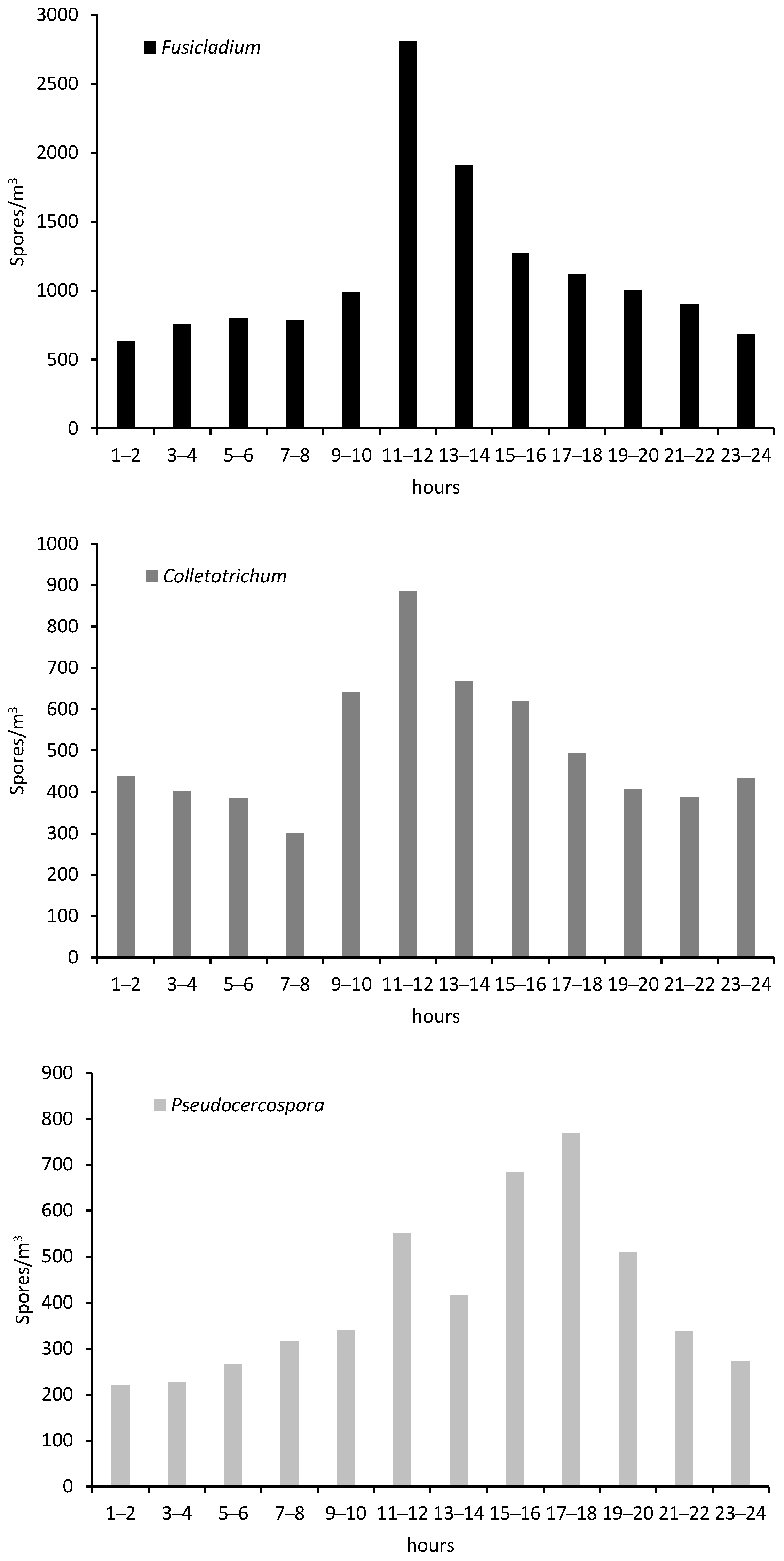Abstract
The most common fungal diseases of Olea europaea are olive leaf spot (Fusicladium oleagineum), anthracnose (Colletotrichum spp.) and cercospora leaf spot (Pseudocercospora cladosporioides). To know the fungal load during the vegetative olive cycle, an aerobiological and phenological study was conducted in an olive grove in North-West Spain. For the aerobiological study, the Spanish Aerobiological Network protocol was followed using a Hirst-type spore trap. The goal of the study was to assess the spore concentrations in the atmosphere of the olive grove and their relationship with the meteorological parameters by applying statistical procedures, including a Cluster analysis, Spearman’s correlation test and PCA analysis. The most abundant spores belong to Fusicladium, registering the double of values than Colletotrichum and Pseudocercospora. The hours with the highest spores’ presence were from 11:00 to 12:00 for Fusicladium and Colletotrichum, and from 17:00 to 18:00 for Pseudocercospora. The Spearman’s and PCA test showed a positive association between temperature and relative humidity with the spores’ concentrations. The combination of meteorological, phenological and aerobiological parameters is a useful tool to understand the ecological behavior of the considered phytopathogenic fungal spores in order to develop futures strategies for the integrated management of fungal olive diseases in areas at the limit of this tree distribution.
1. Introduction
Olive (Olea europaea L.) is one of the most extensively cultivated crops in regions characterized by the Mediterranean climate [1]. In these areas, the olive fruit and the olive oil are the most important products with a high social and economic impact [2]. The meteorological conditions of the Mediterranean basin do not allow a high incidence of phytopathogenic fungi in the crop. However, in recent years an increase in the cultivated area of olive trees was registered in the North-western Spain in areas of the Miño and Sil basins [3]. As a result of the Oceanic climate with Mediterranean influence, this area presents ideal temperature and humidity conditions for the development of phytopathogenic fungi. A large number of pathogenic fungi can affect the olive tree crop, with great variability in terms of diffusion and severity [1]. In our study, we will focus on three of the most important fungal pathogens, Fusicladium oleagineum (Cast.) Ritschel & Braun, Colletotrichum spp. and Pseudocercospora cladosporioides (Sacc.) Braun.
Fusicladium oleagineum is the most common aerial fungal that affects olive tree [1]. The disease known as olive leaf spot induced by this fungus caused typical round spots on the leaves, green-black in color, often surrounded by a yellowish or red-purple halo [1]. This fungus is widespread in the Mediterranean basin and in other temperate and subtropical areas in the world [4]. Nevertheless, in areas where olives are also grown, such as North America, Northern Europe, and South Asia, the presence of this pathogen has not been reported [5]. The period of greatest influence of this disease takes place between spring budding and green fruit, passing through full flowering and fruit set [6]. The Fusicladium oleagineum is also able to affect again the crop at the end of the cycle during the harvest and autumn budding [6]. The fungus can attack petioles and fruits, followed by intense defoliation, which finishes with the weakening of the tree. In fact, this fungus is one of the major causes of defoliation in the olive tree [6]. This disease is the most common disease in the Spanish olive grove, responsible for 6% of the losses, with expenses of 200 million euros in fungicides that could be increased during rainy years [6,7].
Several species of Colletotrichum have been noted as responsible of the olive anthracnose, highlighting C. acutatum (s. lat.) and C. gloesporioides (s. lat.) [1,8]. Different Colletorichum spp. has been reported around the world. In American cultivars of Argentina [9], Brazil [10], Uruguay [11] and USA [12]. In Europe it was reported in France [13], Greece [14], Italy [15,16], Portugal [17,18,19], Spain [20] and Montenegro [21]. In African plots of South Africa [22], Morocco [23] and Tunisia [24,25]. In Asian cultivars of Egypt [26], China [27], Japan [28] Iran [29,30] and India [31], and in Oceania in Australia [32,33]. In our latitude this disease is common in the Western Mediterranean basin, known in Spain with the name of “aceituna jabonosa”, in Portugal as “gafa” and in Italy as “lebbra” [34]. The incidence of this disease takes place on winter and during the final stages of the olive cycle, from the moment when the fruits are green until their maturation. Anthracnose directly affects the fruits, causing circular, dark, sunken necroses, on which slimy, orange masses of spores are produced under high humidity conditions leading to premature fruit drop or mummification [1,34]. Moreover, the fungus can attack young branches producing their necrosis and defoliation [35,36,37]. Nowadays, anthracnose is the disease that most affects the quality of the oil (colored oil) being “Picual” the most susceptible variety [36,38].
Pseudocercospora cladosporioides is the causal agent of the cercospora leaf spot that causes severe defoliation [39]. Since P. cladosporioides is considered a less aggressive pathogen than the other two fungus only few studies were focused on this disease [40,41,42]. This fungus has been found in large olive trees cultivated areas around the world, for example, in Spain [43], Morocco [44], China, North America, Siberia [45], United Kingdom [46], Greece [47], India [48], Japan [49] and New Zealand [50] among others. Foliar symptoms consist of irregular chlorotic spots on the upper surface and diffuse grayish or leaden spots on the underside. The fungus can also affect the fruit, so in some cases the “edible” olive harvest has been left for oil, although it is of poorer quality [51,52].
All the aforementioned diseases affect both the tree and the fruit, resulting in a modification of the conditions in the final product (either olive oil or the fruit of the olive tree itself) which could lead to significant economic losses.
