Leaf and Fruit Nutrient Concentration in Rojo Brillante Persimmon Grown under Conventional and Organic Management, and Its Correlation with Fruit Quality Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Plant Material
2.2. Plant Sampling and Sample Preparation
2.3. Nutrient Determinations
2.4. Determination of Physico-Chemical Parameters
2.5. Agronomic Efficiency
2.6. Statistical Analysis
3. Results and Discussion
3.1. Macronutrients in Leaf and Flesh Fruit under Conventional and Organic Management
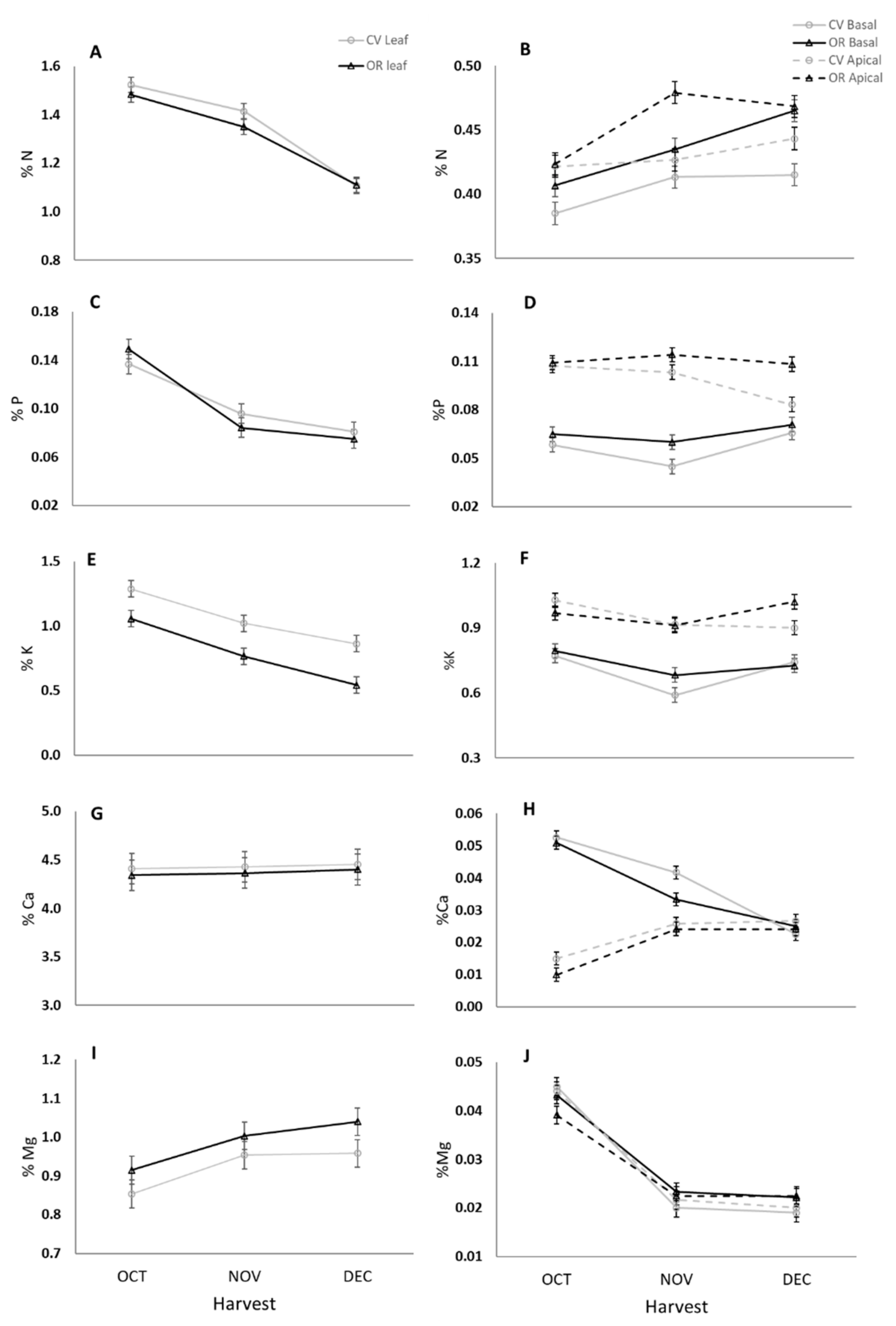
3.1.1. Macronutrients Concentration in Leaves and Fruit
3.1.2. Correlation between Macroelements
3.1.3. Agronomic Efficiency of the Main Macronutrients
3.2. Relation between Macroelements and Fruit Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Available online: www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 16 August 2021).
