Bunch Weight Determination in Relation to the Source-Sink Balance in 12 Cavendish Banana Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Growing Conditions
2.2. Cultivars
- Dwarf type: These are the most cultivated cultivars in the subtropics, especially in the Canary Islands. We monitored the Dwarf Cavendish ‘DC-01’ cultivar in this study. This cultivar was selected for its high fruit number and its high bunch weight in subtropical climate, where it exhibited the highest bunch weight compared to some Giant cultivars (unpublished data).
- Giant type: These are major cultivars cultivated for world export, including the mainstreamed cultivars ‘Grande Naine’ and ‘Williams’. For this study, we monitored nine commercial Giant cultivars that were selected in ‘Grande Naine’ populations: ‘Gua-01’ (considered as the standard ‘Grande Naine’); ‘Gua-02’; ‘Mat-01’; ‘Mat-02’; ‘Mat-03’; ‘Mat-11’; ‘Mat-12’; ‘Cot-01’ and ‘Ruby’. The latter ‘Ruby’ cultivar is nematode-tolerant and has shown resistance to TR4 disease under in vitro conditions.
- Robusta type: These were once major export cultivars that replaced the Gros Michel cultivar, which is highly susceptible to Panama disease. In many exporting areas, they have now been replaced by Giant Cavendish types, which are shorter and produce larger bunches. We monitored two cultivars: ‘Ame-01’, an American cultivar that used to be mostly cropped in Latin America, and ‘Poyo’ that used to be traditionally cultivated in the West Indies.