The aim of the survey is to study for first time the concentrations of Fusicladium oleagineum, Colletotrichum spp. and Pseudocercospora cladosporioides spores in the air of an olive grove placed in the Northwestern Spain. The relationship of the spore concentration with the phenology of the tree and the meteorology will be also assessed in order to be able to know the potential capacity of the three fungi for the infection of the olive groves in this area with potential meteorological conditions for the fungal development.
2. Materials and Methods
The study has been carried out in a ten-year-old olive grove located in Ourense (North-western Spain; 42°18′42″ N, 7°54′47″ W) throughout the year 2020 (Figure 1). This area is located 143 m above sea level and has a transitional climate between the Mediterranean and the Atlantic, but with mild summers [53].
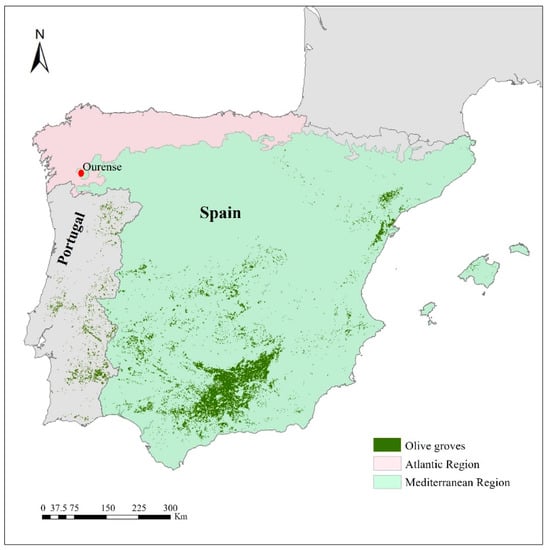
Figure 1.
Sampling location area (Ourense), green zones indicate the olive land use by CORINE land cover 2018. Bioclimatic regions of the studied area were provided by www.miteco.gob.es (15 November 2021).
To carry out the monitoring of the atmospheric fungal spores (Fusicladium oleagineum, Colletotrichum spp. and Pseudocercospora cladosporioides), a Lanzoni VPPS 2010 Hirst type located in the center of the olive orchard was used. Fungal spores were studied along the olive reproductive season (from 1 April to 28 October). Spores count was conducted according to the model proposed by the REA (Spanish Aerobiological Network) [54]. Through this method we obtain total values expressed as spores and daily mean values expressed as spores/m3 following the specifications pointed by Galán et al. [55].
For the identification of the spores under the microscope, the following indications have been followed regarding their morphological characteristics. Fusicladium oleagineum conidia are usually bicellular, obpyriform, light brown in color, truncated at the base and narrower and elongated at the apex, with significant variability in size, ranging between 15–30 µm in length and 4–15 µm in width. The shape of the Colletotrichum conidia showed a predominance of the cylindrical and fusiform forms (wedge-shaped). The length of the conidium ranged from 18.40 µm to 25.25 µm, the width was also variable between 9.80 µm to 20.0 µm. Pseudocercospora cladosporioides present conidia cylindrical, elongated, with several septa and irregular curves, the base truncated and the apical end rounded. The mean length of the conidia was highly variable, with values ranging from 63.8 µm to 37.1 µm. In the case of width, the values were a bit more homogeneous, ranging from 4.3 µm to 3.1 µm [44].
Phenological study was performed by means a weekly visit to the olive grove from March until the olive harvest. The number of visits to the grove were increased to two times a week during the flowering stage. A total of 22 trees (11 of the ‘Picual’ variety and 11 belonging to the ‘Arbequina’ variety) were selected and studied in order to carry out the phenological survey. The BBCH scale developed by Meier et al. [56] and adapted to olive trees by Sanz-Cortés et al. [57] was used for the phenological study. With the aim to design a phenological calendar, we considered the beginning of each stage when the half of the marked trees reach this stage. The specific date for each phase was calculated as an average of the 22 marked olive trees.
Weather data were sampled with a HOBO micro station located on the olive grove (maximum, minimum and average temperature, dew point and relative humidity) with the exception of the wind speed and rainfall variables, obtained from the nearest MeteoGalicia meteorological station (6 km).
In order to calculate the intraday spores’ distribution, we have taken into account the days that exceeded the mean of the sampling period, and the rainy days were excluded. The resulting days were used to calculate the sum for every two hours.
A hierarchical cluster analysis to categorize the different phenological stages was conducted. The number of clusters was determined using: (i) the squared Euclidean distance as a distance measure and (ii) the Nearest Neighbor method as a linking method. In order to perform the cluster analysis, we use the spores and meteorogical data during the study period. In order to determine the influence of the main meteorological variables on airborne spore concentrations, a Spearman’s correlation test was applied, as the considered variables do not show a normal distribution. The normality of the data has been verified with the Kolmogorov-Smirnov test and the significance level for the three spore types has been p = 0.000, therefore the data does not follow a normal distribution. Finally, a Principal Component Analysis (PCA) was conducted in order to assess the meteorological influence of all variables as a whole in the fungus. For this analysis we considered for each phenological stage the maximum, average, and minimum temperatures, relative humidity, rainfall, dew point and wind-speed meteorological parameters. The IBM SPSS statistics 24 package was used for the statistical analysis.
3. Results
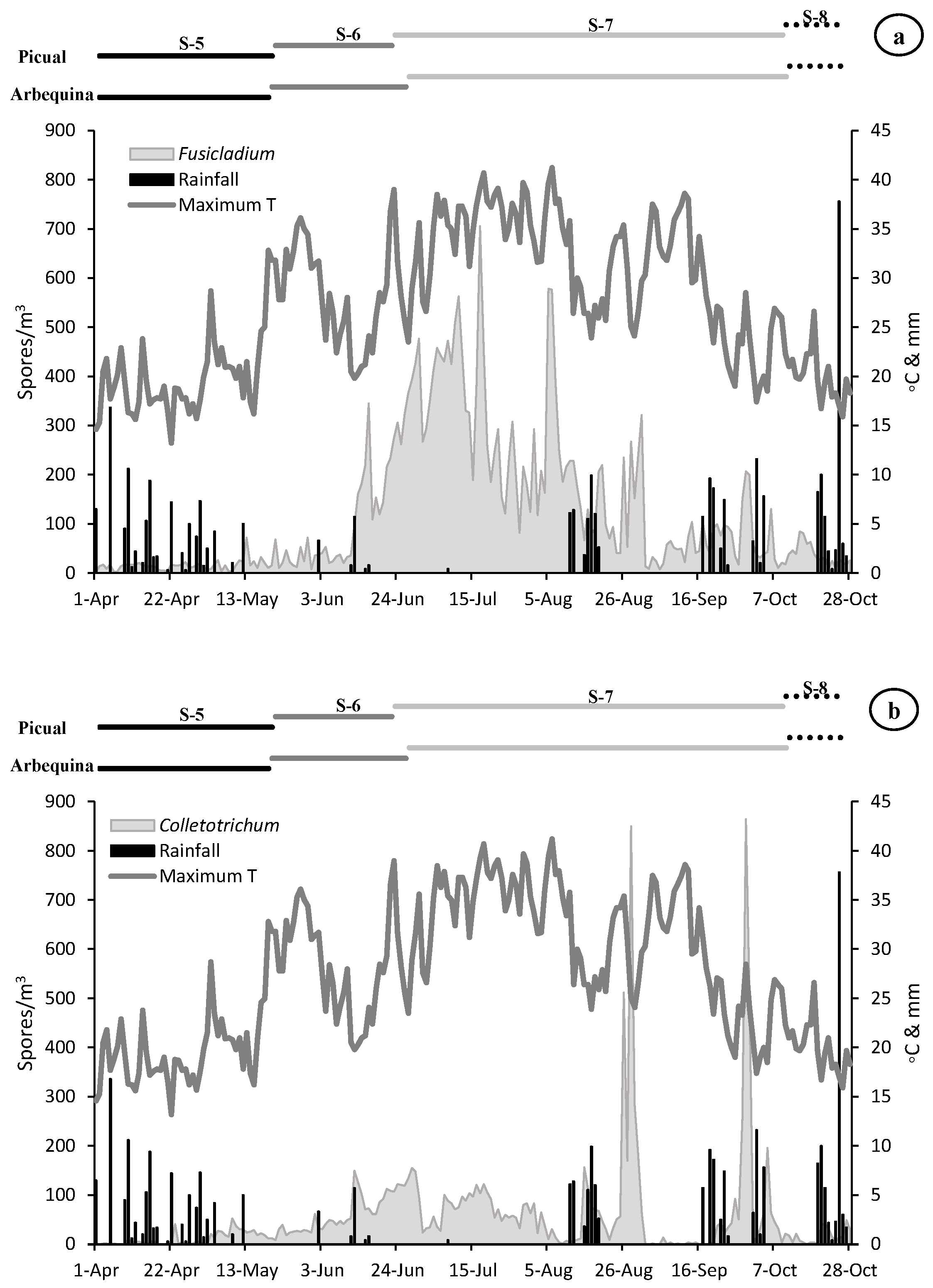
The olive vegetative cycle lasted 210 days. Stage 5 (inflorescences development) took place from the end March to mid-May with an average length of 50 days. The stage 6 (flowering), the most important phenological stage, had a duration around 36 days starting on the second ten days of May and ending on the last days of June. The longest length was recorded for the stage 7 (fruit development) with 109 days from the last days of June to the first fortnight of October. Finally, stage 8 (fruit ripening) covers the period from second fortnight of October to the end of this month, with a mean duration of 17 days. There are no significant phenological differences between the ‘Arbequina’ and ‘Picual’ varieties, with the exception of 5 days in the length of stage 6 (flowering) (Table 1, Figure 2).