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 8 July 2021).
- Arnal, L.; Del Río, M.A. Effect of cold storage and removal astringency on quality of persimmon fruit (Diospyros kaki, L.) cv. Rojo brillante. Food Sci. Technol. Int. 2004, 10, 179–185. [Google Scholar] [CrossRef]
- Giordani, E.; Picardi, E.; Radice, S. Morfología y fisiología. In El cultivo de Caqui; Badenes, M.L., Intrigliolo, D.S., Salvador, A., Civera, A.V., Eds.; IVIA: Valencia, Spain, 2015; pp. 35–54. [Google Scholar]
- Salvador, A.; Arnal, L.; Monterde, A.; Cuquerella, J. Reduction of chilling injury symptoms in persimmon fruit cv. “Rojo Brillante” by 1-MCP. Postharvest Biol. Technol. 2004, 33, 285–291. [Google Scholar] [CrossRef]
- El-Gioushy, S.F. Productivity, Fruit Quality and Nutritional Status of Washington Navel Orange Trees as Influenced by Foliar Application with Salicylic Acid and Potassium Silicate Combinations. J. Hortic. Sci. Ornam. Plants 2016, 8, 98–107. [Google Scholar]
- Jivan, C.; Sala, F. Relationship between tree nutritional status and apple quality. Hortic. Sci. 2014, 41, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Milošević, T.; Milošević, N.; Glišić, I.; Nikolić, R.; Milivojević, J. Early tree growth, productivity, fruit quality and leaf nutrients content of sweet cherry grown in a high density planting system. Hortic. Sci. 2015, 42, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.J.; Smith, G.S. Seasonal changes in the composition, distribution and accumulation of mineral nutrients in persimmon fruit. Sci. Hortic. 1990, 42, 99–111. [Google Scholar] [CrossRef]
- George, A.P.; Nissen, R.J.; Broadley, R.H.; Collins, R.J. Improving the nutritional management of non-astringent persimmon in subtropical Australia. Acta Hortic. 2003, 601, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Ahn, G.; Lee, Y.; Kang, S. Effect of Different Autumnal Nitrogen Application Dates on Fruit Characteristics and Storage Reserves of ’Fuyu’ Persimmon. Hortic. Environ. Biotechnol. 2008, 49, 25–29. [Google Scholar]
- Choi, S.T.; Park, D.S.; Kang, S.M.; Cho, Y.C. Effect of fruit-load on the growth, absorption, and partitioning of inorganic nutrients in young “Fuyu” persimmon trees. Sci. Hortic. 2010, 126, 408–412. [Google Scholar] [CrossRef]
- Choi, S.T.; Kang, S.M.; Park, D.S.; Hong, K.P.; Rho, C.W. Combined effects of leaf/fruit ratios and N and K fertigation levels on growth and distribution of nutrients in pot-grown persimmon trees. Sci. Hortic. 2011, 128, 364–368. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lim, C.S.; Kang, S.M.; Cho, J.L. Root storage of nitrogen applied in autumn and its remobilization to new growth in spring of persimmon trees (Diospyros kaki cv. Fuyu). Sci. Hortic. 2009, 119, 193–196. [Google Scholar] [CrossRef]
- Liebisch, F.; Max, J.F.J.; Heine, G.; Horst, W.J. Blossom-end rot and fruit cracking of tomato grown in net-covered greenhouses in Central Thailand can partly be corrected by calcium and boron sprays. J. Plant Nutr Soil Sci. 2009, 172, 140–150. [Google Scholar] [CrossRef]
- Kim, W.S.; Chung, S.J.; Kim, K.Y.; DeJong, T.; Choi, H.S. Relationships between Ca, K and Mg concentration and browning of blossom end part of “Fuyu” sweet persimmon during MA storage. Adv. Hortic. Sci. 2002, 16, 95–100. [Google Scholar]