2.3. Measurements
2.3.1. Plant Phenology and Morphology
2.3.2. Bunch Characteristics
2.3.3. Bunch Weight Determination
2.4. Statistical Analysis
3. Results
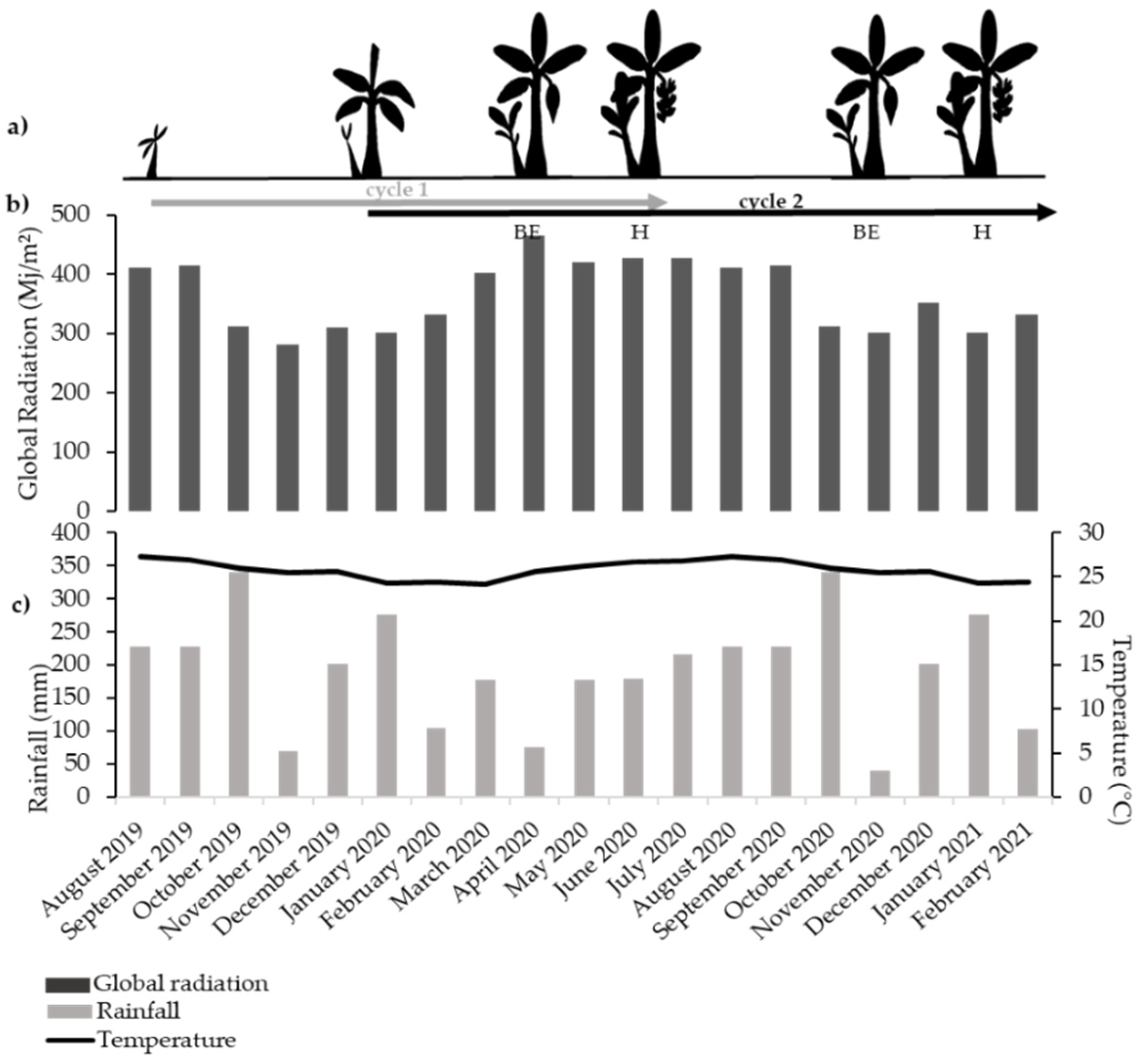
3.1. Climatic Conditions of the Experiment
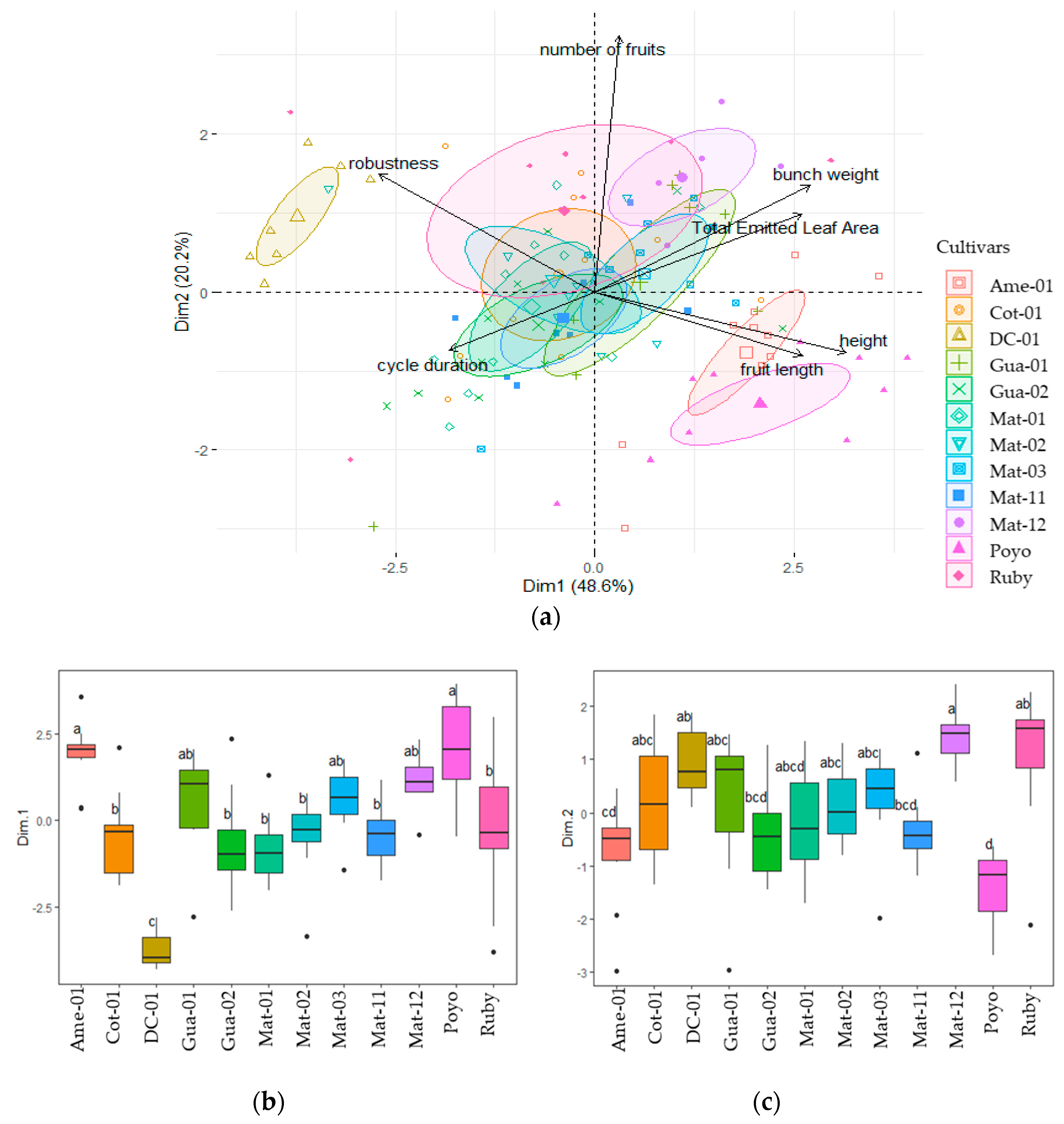
3.2. Multivariate Analysis of Descriptive Variables
3.2.1. First Cycle
3.2.2. Second Cycle
3.3. Bunch Characteristics and Cycle Duration
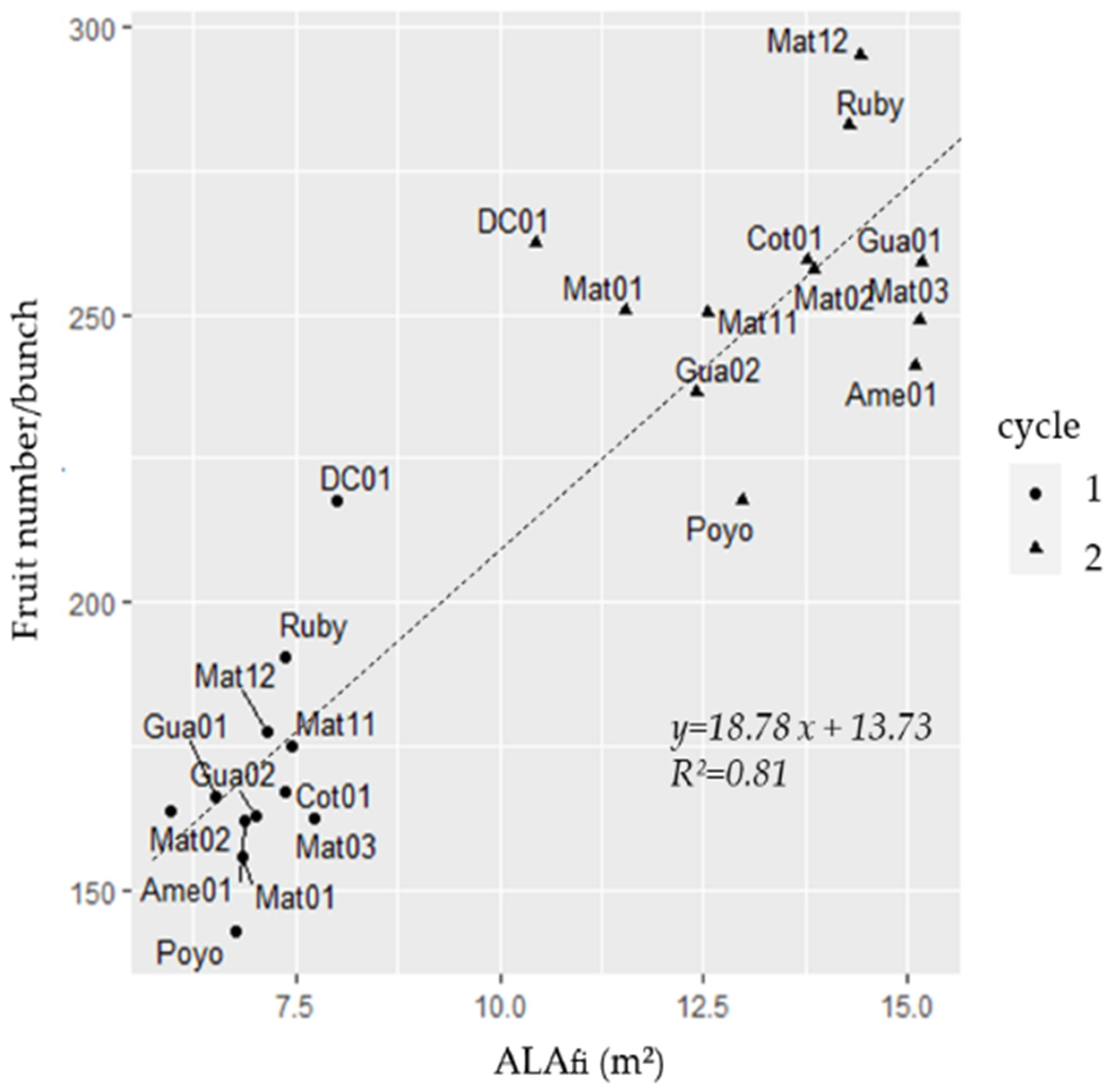
3.4. Fruit Number Determination According to the Fruit Number/ALAfi Ratio
3.5. Biomass Allocated to the Bunch: Source-Sink Balance, Carbohydrate Reserves and Bunch-Sucker Biomass Partitioning
4. Discussion
4.1. Bunch Weight in Source- vs. Sink-Limiting Conditions
4.2. Bunch Weight and Fruit Number Determination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, J.C.; Nel, D.J.; Eckstein, K. A Field Comparison of Ten Cavendish Sub-Group Banana Cultivars and Selections (Musa AAA) over Four Crop Cycles in the Subtropics. J. Hortic. Sci. 1993, 68, 511–521. [Google Scholar] [CrossRef]
- Stover, R.H. ’Valery’ and ’Grand Nain’: Plant and Foliage Characteristics and a Proposed Banana Ideotype. Trop. Agric. Trinidad 1982, 59, 303–306. [Google Scholar]