Table 1.
Start, end date and duration (in days) of the four reproductive phenological stages throughout the year 2020.
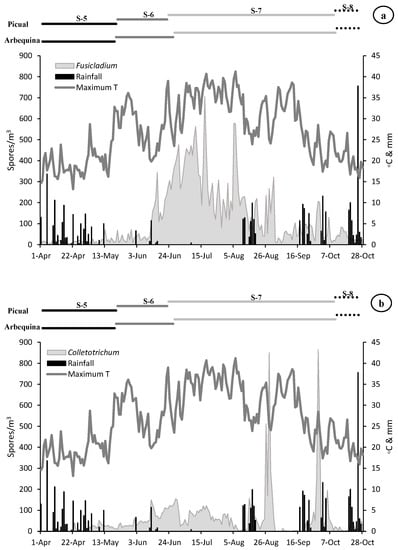
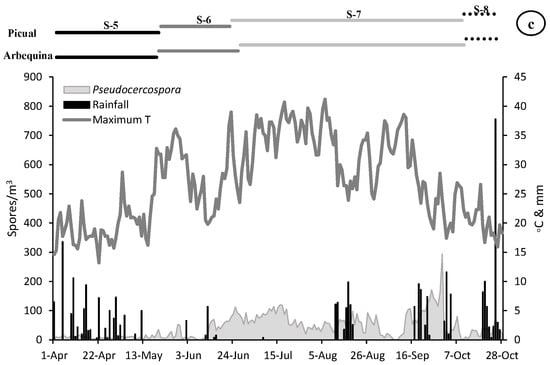
Figure 2.
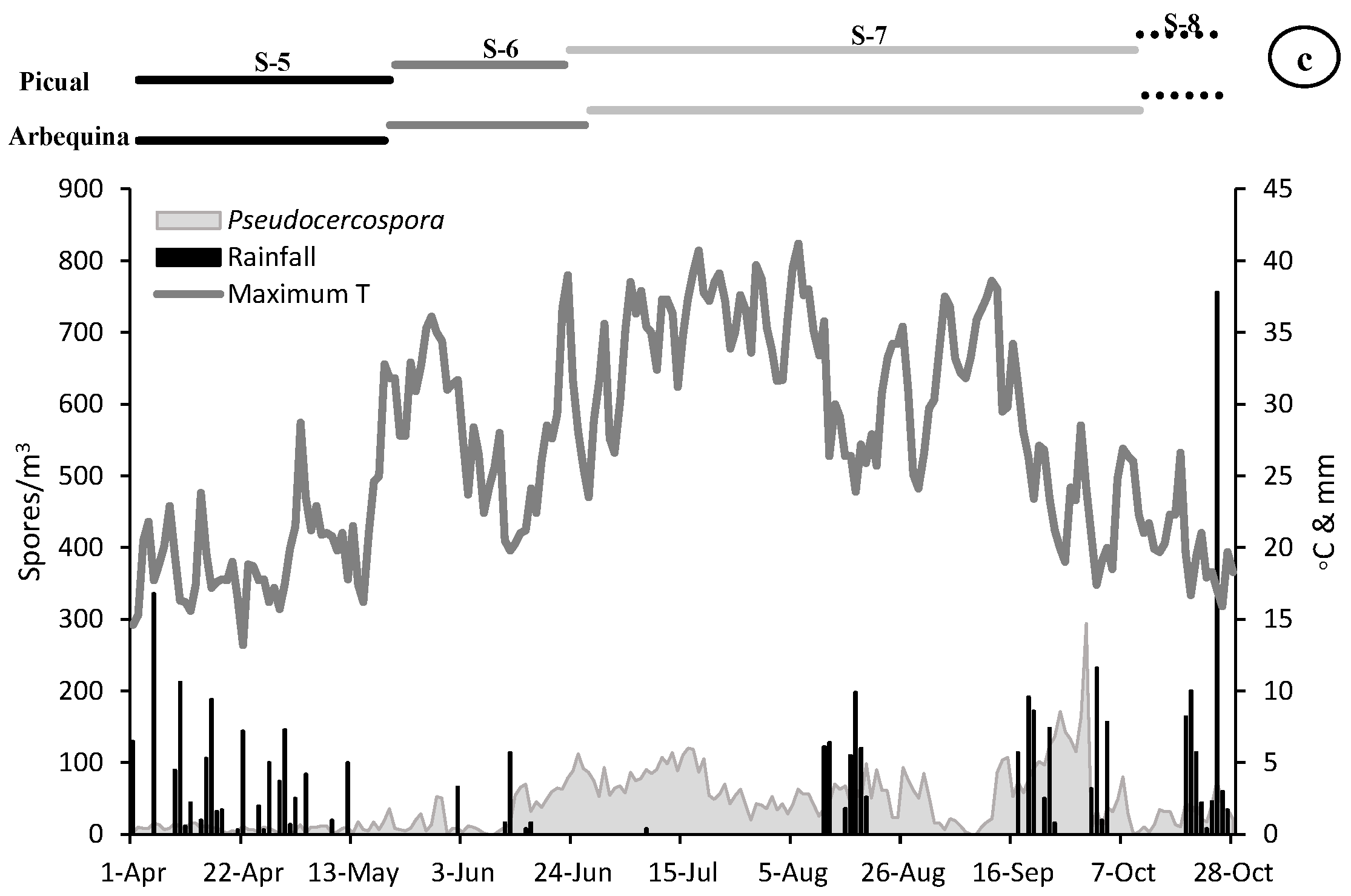
Dinamycs of the Fusicladium (a), Colletotrichum (b) and Pseudocercospora (c) aerobiological fungal spores (grey area) and daily meteorological parameters (maximum temperature in lines and rainfall in bars). On the top, length of the main phenological stages for Arbequina and Picual varieties (S5-Stage 5: Inflorescence emergence, S6-Stage 6: Flowering, S7-Stage 7: Fruit development and S8-Stage 8: Maturity of fruit).
The presence of airborne spores in the olive grove was continuously detected during the reproductive cycle of olive tree. The most abundant spores during the whole vegetative cycle belonged to Fusicladium with 25,536 spores, followed by Colletotrichum with 11,420 and Pseudocercospora with 9015 total spores. During the first stage sampled (stage 5: inflorescences development) a very low value of all the spore types was recorded. In the stage 6 (flowering) we detected an increasing trend in the spore concentrations, being greater in the case of Fusicladium which registers values around twice times higher than Colletotrichum, which also showed values twice times higher than Pseudocercospora. During the stage 7 (fruit development) a considerable increase in the concentration of all spore types was detected, mainly Fusicladium with the maximum value of the entire campaign of around 21,000 spores/m3. The Colletotrichum and Pseudocercospora spores registered similar values of around 8000 spores/m3. Finally, during the stage 8 (fruit ripening), a large reduction in the number of registered spores was detected, with values around 500 spores/m3 for Fusicladium and Pseudocercospora and the minimum in the case of Colletotrichum with 200 spores/m3 (Table 2, Figure 2).

Table 2.
Total spore concentrations on each phenological stages (spores), maximum daily value of each spore type (spores/m3) and date of maximum value during the principal BBCH growth stages (S-5: Inflorescence emergence; S-6: Flowering; S-7: Development of fruits and S-8: Maturity of fruit) during the study year.
When we analyze the maximum daily spore peak, we observed that for the three phytopathogens the maximum peak took place during the stage 7 (fruit development). In the case of Fusicladium on 17 July with 706 spores/m3, for Colletotrichum on 29 August with 865 spores/m3 and Pseudocercospora registered the peak date on 30 September with 294 spores/m3. It should be noted that in the transcendental stage 6 (flowering) for fruiting and final harvest, high concentrations of spores were registered during the second half of June with 345 spores/m3, 150 spores/m3 and 88 spores/m3 for Fusicladium, Colletotrichum and Pseudocercospora, respectively (Table 2, Figure 2).
In order to analyze the intraday spores’ distribution, we can observe a repeated marked dynamic for Fusicladium and Colletotrichum with higher concentrations between 11 and 12 am. In contrast, Pseudocercospora was more abundant during the first hours of the afternoon, between 15–16 h or 17–18 h (Figure 3).
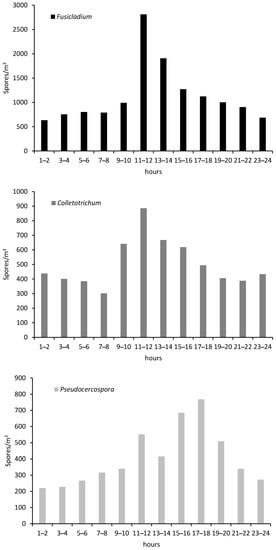
Figure 3.
Intraday variation of Fusicladium, Colletotrichum and Pseudocercospora spores in the crop during the study year.