- Ferri, V.C.; Rombaldi, C.V.; Silva, J.A.; Pegoraro, C.; Nora, L.; Antunes, P.L.; Girardi, C.L.; Tibola, C.S. Boron and calcium sprayed on “Fuyu” persimmon tree prevent skin cracks, groove and browning of fruit during cold storage. Cienc. Rural 2008, 38, 2146–2150. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Sun, N.; Feng, L.; Meng, L.; Qin, R. Effects of calcium and boron elements on top rot of fruit physiological diseases in persimmon (Diospyros kaki Thunb.). Acta Hortic. 2013, 996, 333–338. [Google Scholar] [CrossRef]
- Casero, T.; Torres, E.; Alegre, S.; Recasens, I. Macronutrient accumulation dynamics in apple fruits. J. Plant Nutr. 2017, 40, 2468–2476. [Google Scholar] [CrossRef]
- Bourn, D.; Prescott, J. A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit. Rev. Food Sci. Nutr. 2002, 42, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alcántara, B.; Martínez-Cuenca, M.R.; Bermejo, A.; Legaz, F.; Quiñones, A. Liquid organic fertilizers for sustainable agriculture: Nutrient uptake of organic versus mineral fertilizers in citrus trees. PLoS ONE 2016, 11, 1–20. [Google Scholar]
- Puig-Montserrat, X.; Stefanescu, C.; Torre, I.; Palet, J.; Fàbregas, E.; Dantart, J.; Arrizabalaga, A.; Flaquer, C. Effects of organic and conventional crop management on vineyard biodiversity. Agric. Ecosyst Environ. 2017, 243, 19–26. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Organic and conventional foods: Differences in nutrients. Ital. J. Food Sci. 2016, 28, 565–578. [Google Scholar]
- Toselli, M. Nutritional implications of organic management in fruit tree production. Acta Hortic. 2010, 868, 41–48. [Google Scholar] [CrossRef]
- Yu, X.; Guo, L.; Jiang, G.; Song, Y.; Muminov, M.A. Advances of organic products over conventional productions with respect to nutritional quality and food security. Shengtai Xuebao/Acta Ecol. Sin. 2018, 38, 53–60. [Google Scholar] [CrossRef]
- Comité de Agricultura Ecológica de la Comunitat Valenciana (CAECV). Available online: https://www.caecv.com/ (accessed on 16 August 2021).
- Keller, J.; Karmeli, D. Trickle irrigation design parameters. Trans. ASAE 1974, 17, 678–684. [Google Scholar] [CrossRef]
- Doorenbos, J.; Pruitt, W.O. Crop water requirements. Irrig. Drain. Pap. 1977, 24, 144. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration (guidelines for computing crop water requirements). Irrig. Drain. Pap. 1998, 56, 1–15. [Google Scholar]
- Castel, J.R.; Buj, A.C. Growth and evapotranspiration of young drip-irrigated clementine trees. In Proceedings of the 7th International Citrus Congress, Acireale, Italy, 8–13 March 1992; pp. 2, 651–656. [Google Scholar]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis; Sparks, D.L., Ed.; Chemical methods; Oil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Jiménez-Cuesta, M.; Cuquerella, J.; Martínez-Jávega, J.M. Determination of a color index for citrus fruit degreening. Proc. Int. Soc. Citric. 1981, 2, 750–753. [Google Scholar]
- Clark, C.J.; Smith, G.S. Seasonal changes in the mineral nutrient content of persimmon leaves. Sci. Hortic. 1990, 42, 85–97. [Google Scholar] [CrossRef]
- Enab, H.; Mikhael, G. Replacing Nitrogen Fertilization by Using Organic and Biofertilizers on Costata Persimmon Trees. J. Product. Dev. 2018, 23, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Rehalia, A.S.; Sandhu, R.D. Standardization of foliar sampling technique for macro-nutrients in persimmon (Diosypros kaki L.) cv. Hachiya. Acta Hortic. 2005, 696, 265–268. [Google Scholar] [CrossRef]
