybean in Ohio. Phytopathology 2017, 107, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Khangura, R.; Beard, C. Managing Sclerotinia Stem Rot in Canola. Department of Agriculture and Food. Australian Government. 2015. Available online: https://www.agric.wa.gov.au/canola/managing-sclerotinia-stem-rot-canola (accessed on 8 February 2022).
- Ma, H.X.; Chen, Y.; Wang, J.X.; Yu, W.Y.; Tang, Z.H.; Chen, C.; Zhou, M.G. Activity of carbendazim, dimethachlon, iprodione, procymidone and boscalid against Sclerotinia stem rot in Jiangsu Province of China. Phytoparasitica 2009, 37, 421–429. [Google Scholar] [CrossRef]
- Benigni, M.; Bompeix, G. Chemical and biological control of Sclerotinia sclerotiorum in witloof chicory culture. Pest Manag. Sci. 2010, 66, 1332–1336. [Google Scholar] [CrossRef]
- Attanayake, R.N.; Jiang, D.H.; del Rio Mendoza, L.; Chen, W. Genetic diversity and population differentiation of Sclerotinia sclerotiorum collected from canola in China and in USA. Phytopathology 2011, 101, S10. [Google Scholar]
- Kuang, J.; Hou, Y.P.; Wang, J.X.; Zhou, M.G. Sensitivity of Sclerotinia sclerotiorum to fludioxonil: In vitro determination of baseline sensitivity and resistance risk. Crop Prot. 2011, 30, 876–882. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hou, Y.P.; Wang, J.X.; Yang, G.F.; Liang, X.Y.; Zhou, M.G. Activity of a novel strobilurin fungicide benzothiostrobin against Sclerotinia sclerotiorum. Pestic. Biochem. Phys. 2014, 115, 32–38. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, X.Y.l.; Di, Y.L.; Liang, H.J.; Zhu, F.X. Baseline sensitivity and control efficacy of DMI fungicide epoxiconazole against Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2015, 141, 237–246. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Activity of boscalis fenhexamid, fluazunam fludioxonil, and vinclozolin on growth od Sclerotinia minor and S. sclerotirum od Sclerotinia stem rot of canola. Plant Dis. 2004, 88, 665–668. [Google Scholar] [CrossRef] [Green Version]
- Bradley, C.A.; Lamey, H.A.; Endres, G.J.; Henson, R.A.; Hanson, B.K.; McKay, K.R.; Halvorson, M.; LeGare, D.G.; Portter, P.M. Efficacy of fungicides for control of Sclerotinia stem rot of canola. Plant Dis. 2006, 90, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Liang, H.J.; Di, Y.L.; You, H.; Zhu, F.X. Stimulatory effects of sublethal doses of dimethachlon on Sclerotinia sclerotiorum. Plant Dis. 2014, 98, 1364–1370. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.P.; Mao, X.W.; Wu, L.Y.; Wang, J.X.; Mi, B.; Zhou, M.G. Impact of fluazinam on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Phys. 2019, 155, 81–89. [Google Scholar] [CrossRef]
- Albert, D.; Dumonceaux, T.; Carisse, O.; Beaulieu, C.; Filion, M. Combining Desirable Traits for a Good Biocontrol Strategy against Sclerotinia sclerotiorum. Microorganisms 2022, 10, 1189. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Muhayimana, S.; Xiong, H.; Liu, X.; Huang, Q. Antifungal effects of 3-(2-pyridyl)methyl-2-(4-chlorphenyl) iminothiazolidine against Sclerotinia sclerotiorum. Pest Manag. Sci. 2020, 76, 2978–2985. [Google Scholar] [CrossRef]
- Ryley, M.J.; Kyei, N.A.; Tatnell, J.R. Evaluation of fungicides for the management of sclerotinia blight of peanut. Aust. J. Agric. Res. 2000, 51, 917–924. [Google Scholar] [CrossRef]
- Smith, D.L.; Garrison, M.C.; Hollowell, J.E.; Isleib, T.G.; Shew, B.B. Evaluation of application timing and efficacy of the fungicides fluazinam and boscalid for control of Sclerotinia blight of peanut. Crop Prot. 2008, 27, 823–833. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liu, H.P.; Li, X.F.; Han, J.C.; Liu, H.Q. Biological, physiological and biochemical characteristics of procymidone-resistant Sclerotinia sclerotiorum. Chin. Agric. Sci. Bull. 2010, 26, 277–281. [Google Scholar]
- Lehner, M.S.; Paula Júnior, T.J.; Silva, R.A.; Vieira, R.F.; Carneiro, J.E.S.; Schnabel, G.; Mizubuti, E.S.G. Fungicide sensitivity of Sclerotinia sclerotiorum: A thorough assessment using discriminatory dose, EC50, high-resolution melting analysis, and description of new point mutation associated with thiophanate-methyl resistance. Plant Dis. 2015, 99, 1537–1543. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.L.; Chapman, S.; Conley, S.P. Evaluation of “curative” fungicide treatments for control of Sclerotinia stem rot of soybean in Wisconsin, 2014. Plant Dis. Manag. Rep. 2015, 9, FC028. [Google Scholar]
- Mueller, B.; Smith, D.L.; Willbur, J.; Chapman, S. Evaluation of foliar fungicides for control of Sclerotinia stem rot of soybean in Arlington Wisconsin, 2015. Plant Dis. Manag. Rep. 2016, 10, FC050. [Google Scholar]
- Mueller, D.S.; Bradley, C.A.; Grau, C.R.; Gaska, J.M.; Kurle, J.E.; Pedersen, W.L. Application of thiophanate-methyl at different host growth stages for management of Sclerotinia stem rot in soybean. Crop Prot. 2004, 23, 983–988. [Google Scholar] [CrossRef]
- Paula Júnior, T.J.D.; Vieira, R.F.; Rocha, P.R.R.; Bernardes, A.; Costa, É.L.; Carneiro, J.E.S.; Vole, F.X.R.; Zambolim, L. White mold intensity on common bean in response to plant density, irrigation frequency, grass mulching, Trichoderma spp., and fungicide. Summa Phytopathol. 2009, 35, 44–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antwi-Boasiako, A.; Wang, Y.; Dapaah, H.K.; Zhao, T. Mitigating against Sclerotinia Diseases in Legume Crops: A Comprehensive Review. Agronomy 2022, 12, 3140. https://doi.org/10.3390/agronomy12123140
Antwi-Boasiako A, Wang Y, Dapaah HK, Zhao T. Mitigating against Sclerotinia Diseases in Legume Crops: A Comprehensive Review. Agronomy. 2022; 12(12):3140. https://doi.org/10.3390/agronomy12123140
Chicago/Turabian StyleAntwi-Boasiako, Augustine, Yu Wang, Harrison Kwame Dapaah, and Tuanjie Zhao. 2022. "Mitigating against Sclerotinia Diseases in Legume Crops: A Comprehensive Review" Agronomy 12, no. 12: 3140. https://doi.org/10.3390/agronomy12123140
APA StyleAntwi-Boasiako, A., Wang, Y., Dapaah, H. K., & Zhao, T. (2022). Mitigating against Sclerotinia Diseases in Legume Crops: A Comprehensive Review. Agronomy, 12(12), 3140. https://doi.org/10.3390/agronomy12123140