With the aim of relate the presence of spore concentrations in the atmosphere with the weather variables, a similar very clear pattern was registered for the three species (Figure 2). At the beginning of the campaign (stage 5), relatively low temperature values and abundant rainfall were registered, coinciding with a period with a scarce presence of spores in the air (Table 3, Figure 2). In stage 6 (flowering) we observed a very sharp decrease in rainfall (8 mm) accompanied by an increase in temperatures (average temperature of 18.31 °C) which can induce adequate flowering process. The maximum peak of the three spore types was registered during stage 7 (fruit development). In the case of Fusicladium the peak took place on 17 July with 706 spores/m3 coinciding with soft temperatures around 24.30 °C and absence of rainfall. For Colletotrichum the maximum peak took place on 29 August with 865 spores/m3 under conditions of average temperature of 14.60 °C and high relative humidity (80.60%). Finally, in the case of Pseudocercospora the spore peak was registered on 30 September with 294 spores/m3 when the average temperature was 14.50 °C accompanied by high relative humidity (83.00%) values and slight rainfall during the previous days (Figure 2).

Table 3.
Main meteorological parameters—Daily mean values of maximum, minimum and average temperatures (°C), dew point (°C), relative humidity (%), rainfall (mm) and wind speed (m/s)—during the phenological stages, Stage 5: Inflorescence emergence, Stage 6: Flowering, Stage 7: Fruit development and Stage 8: Maturity of fruit.
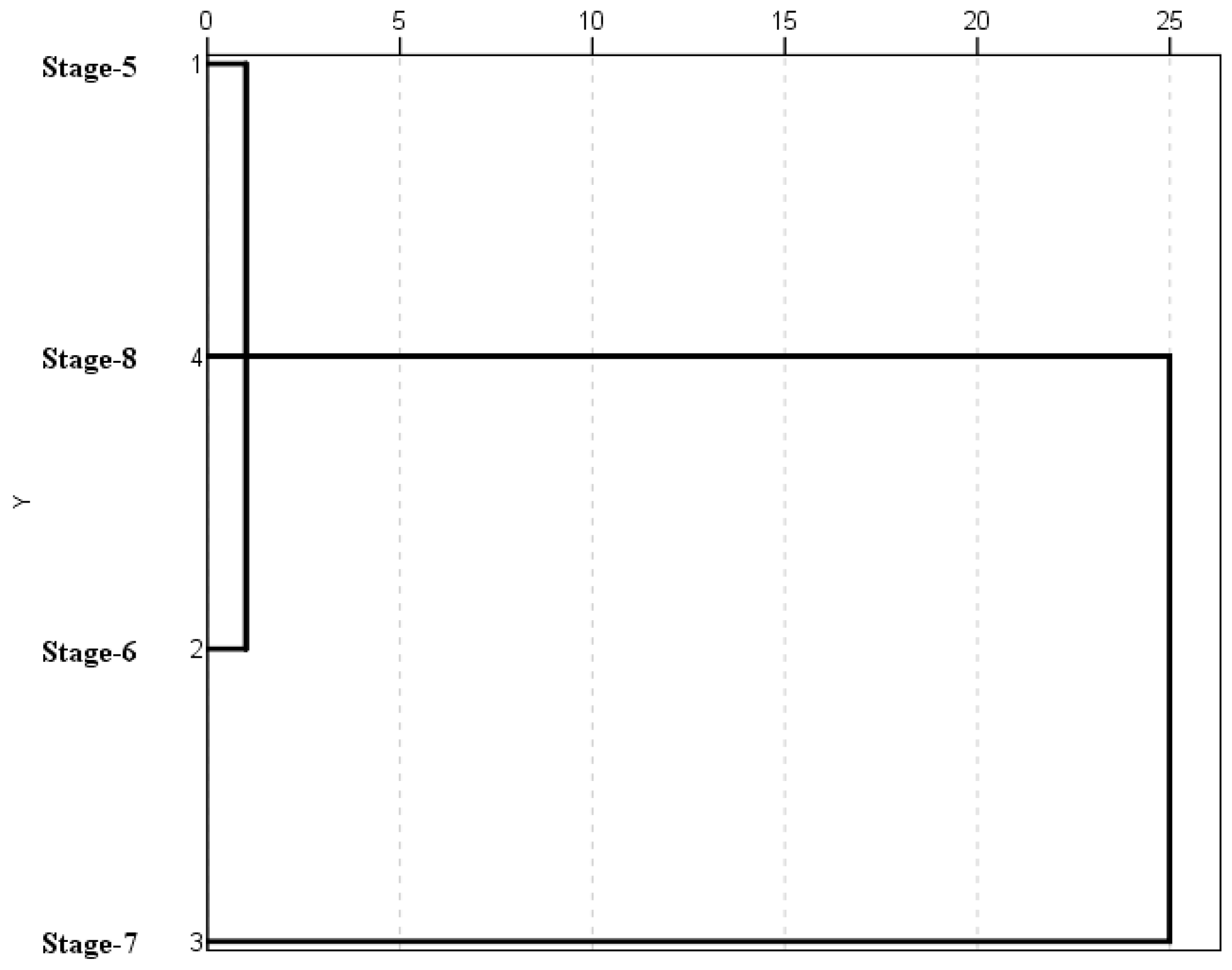
A cluster analysis applied to the spore and weather data on each phenological stage (Figure 4) was performed. Two sample groups were identified. The first clustered stage 5 (inflorescences development), stage 6 (flowering) and stage 8 (fruit ripening) which showed similar conditions. The second group separated stage 7 (fruit development) as consequence of their higher spore concentrations and high average values of maximum, minimum and average temperatures and dew point (Table 2 and Table 3).
Figure 4.
Cluster analysis applied to the spore and weather data.
Furthermore, the influence of the main weather variables on airborne spore presence in each stage was also statistically assessed by means a Spearman correlation test. In the case of Fusicladium a positive correlation coefficient (p < 0.01) was detected between spores and maximum, average and minimum temperatures, dew point and wind speed during stage 7 (fruit development). In contrast, a negative correlation, with the same signification level was observed with relative humidity (p < 0.05) and rainfall. During stage 8 (fruit ripening) a positive correlation (p < 0.05) was registered with maximum temperature whereas with a negative sing and the same signification level with minimum temperature and rainfall. The stronger Spearman correlation for Colletotrichum was observed during the first studied stages (stage 5 and 8). During stage 5 (inflorescences development) a positive correlation coefficient (p < 0.01) was detected between Colletotrichum spores and maximum and average temperatures, and with negative sing (p < 0.05) with relative humidity. In stage 6 (flowering) a negative correlation was detected with maximum (p < 0.01) and average temperature (p < 0.05). During stage 7 (fruit development) a positive correlation coefficient (p < 0.01) was observed between the spores and wind speed. Finally, during stage 8 (fruit ripening) a positive correlation coefficient (p < 0.05) was obtained between Colletotrichum spores and minimum temperature, and negative with maximum temperature. In the case of Pseudocercospora a negative correlation coefficient (p < 0.05) was obtained between the spore concentrations and wind speed in stages 5 (inflorescences development) and 6 (flowering). In stage 8 (fruit ripening) a positive correlation (p < 0.01) was observed between Pseudocercospora spores and rainfall, and negative (p < 0.05) with maximum temperature (Table 4).

Table 4.
Spearman’s correlation between three fungal spore’s concentration and weather parameters in each phenological stage (Stage 5 (S-5): Inflorescence emergence; Stage 6 (S-6): Flowering; Stage 7 (S-7): Fruit development; Stage 8 (S-8): Maturity of fruit). Signification level (* p < 0.05, ** p < 0.01).