- Pomares, F.; Gris, V.; Albiach, M.R. Fertilización. In El Cultivo de Caqui; Badenes, M.L., Intrigliolo, D.S., Salvador, A., Civera, A.V., Eds.; IVIA: Valencia, Spain, 2015; pp. 139–174. [Google Scholar]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Strik, B.C. Seasonal variation in mineral nutrient content of primocane-fruiting blackberry leaves. HortScience 2015, 50, 540–545. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, Y.; Gu, J.; Wang, Z.; Guo, D. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 177, 333–344. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal accumulation of mineral nutrients by kiwifruit. Fruit. New Phytol. 1988, 108, 399–409. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S.; Gravett, I.M. Seasonal Accumulation of Mineral Nutrients by Tamarillo. Fruit 1989, 40, 203–213. [Google Scholar]
- Liu, G.; Chen, Y.; He, X.; Yao, F.; Guan, G.; Zhong, B.; Zhou, G. Seasonal changes of mineral nutrients in the fruit of navel orange plants grafted on trifoliate orange and citrange. Sci. Hortic. 2020, 264, 109–156. [Google Scholar] [CrossRef]
- Tagliavini, M.; Scandellari, F. Methodologies and concepts in the study of nutrient uptake requirements and partitioning in fruit trees. Acta Hortic. 2013, 984, 47–56. [Google Scholar] [CrossRef]
- Ben-Arie, R.; Zilkah, S.; Klein, I.; Gamrasni, D. Persimmon and environment: Soil and water management for high quality fruit production. Adv. Hortic. Sci. 2008, 22, 286–293. [Google Scholar]
- Zanotelli, D.; Rechenmacher, M.; Guerra, W.; Cassar, A.; Tagliavini, M. Seasonal uptake rate dynamics and partitioning of mineral nutrients by bourse shoots of field-grown apple trees. Eur. J. Hortic. Sci. 2014, 79, 203–211. [Google Scholar]
- Glew, R.H.; Ayaz, F.A.; Vanderjagt, D.J.; Millson, M.; Dris, R.; Niskanen, R. A research note mineral composition of medlar (Mespilus germanica) fruit at different stages of maturity. J. Food Qual. 2003, 26, 441–447. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of Nitrogen with Other Nutrients and Water: Effect on Crop Yield and Quality, Nutrient Use Efficiency, Carbon Sequestration, and Environmental Pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Lowland rice response to nitrogen fertilization. Commun. Soil Sci. Plant Anal. 2001, 32, 1405–1429. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.R.; Grunes, D.L.; Sumner, M.E. Nutrient interactions in soil and plant nutrition. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: New York, NY, USA, 2000; pp. D89–D104. [Google Scholar]
- Grunes, D.L.; Huang, J.W.; Smith, F.W.; Joo, P.K.; Hewes, D.A. Potassium effects on minerals and organic acids in three cool-season grasses. J. Plant Nutr. 1992, 15, 1007–1025. [Google Scholar] [CrossRef]
- George, A.; Nissen, B.; Broadley, R. Persimmon Nutrition; A practical guide to improving fruit quality and production; Department of Primary Industries: Queenland, Australia, 2005. [Google Scholar]
- Marcelle, R.D. Mineral nutrition and plant quality. Acta Hortic. 1995, 383, 219–226. [Google Scholar] [CrossRef]