- Risède, J.-M.; Achard, R.; Brat, P.; Chabrier, C.; Damour, G.; Guillermet, C.; de Lapeyre, L.; Loeillet, D.; Lakhia, S.; Meynard, P.; et al. Chapitre 6. La Transition Agro-Écologique Des Systèmes de Culture de Bananes Cavendish Aux Antilles Françaises. In La Transition Agro-Ecologiqes des Agricultures du Sud; Agricultures et défis du monde; Éditions Quæ: Versailles, France, 2019; p. 368. [Google Scholar]
- Simmonds, N.W. Varietal Identification in the Cavendish Group of Bananas. J. Hortic. Sci. 1956, 29, 81–86. [Google Scholar] [CrossRef]
- Stover, R.H. Variation and Cultivar Nomenclature in Musa, AAA Group, Cavendish Subgroup. Fruits 1988, 43, 353–357. [Google Scholar]
- Cabrera Cabrera, J.; Galán Saúco, V. Evaluation of the Banana Cultivars Zelig, Grande Naine and Gruesa under Different Environmental Conditions in the Canary Islands. Fruits 2005, 60, 357–369. [Google Scholar] [CrossRef]
- Eckstein, K.; Fraser, C.; Husselmann, J.; Temple Murray, N. The Evaluation of Promising New Banana Cultivars. Acta Hortic. 1998, 490, 57–69. [Google Scholar] [CrossRef]
- Fonsah, E.G.; Adamu, C.A.; Okole, B.N.; Mullinix, B.G. Field Evaluation of Cavendish Banana Cultivars Propagated Either by Suckers or by Tissue Culture, over Six Crop Cycles in the Tropics. Fruits 2007, 62, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Galán Saúco, V.; Cabrera Cabrera, J.; Hernàndez, D.; Rodriguez Pastor, M.C. Evaluation of Medium-Height Cavendish Banana Cultivars under the Subtrpical Conditions of the Canary Islands. Acta Hortic. 1998, 490, 103–116. [Google Scholar] [CrossRef]