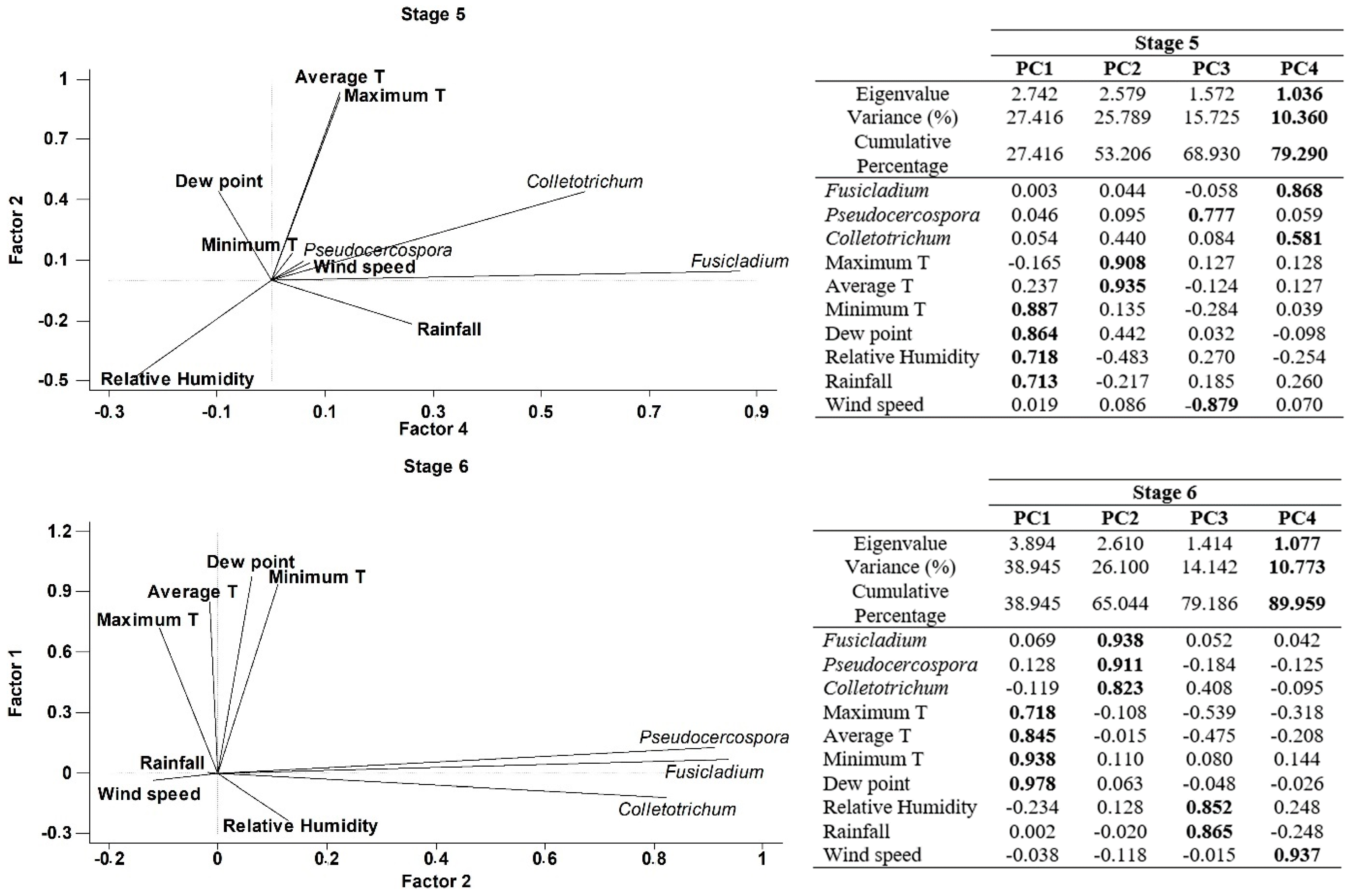
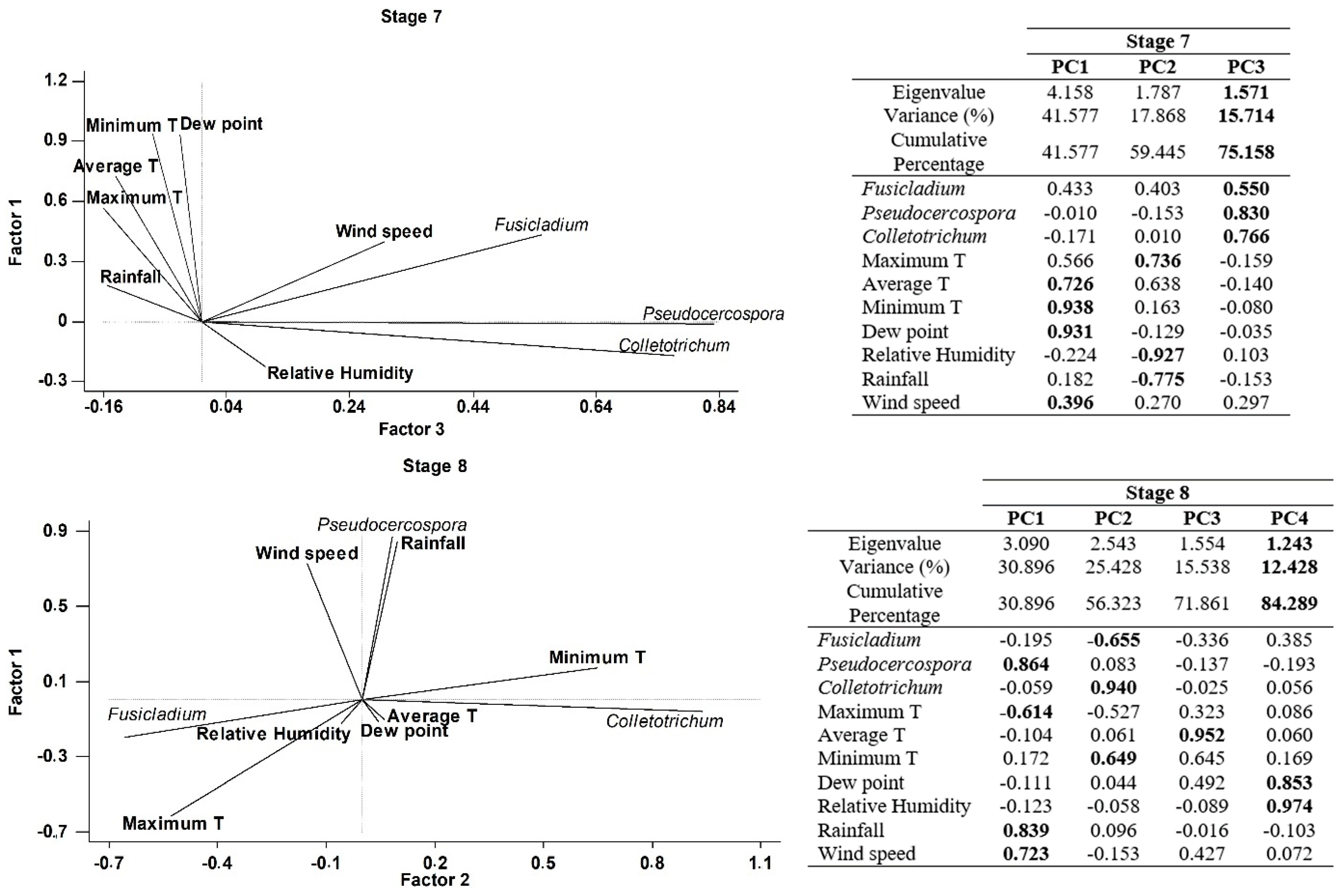
Finally, a Principal component analysis (PCA) was performed in order to better understand the joint influence of all meteorological parameters with the airborne spore concentrations on each phenological stages. Four principal components have been extracted on Stage 5 (inflorescences development), since they had eigenvalues greater than or equal to 1.0 and accounting together for around 79.29% of the variability in the original data (Figure 5). Most of the variables have significant positive loadings in the first four Principal Components (PCs). In general, the four PCs were correlated as follows: Component 1 (minimum temperature, dewpoint, relative humidity and rainfall), Component 2 (maximum and average temperatures), Component 3 (Pseudocercospora and Wind speed), Component 4 (Fusicladium and Colletrotrechum). In the case of the stage 6 (flowering), we have extracted four principal components accounting for around 89.96% of the variability in the original data (Figure 5). Generally, the four PCs were correlated as follows: Component 1 (maximum, averge and minimum temperatures and dewpoint), Component 2 (Fusicladium, Pseudocercospora and Colletotrechum), Component 3 (relative humidity and rainfall), Component 4 (wind speed). In stage 7 (fruit development) we extracted three principal components accounting for around 75.16% of the variability in the original data (Figure 5). As a general form, the three PCs were correlated as follows: Component 1 (average and minimum temperatures, dewpoint and wind speed), Component 2 (maximum temperature, relative humidity and rainfall) and Component 3 (Fusicladium, Pseudocercospora and Colletotrechum). Finally, four principal components have been extracted in stage 8 (fruit ripening), since they had eigenvalues greater than or equal to 1.0 and they accounted for together around 84.29% of the variability in the original data (Figure 5). In general, the four PCs were correlated as follows: Component 1 (Pseudocercospora, maximum temperature, rainfall and wind speed), Component 2 (Fusicladium, Colletrotrechum and minimum temperature), Component 3 (average temperature), Component 4 (dew point and relative humidity). In general, the results of PCA analysis show a high degree of positive association between the daily spore concentrations and the meteorological parameters (Figure 5).
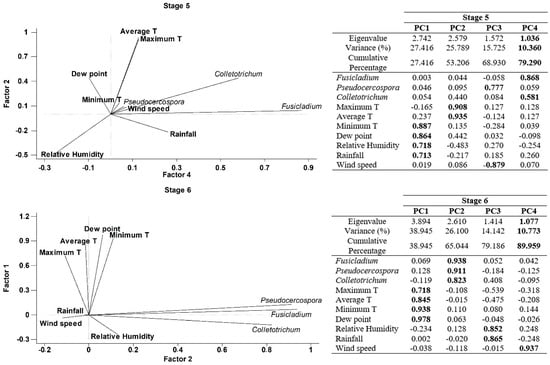
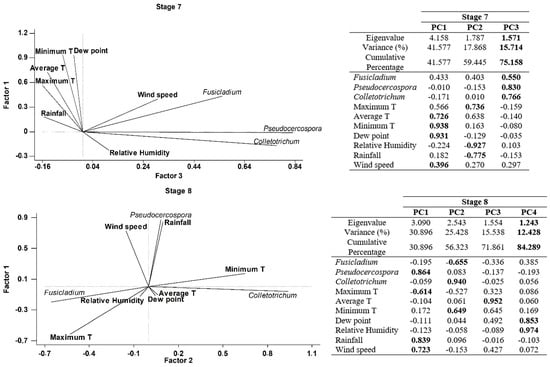
Figure 5.
Principal Component Analysis on each phenological stage, Stage 5: Inflorescence emergence; Stage 6: Flowering; Stage 7: Fruit development and Stage 8: Maturity of fruit for three studied fungus and the main weather variables. (PC1) Principal component one, (PC2) Principal component two, (PC3) Principal component three and (PC4) Principal component four. In bold the highest weights in each principal component.