- Madani, B.; Wall, M.; Mirshekari, A.; Bah, A.; Muda Mohamed, M.T. Influence of calcium foliar fertilization on plant growth, nutrient concentrations, and fruit quality of papaya. HortTechnology 2015, 25, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Brunetto, G.; De Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. The Role of Mineral Nutrition on Yields and Fruit Quality in Grapevine, Pear and Apple. Rev. Bras. De Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- De Ponti, T.; Rijk, B.; Van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Li, W.; Yang, M.; Wang, J.; Wang, Z.; Fan, Z.; Kang, F.; Wang, Y.; Luo, Y.; Kuang, D.; Chen, Z.; et al. Agronomic responses of major fruit crops to fertilization in China: A meta-analysis. Agronomy 2020, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; Huber, J.A.; Gerl, G.; Hülsbergen, K.J. Nitrogen balances and nitrogen-use efficiency of different organic and conventional farming systems. Nutr. Cycl. Agroecosystems 2016, 105, 1–23. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Alves, B.; Aulakh, M.; Bekunda, M.; Cai, Z.; Drinkwater, L.; Oenema, O. Crop, environmental, and management factors affecting nitrogen use efficiency. In Agriculture and the Nitrogen Cycle; Mosier, A.R., Syers, J.K., Freney, J., Eds.; Island Press: Washington, DC, USA, 2004; Volume 65, pp. 19–33. [Google Scholar]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional farming system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Besada, C.; Larrea, V.; Quiles, A.; Pérez-Munuera, I. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. “Rojo Brillante”. Postharvest Biol. Technol. 2007, 46, 181–188. [Google Scholar] [CrossRef]
- Besada, C.; Salvador, A. Postharvest Handling of Persimmon Fruit. In Mechanism and Action of Phytoconstituents; Awaad, A.S., Kaushik, G., Govil, J.N., Eds.; Studium Press Pvt. Ltd.: New Delhi, India, 2011; pp. 111–137. [Google Scholar]
- Salvador, A.; Arnal, L.; Carot, J.M.; Carvalho, C.P.; Jabaloyes, J.M. Influence of different factors on firmness and color evolution during the storage of persimmon cv. “Rojo Brillante”. J. Food Sci. 2006, 71, S169–S175. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Besada, C.; Hernando, I.; Appezzato-da-Glória, B.; Quiles, A.; Salvador, A. Microstructural changes while persimmon fruits mature and ripen. Comparison between astringent and non-astringent cultivars. Postharvest Biol. Technol. 2016, 120, 52–60. [Google Scholar] [CrossRef]
- Taira, S.; Ono, M. Reduction of astringency in persimmon caused by adhesion of tannins to cell wall fragments. Acta Horticuturae 1996, 436, 235–242. [Google Scholar] [CrossRef]
- El-Razek, A.; Treutter, E. Effect of nitrogen and potassium fertilization on productivity and fruit quality of ‘crimson seedless’ grape. Agric. Biol. J. North Am. 2011, 2, 330–340. [Google Scholar] [CrossRef]
- Pacheco, C.; Calouro, F.; Santos, F.; Vieira, S.; Rodrigues, S.; Neves, N.; Curado, F. Influence of nitrogen and potassium on yield, fruit quality and mineral composition of kiwifruit. Int. J. Energy Environ. 2008, 2, 9–15. [Google Scholar]
- Choi, S.T.; Park, D.S.; Kang, S.M.; Kang, S.K. Influence of leaf-fruit ratio and nitrogen rate on fruit characteristics, nitrogenous compounds, and nonstructural carbohydrates in young persimmon trees. HortScience 2012, 47, 410–413. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Conventional Orchards | Organic Orchards |
|---|---|---|
| Sand (%) | 28.1 | 22.4 |
| Silt (%) | 37.1 | 54.9 |
| Clay (%) | 34.8 | 22.7 |
| USDA Classification | Clay Loam | Silty Loam |
| pH | 8.4 | 7.6 |