4. Discussion
The knowledge of the ecological dispersion mechanisms of the phytopathogenic fungus in the crops has become a key tool to detect the risk of diseases in the plants. Therefore, the identification and quantification of the spore load in the atmosphere as bioindicators of pathogen presence, and their relationship with the meteorological and geographical data allows us to prevent the frequency and severity of diseases early [58]. Several studies detected a high degree of association between the aerial conidia concentration and the lesion density of the plants one week later, which shows the relation of disease development with spore concentrations in the atmosphere of the crop during previous periods [59,60,61]. The learning of the different ecological processes behavior of phytopathogenic fungus in the atmosphere of the crop is very important in regions where the cultivated area tend to the increase before the pressure of disease could be high. Mainly in areas located at the limit of the distribution of the olive cultivation, such as the study area where the boundary of the Eurosiberian and Mediterranean converge, and the effects of climate change could be more evident [62]. The impact caused by the imminent climate change in the future appearance of diseases in areas where they did not occur previously should be considered [58]. The increases in temperature and variations in precipitation can favor an increase in the proliferation of fungi and bacteria and, therefore, an increase in diseases that affect crops [63]. Infection, sporulation and dispersal processes of many of these species depends on humidity and temperature [64,65]. A large number of studies in this field have been conducted on different crops [66]. However, the number of them considering the cultivation of the olive tree is very limited which highlights the importance of our research. New findings about the life cycle and the temperature and humidity requirements for the main olive phytopathogenic fungi at the limit of the distribution of the tree were obtained.
The Fusicladium production of conidia requires conditions of high humidity (RH > 98%) accompanied by temperatures until 27 °C depending on the phenological phase [37]. However, the conidia can remain dormant on the leaf for several months, with characteristic spots appearing after 15–20 min from their germination. In our study, the highest Fusicladium spore concentrations were detected in stage 7 (fruit development) also showing high values in stage 6 (flowering) as other authors pointed out [6]. High levels of infection on leaves before flowering can induce flower drop and poor fruiting [67].
Colletotrichum conidia remain dormant at low winter temperatures in “mummified” olives that have remained on the tree for about a year (more rarely on the branches). With spring rains and optimal temperatures (15–25 °C) they separate from the conidium and are dispersed by raindrops to new inflorescences. The infection begins at the peduncle of the fruit (an area where dew water drops accumulate) and affects the base of the fruit. In a few days the first symptoms appear as they penetrate the epidermis of the young fruits and in conditions of high humidity. Colletotrichum spores need a lower amount of heat and can occur in spring-early summer if there is precipitation and enough humidity [34]. Moreover, in the wet autumns the conidia reactivate and exudes a pinkish jelly (soapy appearance) with a large number of conidia that reinitiate the infection. In our study the Colletotrichum higher spore concentrations were registered during stage 7 (fruit development). Previous studies also reported an increase of the incidence of affected fruit by this fungus from late autumn to winter [68] during the months of October to December under Mediterranean conditions [69].
The Pseudocercospora life cycle takes place at the same time that the leaf spot produced by F. oleagineum and is the less studied of all three fungi. In general, it is quite similar to the “repilo” cycle (Fusicladium oleagineum), with foliar and fruit involvement. The cycle includes survival in the soil as a chlamydospore and a long incubation period as a biotroph in the epidermis of the leaf after the anticipated fall of the leaf caused by the fungus. The range of temperatures in which the development of the pathogen is favored are 5–30 °C. In the present study, Pseudocercospora appears in greater quantity in the stage 7 showing a similar tendency to Fusicladium but with a lower total number of spores, which coincides with previous studies that show a life cycle of both fungi occurring at the same time [1].
Regarding the presence of spores throughout the day, we observe a repeated trend for Fusicladium and Colletotrichum, with a lower number of spores during the first hours of the day that gradually increases until reaching the maximum in the bi-hourly band of 11 to 12 a.m. From this moment, the tendency is to a decrease of the spore concentrations. On the other hand, the highest Pseudocercospora concentrations were in the afternoon, between 17 and 18 h. Several authors pointed higher spore concentration during diurnal hours for other phytopathogenic spores as Alternaria and Penicillium airborne spores [70] or Curvularia [71] in cultivations of Cuba. In crops such as Vitis vinifera, the main phytopathogens such as Botrytis and Erysiphe registered the highest concentrations in the early afternoon while in the case of Plasmopara at dawn [72].
The presence of fungal spores in the atmosphere is strongly influenced by the meteorological parameters. The results obtained showed that at the beginning of the olive tree cycle (stage 5) a period of abundant rainfall and relatively low temperatures took place, so the presence of spores is low, as it was noted for studies conducted by Nigro et al. [1]. Afterward, a decrease in rainfall and an increase in temperatures takes place, causing the favorable conditions for the development of the conidia, inducing the maximum concentrations of spores during stage 7. Our results are consistent with previous studies in which it is noted that the presence of water (but without high rainfall) and moderately high temperatures (around 25 °C) favor the infection of the studied fungus [9,37,44]. It would be interesting to conduct more studies focused on this subject in order to compare our data with the obtained in different places with different climate and biogeographical conditions. In order to better understand the associations between the main phytopathogenic spores of the olive tree and the meteorological variables, a Spearman correlation analysis was conducted. Fusicladium showed significant and positive correlations with the wind and the temperature related variables during stages 7 and 8, while negative with the water related variables. In the case of Colletotrichum we found significant and positive correlations with the temperatures during stages 5 and 8 and wind speed during stage 7. The correlation with the temperature of the months of April and May has been previously noted, being associated with a higher incidence of the disease in late winter and early spring [73]. During April and May, olive trees are in the stages between flowering and fruit set, and therefore no susceptible fruits are present on the trees. High temperatures registered during this period induce an early ripening of the fruit. Several authors noted that early fruit ripening increases the infection susceptibly during the months of October and November when conditions are favorable for successive anthracnose attacks [74]. Colletotrichum spores need a lower amount of heat, rainfall and relative humidity in spring-early summer [73]. Pseudocercospora showed significant and positive correlations with rainfall and negative with the temperature related variables during stage 8 and wind speed in stages 5 and 6. These results are according with the data pointed by several authors, suggesting a high P. cladosporioides in vitro activity between 5 and 35 °C, and mild temperatures in natural conditions [44]. This finding is in concordance with the annual seasons (autumn and winter) in which the pathogen causes infection under Mediterranean conditions [75]. In other crops such as Vitis vinifera, phytopathogenic spores such as Botrytis, Erysiphe or Plasmopara also showed significative and positive correlations with temperatures and negatives with relative humidity and rainfall [72]. Finally, Principal component analysis (PCA) was performed in order to better understand the influence of all weather variables in conjunction with the three fungal spore concentrations on each stage studied. Largely, the spore concentrations showed a positive correlation with temperatures and relative humidity and negative relation with rainfall and wind speed. The similar behavior has been pointed out by several author in other environments, such as for Aspergillus/Penicillium airborne spores [70] or other phytopathogen spores such as Botrytis, Erysiphe or Plasmopara in Vitis vinifera crops [76].
5. Conclusions
The duration of the olive reproductive cycle from stage 5 (inflorescence development) to stage 8 (fruit maturation) registered 210 days in a placed situated between the Atlantic and Mediterranean biogeographical region. The total Fusicladium spore concentration in the atmosphere of the grove was the double than the registered for the Colletotrichum and Pseudocercospora fungus. The hours of higher abundance of spores during the day were from 11:00 to 12:00 h for Fusicladium and Colletotrichum and yet from 17:00 to 18:00 h for Pseudocercospora. The Spearman correlation and PCA analysis showed a high degree of positive association between spore concentrations and temperatures and relative humidity during stages 5 and 6, between spore concentrations and wind speed and relative humidity in stage 7 and finally during stage 8 between Fusicladium and maximum temperature and relative humidity, Colletotrichum and average temperature and dew point, and Pseudocercospora and rainfall. The combination of meteorological, phenological and aerobiological parameters is a useful tool to understand the ecological behavior of the considered phytopathogenic fungal spores in order to develop futures strategies for the integrated management of fungal olive diseases in areas at the limit of the tree distribution.