| MO (%) | 0.94 | 3.14 |
| Norg (%) | 0.05 | 0.14 |
| C/N | 11.47 | 13.05 |
| Soluble POlsen 1 (ppm) | 15.2 | 18.0 |
| Casse 2 (meq/L) | 6.81 | 7.43 |
| Mgsse 2 (meq/L) | 2.89 | 2.43 |
| Ksse 2 (meq/L) | 0.31 | 0.35 |
| Management | Chemical Compound (kg year−1 ha−1) | |||||
|---|---|---|---|---|---|---|
| N | P2O5 | K2O | CaO | MgO | FLUIDOGAMA® 1 | |
| Conventional | 170 | 74 | 155 | 0.0 | 0.0 | 0.0 |
| Organic | 27 | 1 | 27 | 0.5 | 0.0 | 840 |
| Significance | ||||||
|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | ||
| Leaf | A: Harvest | 0.000 * | 0.000 * | 0.000 * | ns | 0.004 * |
| B: Management | ns | ns | 0.000 * | ns | 0.030 * | |
| AB: | ns | ns | ns | ns | ns | |
| Fruit | A: Harvest | 0.000 * | ns | 0.000 * | 0.000 * | 0.000 * |
| B: Management | 0.000 * | 0.000 * | ns | 0.019 * | ns | |
| C: Fruit Part | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ns | |
| AB: | ns | ns | ns | ns | 0.034 * | |
| AC: | ns | 0.000 * | ns | ns | ns | |
| BC: | ns | ns | ns | ns | ns | |
| ABC: | ns | ns | 0.018 * | ns | ns | |
| Organ | Element | Leaf | Fruit Basal Area | Fruit Apical Area | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | N | P | K | Ca | Mg | N | P | K | Ca | Mg | ||
| Leaf | N | |||||||||||||||
| P | 0.701 * | |||||||||||||||
| K | 0.777 ** | 0.670 * | ||||||||||||||
| Ca | −0.413 | 0.202 | −0.164 | |||||||||||||
| Mg | −0.515 | −0.432 | −0.772 ** | 0.329 | ||||||||||||
| Fruit basal area | N | −0.458 | −0.477 | −0.815 ** | 0.237 | 0.919 *** | ||||||||||
| P | −0.404 | −0.036 | −0.439 | 0.633 | 0.457 | 0.554 | ||||||||||
| K | 0.277 | 0.203 | 0.205 | 0.157 | 0.308 | 0.201 | −0.198 | |||||||||
| Ca | 0.827 *** | 0.805 ** | 0.844 *** | −0.127 | −0.749 ** | −0.733 * | −0.306 | −0.034 | ||||||||
| Mg | 0.385 | 0.706 | 0.566 | 0.431 | −0.468 | −0.455 | 0.214 | −0.066 | 0.712 *** | |||||||
| Fruit apical area | N | −0.523 | −0.614 * | −0.808 ** | −0.116 | 0.617 ** | 0.679 * | 0.353 | −0.448 | −0.612 * | −0.454 | |||||
| P | 0.475 | 0.29 | 0.122 | −0.292 | −0.072 | −0.112 | −0.287 | −0.090 | 0.509 | 0.198 | 0.554 | |||||
| K | 0.120 | 0.264 | 0.168 | 0.515 | -0.038 | 0.062 | 0.434 | 0.088 | 0.301 | 0.521 | 0.201 | -0.198 | ||||
| Ca | −0.542 | −0.812 ** | −0.285 | −0.346 | −0.088 | −0.048 | −0.152 | −0.417 | −0.505 | −0.568 | −0.733 * | −0.306 | −0.034 | |||
| Mg | 0.599 | 0.723 | 0.659 | 0.247 | −0.520 | −0.511 | −0.005 | −0.010 | 0.878 * | 0.894 ** | −0.455 | 0.214 | −0.066 | 0.712 * | ||
| Organ | Harvest | Ca/(K+Mg) | N/Ca | ||
|---|---|---|---|---|---|
| Conventional | Organic | Conventional | Organic | ||
| Leaf | October | 2.06 | 2.20 | 0.35 | 0.34 |
| November | 2.24 | 2.47 | 0.32 | 0.31 | |
| December | 2.44 | 2.78 | 0.25 | 0.25 | |
| Fruit basal area | October | 0.06 | 0.06 | 7.33 | 8.00 |
| November | 0.07 | 0.05 | 9.92 | 13.05 | |
| December | 0.03 | 0.03 | 18.44 | 18.60 | |
| Fruit apical area | October | 0.01 | 0.01 | 28.11 | 42.33 |
| November | 0.03 | 0.03 | 16.52 | 19.83 | |
| December | 0.03 | 0.02 | 16.52 | 19.38 | |
| Management | Orchard (m2) | Yield (kg)/Orchard | Yield (kg)/ha |
|---|---|---|---|
| Conventional | 30 | 174 b | 44,452 b |
| Organic | 40 | 97 a | 33,418 a |
| Management | N | P | K |
|---|---|---|---|
| Conventional | 262 a | 600 | 286 a |
| Organic | 1243 b | ∞ | 1227 b |
| Harvest | Management | Weight | CI | Firmness | ST | TSS |
|---|---|---|---|---|---|---|