Author Contributions
Conceptualization, M.F.-G. and F.J.R.-R.; methodology, M.F.-G., E.G.-F. and F.J.R.-R.; formal analysis, A.G., M.F.-G., M.A.-C., E.G.-F. and L.C.; data curation, A.G., J.A.C.R., E.G.-F. and L.C.; writing—original draft preparation, A.G. and M.F.-G.; writing—review and editing, E.G.-F., J.A.C.R., M.A.-C., L.C., M.F.-G. and F.J.R.-R.; supervision, M.F.-G. and F.J.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nigro, F.; Antelmi, I.; Sion, V. Integrated control of aerial fungal diseases of olive. Acta Hortic. 2018, 327–332. [Google Scholar] [CrossRef]
- Galán, C.; García-Mozo, H.; Vázquez, L.; Ruiz-Valenzuela, L.; Díaz de la Guardia, C.; Trigo, M.M. Heat requirement for the onset of the Olea europaea L. pollen season in several sites of Andalusia and the effect of the expected future climate change. Int. J. Biometeorol. 2005, 49, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Fernández-González, M.; Vázquez-Ruiz, R.A.; Rodríguez-Rajo, F.J.; Aira, M.J. Reproductive Biology of Olive Trees (Arbequina cultivar) at the Northern Limit of Their Distribution Areas. Forests 2021, 12, 204. [Google Scholar] [CrossRef]
- Graniti, A. Olive scab: A review. EPPO Bull. 1993, 23, 377–384. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Armengol, J.; Vittorio, R. Biology and Epidemiology of Venturia Species Affecting Fruit Crops: A Review. Front. Plant Sci. 2017, 8, 1496. [Google Scholar] [CrossRef]
- Viruega, J.R.; Moral, J.; Roca, L.F.; Navarro, N.; Trapero, A. Spilocaea oleagina in Olive groves of Southern Spain: Survival, Inoculum Production, and Dispersal. Plant Dis. 2013, 97, 1549–1556. [Google Scholar] [CrossRef]
- López-Doncel, L.M.; Viruega, J.R.; Trapero-Casas, A. Respuesta del olivo a la inoculación con Spilocaea oleagina. Bol. Sanid. Veg. Plagas 2000, 26, 349–363. [Google Scholar]
- Cacciola, S.; Faedda, R.; Sinatra, F.; Agosteo, G.; Schena, L.; Frisullo, S.; Magnano di San Lio, G. Olive anthracnose. J. Plant Pathol. 2012, 94, 29–44. [Google Scholar]
- Brancher, N.; Pérez, B.A.; Matías, C.; Otero, L.; Oriolani, E.; Aybar, V.E.; Roca, M. Olive (Olea europea L.) pathologies and pests in Catamarca province, Argentina. Acta Hortic. 2008, 949, 317–321. [Google Scholar] [CrossRef]
- Duarte, H.S.S.; Cabral, P.G.C.; Pereira, O.L.; Zambolim, L.; Gonçalves, E.D.; Vieira Neto, J.; Zambolim, E.M.; Sergeeva, V. First report of anthracnose and fruit mummification of olive fruit (Olea europaea) caused by Colletotrichum acutatum in Brazil. Plant Pathol. 2010, 59, 1170. [Google Scholar] [CrossRef]
- Acosta, D.R. Investigaciones fitopatológicas. Min. Ind. Dir. Agron. Publ. Mens. 1932, 4, 1–18. [Google Scholar]
- Pontis, R.E.; Hansen, H.N. Olive anthracnose in the United States. Phytopathology 1942, 32, 642–644. [Google Scholar]
- Bompeix, G.; Julio, E.V.R.; Phillips, D.H. Glomerella cingulate (Stoneman) Spaulding et V. Schrenk. In European Handbook of Plant Diseases; Smith, I.M., Dunez, J., Lelliot, R.A., Phillips, D.H., Archer, S.A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1988; pp. 325–327. [Google Scholar]
- Iliadi, M.K.; Tjamos, E.; Antoniou, P.; Tsitsigiannis, D.I. First report of Colletotrichum acutatum causing anthracnose on olives in Greece. Plant Dis. 2018, 102, 820. [Google Scholar] [CrossRef]
- Agosteo, G.E.; Magnano di San Lio, G.; Frisullo, S.; Cacciola, S.O. Characterisation of the causal agent of olive anthracnose in southern Italy. Acta Hortic. 2002, 586, 713–716. [Google Scholar] [CrossRef]
- Mosca, S.; Li Destri Nicosia, M.G.; Cacciola, S.O.; Schena, L. Molecular analysis of Colletotrichum species in the carposphere and phyllosphere of olive. PLoS ONE 2014, 9, e114031. [Google Scholar] [CrossRef]
- Talhinhas, P.; Neves-Martins, J.; Oliveira, H.; Sreenivasaprasad, S. The distinctive population structure of Colletotrichum species associated with olive anthracnose in the Algarve region of Portugal reflects a host–pathogen diversity hot spot. FEMS Microbiol. Lett. 2009, 296, 31–38. [Google Scholar] [CrossRef]
- Talhinhas, P.; Mota-Capitao, C.; Martins, S.; Ramos, A.P.; Neves-Martins, J.; Guerra-Guimaraes, L.; Várzea, V.; Silva, M.C.; Sreenivasaprasad, S.; Oliveira, H. Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal. Plant Pathol. 2011, 60, 483–495. [Google Scholar] [CrossRef]
- Talhinhas, P.; Gonçalves, E.; Sreenivasaprasad, S.; Oliveira, H. Virulence diversity of anthracnose pathogens (Colletotrichum acutatum and C. gloeosporioides complexes) on eight olive cultivars commonly grown in Portugal. Eur. J. Plant Pathol. 2015, 142, 73–83. [Google Scholar] [CrossRef]
- Martín, M.P.; García-Figueres, F. Colletotrichum acutatum and C. gloeosporioides cause anthracnose on olives. Eur. J. Plant Pathol. 1999, 105, 733–741. [Google Scholar] [CrossRef]
- Vucinic, Z.; Latinovic, J.; Metzidakis, I.T.; Voyiatzis, D.G. Colletotrichum gloeosporioides, a new olive (Olea europaea L.) parasite in Yugoslavia. Acta Hortic. 1999, 474, 577–579. [Google Scholar] [CrossRef]
- Gorter, G.J.M.A. Anthracnose fungi of olives. Nature 1956, 178, 1129–1130. [Google Scholar] [CrossRef]
- Achbani, E.A.; Benbouazza, A.; Douira, A. First report of olive anthracnose, caused by Colletotrichum gloeosporioides, in Morocco. Atlas J. Biol. 2013, 2, 171–174. [Google Scholar] [CrossRef]
- Chattaoui, M.; Raya, M.C.; Bouri, M.; Moral, J.; Perez-Rodriguez, M.; Trapero, A.; Msallem, M.; Rhouma, A. Characterization of a Colletotrichum population causing anthracnose disease on olive in northern Tunisia. J. Appl. Microbiol. 2016, 120, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, A.; Triki, M.A.; Msallem, M. First report of olive anthracnose caused by Colletotrichum gloeosporioides in Tunisia. Phytopathol. Mediterr. 2010, 49, 95–98. [Google Scholar]
- Embaby, E.-S. Anthracnose disease (Colletotrichum sp.) affecting olive fruit quality and its control in Egypt. J. Agric. Technol. 2014, 10, 1289–1306. [Google Scholar]
- Margarita, L.; Porta-Puglia, A.; Quacquarelli, A. Colletotrichum acutatum, nuovo patogeno dell’olivo in Cina e confronto con l’agente della “lebbra” dell’olivo. Ann. Ist. Sperim. Patol. Veg. 1986, 11, 125–133. [Google Scholar]
- Hemmi, T.; Kurata, S. Contributions to the knowledge of anthracnose of plants II, on Gloeosporium olivarum Alm. causing the olive anthracnose. J. Soc. Trop. Agric. Taiwan 1935, 6, 573–583. [Google Scholar]
- Sanei, S.J.; Razavi, S.E. Differentiation of olive Colletotrichum gloeosporioides populations on the basis of vegetative compatibility and pathogenicity. Afr. J. Agric. Res. 2011, 6, 2099–2107. [Google Scholar]
- Sanei, S.J.; Razavi, S.E. Survey of olive fungal disease in north of Iran. Annu. Rev. Res. Biol. 2012, 2, 27–36. [Google Scholar]
- Sharma, R.L.; Kaul, J.L. Field evaluation of fungicide for control of olive anthracnose. Indian J. Mycol. Plant Pathol. 1990, 20, 185–187. [Google Scholar]
- Schena, L.; Mosca, S.; Cacciola, S.O.; Faedda, R.; Sanzani, S.M.; Agosteo, G.E.; Sergeeva, V.; Magnano di San Lio, G. Species of the Colletotrichum gloeosporioides and C. boninense complexes associated with olive anthracnose. Plant Pathol. 2014, 63, 437–446. [Google Scholar] [CrossRef]
- Whitelaw-Weckert, M.; Curtin, S.J.; Huang, R.; Steel, C.C.; Blanchard, C.L.; Roffey, P.E. Phylogenetic relationships and pathogenicity of Colletotrichum acutatum isolates from grape in subtropical Australia. Plant Pathol. 2007, 56, 448–463. [Google Scholar] [CrossRef]
- Talhinhas, P.; Loureiro, A.; Oliveira, H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018, 19, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Moral, J.; Bouhmidi, K.; Trapero, A. Caracterización morfológica y cultural de aislados de Colletotrichum spp. Causantes de la antracnosis del olivo. Bol. Sanid. Veg. Plagas 2005, 31, 531–548. [Google Scholar]
- Moral, J.; Xaviér, C.; Roca, L.F.; Romero, J.; Moreda, W.; Trapero, A. La Antracnosis del olivo y su efecto en la calidad del aceite. Grasas Y Aceites 2014, 65, e028. [Google Scholar] [CrossRef]
- Castellar-Sánchez, M.A. Plagas y enfermedades del olivo (Olea europaea). Prot. Masas For. 2011, 1, 1–20. [Google Scholar]