| October | Conventional | 242.37 a | −1.26 a | 55.05 a | 0.90 a | 13.84 a |
| Organic | 257.73 a | −1.41 a | 50.17 b | 0.85 a,b | 12.73 b | |
| November | Conventional | 251.90 a | 8.54 b | 42.94 c | 0.79 b | 14.42 a,c |
| Organic | 268.88 a | 9.12 b | 42.41 c | 0.69 b,c | 14.90 c,d | |
| December | Conventional | 248.98 a | 14.04 c | 36.07 d | 0.61 c | 16.10 e |
| Organic | 283.96 a | 13.26 c | 32.96 e | 0.58 c | 15.33 d | |
| ANOVA | A: Harvest | ns | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| B: Management | ns | ns | 0.000 * | 0.047 * | 0.032 * | |
| A x B | ns | ns | 0.007 * | ns | 0.009 * |
| Organ | Nutrient | Weight | CI | Firmness | ST | TSS |
|---|---|---|---|---|---|---|
| Leaf | N | −0.404 | −0.836 *** | 0.834 *** | 0.733 ** | −0.781 ** |
| P | −0.204 | −0.897 *** | 0.807 ** | 0.704 * | −0.818 ** | |
| K | −0.634 | −0.782 ** | 0.873 *** | 0.859 *** | −0.582 | |
| Ca | 0.689 | 0.001 | −0.197 | −0.304 | 0.113 | |
| Mg | 0.901 *** | 0.563 | −0.598 | −0.794 ** | 0.325 | |
| N/Ca | −0.604 | −0.747 ** | 0.751 ** | 0.708 ** | −0.649 | |
| Ca/K+Mg | 0.649 | 0.702 ** | −0.776 ** | −0.716 ** | 0.529 | |
| Basal area | N | 0.823 *** | 0.588 | −0.683 | −0.845 *** | 0.346 |
| P | 0.721 ** | 0.237 | −0.400 | −0.654 | 0.151 | |
| K | 0.192 | −0.161 | 0.188 | −0.027 | −0.357 | |
| Ca | −0.454 | −0.945 | 0.943 *** | 0.879 *** | −0.837 *** | |
| Mg | −0.293 | −0.934 | 0.898 *** | 0.753 ** | −0.805 ** | |
| N/Ca | 0.518 | 0.881 *** | −0.869 *** | −0.876 *** | 0.782 ** | |
| Ca/K+Mg | −0.385 | −0.769 ** | 0.801 ** | 0.795 ** | −0.745 ** | |
| Apical area | N | 0.429 | 0.601 | −0.614 | −0.652 | 0.564 |
| P | 0.011 | −0.542 | 0.491 | 0.410 | −0.595 | |
| K | 0.274 | −0.307 | 0.226 | 0.124 | −0.361 | |
| Ca | −0.224 | 0.722 ** | −0.574 | −0.352 | 0.752 ** | |
| Mg | −0.276 | −0.888 *** | 0.829 *** | 0.715 ** | −0.761 ** | |
| N/Ca | 0.157 | −0.716 ** | 0.537 | 0.349 | −0.705 * | |
| Ca/K+Mg | −0.193 | 0.765 ** | −0.607 | −0.385 | 0.767 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilhena, N.Q.; Quiñones, A.; Rodríguez, I.; Gil, R.; Fernández-Serrano, P.; Salvador, A. Leaf and Fruit Nutrient Concentration in Rojo Brillante Persimmon Grown under Conventional and Organic Management, and Its Correlation with Fruit Quality Parameters. Agronomy 2022, 12, 237. https://doi.org/10.3390/agronomy12020237
Vilhena NQ, Quiñones A, Rodríguez I, Gil R, Fernández-Serrano P, Salvador A. Leaf and Fruit Nutrient Concentration in Rojo Brillante Persimmon Grown under Conventional and Organic Management, and Its Correlation with Fruit Quality Parameters. Agronomy. 2022; 12(2):237. https://doi.org/10.3390/agronomy12020237
Chicago/Turabian StyleVilhena, Nariane Q., Ana Quiñones, Isabel Rodríguez, Rebeca Gil, Paula Fernández-Serrano, and Alejandra Salvador. 2022. "Leaf and Fruit Nutrient Concentration in Rojo Brillante Persimmon Grown under Conventional and Organic Management, and Its Correlation with Fruit Quality Parameters" Agronomy 12, no. 2: 237. https://doi.org/10.3390/agronomy12020237
APA StyleVilhena, N. Q., Quiñones, A., Rodríguez, I., Gil, R., Fernández-Serrano, P., & Salvador, A. (2022). Leaf and Fruit Nutrient Concentration in Rojo Brillante Persimmon Grown under Conventional and Organic Management, and Its Correlation with Fruit Quality Parameters. Agronomy, 12(2), 237. https://doi.org/10.3390/agronomy12020237