- Moral, J.; Ávila, L.M.; López-Doncel, M.; Alsalimiya, R.; Oliveira, F.; Gutiérrez, N.; Benali, A.; Roca, L.F.; Navarro, N.; Bouhmidi, K.; et al. Resistencia a los repilos de distintas variedades de olivo. Vida Rural 2005, 208, 34–41. [Google Scholar]
- Nigro, F.; Ippolito, A.; Gallone, P.; Romanazzi, G.; Carmignano, P.; Laccone, G. Cercosporiosis of olive in Apulia and attempts to control the disease. Acta Hortic. 2002, 586, 773–776. [Google Scholar] [CrossRef]
- Govi, G. La Cercosporiosis o Piombatura dell’Olivo. Ann. Sper. Agrar. 1952, 6, 69–80. [Google Scholar]
- Modugno Pettinari, C. Istopatologia causata dal parassitismo di Cercospora cladosporioides Sacc. su olivo. Boll. Della Stn. Patol. Veg. Roma S. III 1960, 18, 65–77. [Google Scholar]
- Nigro, F.; Ferrara, M. Olive cercosporiosis. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Research Signpost: Thiruvananthapuram, India, 2011; pp. 247–258. [Google Scholar]
- Ávila, A.; Groenewald, J.Z.; Trapero, A.; Crous, P.W. Characterisation and epitypification of Pseudocercospora cladosporioides, the causal organism of Cercospora leaf spot of olives. Mycol. Res. 2005, 109, 881–888. [Google Scholar] [CrossRef]
- Ávila, A.; Romero, J.; Agusti-Brisach, C.; Benali, A.; Roca, L.F.; Trapero, A. Phenotypic and pathogenic characterization of Pseudocercospora cladosporioides, causal agent of cercospora leaf spot of olives. Eur. J. Plant Pathol. 2020, 156, 45–65. [Google Scholar] [CrossRef]
- Chen, M.-M. Forest Fungi Phytogeography: Forest Fungi Phytogeography of China, North America, and Siberia and International Quarantine of Tree Pathogens; Pacific Mushroom Research and Education Center: Sacramento, CA, USA, 2002; 469p. [Google Scholar]
- Henricot, B.; Gorton, C.; Denton, J.; Denton, G. Pseudocercospora cladosporioides, the cause of leaf spot on olive, a pathogen new to the United Kingdom. Plant Pathol. 2009, 58, 803. [Google Scholar] [CrossRef]
- Holevas, C.D.; Chitzanidis, A.; Pappas, A.C.; Tzamos, E.C.; Psallidas, P.G.; Alivizatos, A.; Panagopoulos, C.; Kyriakopoulou, P.; Bem, F.; Lascaris, D.; et al. Disease agents of cultivated plants observed in Greece from 1981 to 1990. Ann. De L’institut Phytopathol. Benaki 2000, 19, 1–96. [Google Scholar]
- Kamal. Cercosporoid Fungi of India; Bishen Singh Mahendra Pal Singh: Dehra Dun, India, 2010; 351p. [Google Scholar]
- Kobayashi, T. Index of Fungi Inhabiting Woody Plants in Japan-Host, Distribution and Literature; Zenkoku-Noson-Kyoiku Kyokai Publishing Co.: Tokio, Japan, 2007; 1227p. [Google Scholar]
- McKenzie, E.H.C. New plant disease records in New Zealand: Miscellaneous fungal pathogens II. N. Z. J. Crop Hortic. Sci. 1990, 18, 65–73. [Google Scholar] [CrossRef]
- Ávila, A.; Benali, A.; Trapero, A. El emplomado del olivo, una grave enfermedad poco conocida. Vida Rural 2004, 198, 32–36. [Google Scholar]
- Ávila, A.; Trapero, A. El Emplomado del Olivo y del Acebuche; Consejería de Medio Ambiente de la Junta de Andalucía: Sevilla, Spain, 2010; p. 8. [Google Scholar]
- Orriols, I.; Vázquez, I.; Losada, A. Variedades gallegas. Terruños 2006, 16, 21–22. [Google Scholar]
- Galán, C.; Cariñanos, P.; Alcázar, P.; Domínguez, E. Spanish Aerobiology Network (REA): Management and Quality Manual; University of Córdoba Publication Service: Córdoba, Spain, 2007; p. 61. [Google Scholar]
- Galán, C.; Ariatti, A.; Bonini, M.; Clot, B.; Crouzy, B.; Dahl, A.; Fernandez-González, D.; Frenguelli, G.; Gehrig, R.; Isard, S.; et al. Recommended terminology for aerobiological studies. Aerobiologia 2017, 33, 293–295. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants, BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Yacer, G.; Meier, V. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- González-Fernández, E.; Kennedy, R.; Osborn, R.; Fernández-González, M.; Rodríguez-Rajo, F.J. Botrytis cinerea airborne conidia and their germination ability assessed by immunological methods in a NW Spain vineyard. Agronomy 2021, 11, 1441. [Google Scholar] [CrossRef]
- Carisse, O.; Savary, S.; Willocquet, L. Spatiotemporal relationships between disease development and airborne inoculum in unmanaged and managed Botrytis leaf blight epidemics. Phytopathology 2008, 98, 38–44. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, E.; Piña-Rey, A.; Fernández-González, M.; Aira, M.J.; Rodríguez-Rajo, F.J. Identification and evaluation of the main risk periods of Botrytis cinerea infection on grapevine based on phenology, weather conditions and airborne conidia. J. Agric. Sci. 2020, 158, 88–98. [Google Scholar] [CrossRef]
- Fernández-González, M.; Piña-Rey, A.; González-Fernández, E.; Aira, M.J.; Rodríguez-Rajo, F.J. First assessment of Goidanich Index and aerobiological data for Plasmopara viticola infection risk management in north-west Spain. J. Agric. Sci. 2019, 157, 129–139. [Google Scholar] [CrossRef]
- Piña-Rey, A.; González-Fernández, E.; Fernández-González, M.; Lorenzo, M.N.; Rodríguez-Rajo, F.J. Climate change impacts assessment on wine-growing bioclimatic transition areas. Agriculture 2020, 10, 605. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Barzman, M.; Booij, K.; Boonekamp, P.; Desneux, N.; Huber, L.; Kudsk, P.; Langrell, S.R.H.; Ratnadass, A.; Ricci, P.; et al. Robust cropping systems to tackle pests under climate change. Agron. Sustain. Dev. 2015, 35, 443–459. [Google Scholar] [CrossRef]
- Huber, L.; Gillespie, T.J. Modeling Leaf Wetness in Relation to Plant Disease Epidemiology. Ann. Rev. Phytopathol. 1992, 30, 553–577. [Google Scholar] [CrossRef]
- Juroszek, P.; von Tiedemann, A. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Huang, C.; Li, N.; Zhang, Z.; Liu, Y.; Chen, X.; Wang, F.; Chen, Q. What Is the Consensus from Multiple Conclusions of Future Crop Yield Changes Affected by Climate Change in China? Int. J. Environ. Res. Public Health 2020, 17, 9241. [Google Scholar] [CrossRef]
- Sergeeva, V.; Braun, U.; Spooner-Hart, R.; Nair, N.G. Observations on spot caused by Fusicladium oleagineum on olives (Olea europaea) in New South Wales, Australia. Australas. Plant Dis. Notes 2009, 4, 26–28. [Google Scholar]
- Moral, J.; Trapero, A. Mummified fruit as a source of inoculums and disease dynamics of olive anthracnose caused by Colletotrichum spp. Phytopathology 2012, 102, 982–989. [Google Scholar] [CrossRef]
- Schena, L.; Abdelfattah, A.; Mosca, S.; Destri Nicosia, M.G.; Agosteo, G.E.; Cacciola, S.O. Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. Eur. J. Plant Pathol. 2017, 149, 337–347. [Google Scholar] [CrossRef]
- Almaguer, M.; Fernández-González, M.; Díaz, L.; Sánchez, K.C.; Rodríguez-Rajo, F.J.; Aira, M.J. Aspergillus and Penicillium spores as urban pathogens of the Havana atmosphere, Cuba. Aerobiologia 2021, 37, 767–783. [Google Scholar] [CrossRef]
- Almaguer, M.; Fernández-González, M.; Díaz, L.; Sánchez, K.C.; Rodríguez-Rajo, F.J.; Aira, M.J. Assessment of airborne Curvularia propagules in the atmosphere of Havana, Cuba. Aerobiologia 2021, 37, 53–69. [Google Scholar] [CrossRef]
- Fernández-González, M.; Rodríguez-Rajo, F.J.; Jato, V.; Aira, M.J. Incidence of fungals in a vineyard of the Denomination of Origin Ribeiro (Ourense—north -western Spain). Ann. Agric. Environ. Med. 2009, 16, 263–271. [Google Scholar]
- Romero, J.; Moral, J.; Gonzalez-Dominguez, E.; Agustí-Brisach, C.; Roca, L.F.; Rossi, V.; Trapero, A. Logistic models to predict olive anthracnose under field conditions. Crop Prot. 2021, 148, 105714. [Google Scholar] [CrossRef]
- Moral, J.; Oliveira, R.; Trapero, A. Elucidation of the disease cycle of olive anthracnose caused by Colletotrichum acutatum. Phytopathology 2009, 99, 548–556. [Google Scholar] [CrossRef]
- Del Moral, J.; Medina, D. El “repilo plomizo” del olivo causado por Cercospora cladosporioides Sacc., enfermedad presente en España. Bol. Sanid. Veg. Plagas 1985, 11, 31–36. [Google Scholar]
- Cortiñas Rodríguez, J.A.; González-Fernández, E.; Fernández-González, M.; Vázquez-Ruiz, R.A.; Aira, M.J. Fungal Diseases in Two North-West Spain Vineyards: Relationship with Meteorological Conditions and Predictive Aerobiological Model. Agronomy 2020, 10, 219. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).