- Nuno, L.; Ribeiro, V.P.; Alcino da Silva, J. Preliminary Studies of Cavendish Banana Cultivars under the Edafoclimatic Conditions of Madeira Island. Acta Hortic. 1998, 490, 85–88. [Google Scholar] [CrossRef]
- Eckstein, K.; Robinson, J.C.; Davie, S.J. Physiological Responses of Banana (Musa AAA; Cavendish Sub-Group) in the Subtropics. III. Gas Exchange, Growth Analysis and Source-Sink Interaction over a Complete Crop Cycle. J. Hortic. Sci. 1995, 70, 169–180. [Google Scholar] [CrossRef]
- Jullien, A.; Munier-Jolain, N.G.; Malézieux, E.; Chillet, M.; Ney, B. Effect of Pulp Cell Number and Assimilate Availability on Dry Matter Accumulation Rate in a Banana Fruit [Musa sp. AAA Group ‘Grande Naine’ (Cavendish Subgroup)]. Ann. Bot. 2001, 88, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Ganry, J. Le développement du bananier en relation avec les facteurs du milieu: Action de la température et du rayonnement d’origine solaire sur la vitesse de croissance des feuilles. Etude du rythme de développement de la plante. Fruits 1980, 35, 727–743. [Google Scholar]
- FAO. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Ganry, J. Détermination “in situ” du stade de transition entre la phase végétative et la phase florale chez le bananier, utilisant le “coefficient de vitesse de croissance des feuilles”. Essai d’interprétation de quelques processus de développement durant la période florale. Fruits 1977, 32, 373–386. [Google Scholar]
- Robinson, J.C.; Nel, D.J. Comparative Morphology, Phenology and Production Potential of Banana Cultivars “Dwarf Cavendish” and Williams’ in the Eastern Transvaal Lowveld. Sci. Hortic. 1985, 25, 149–161. [Google Scholar] [CrossRef]
- Stevens, B.; Diels, J.; Brown, A.; Bayo, S.; Ndakidemi, P.A.; Swennen, R. Banana Biomass Estimation and Yield Forecasting from Non-Destructive Measurements for Two Contrasting Cultivars and Water Regimes. Agronomy 2020, 10, 1435. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Li, T.; Heuvelink, E.; Marcelis, L.F.M. Quantifying the Source–Sink Balance and Carbohydrate Content in Three Tomato Cultivars. Front. Plant Sci. 2015, 6, 416. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Rawat, V.; Rawat, J.M.S.; Tomar, Y.K. Effect of Pruning Intensity on Peach Yield and Fruit Quality. Sci. Hortic. 2010, 125, 218–221. [Google Scholar] [CrossRef]
- Pallas, B.; Mialet-Serra, I.; Rouan, L.; Clément-Vidal, A.; Caliman, J.-P.; Dingkuhn, M. Effect of Source/Sink Ratios on Yield Components, Growth Dynamics and Structural Characteristics of Oil Palm (Elaeis Guineensis) Bunches. Tree Physiol. 2013, 33, 409–424. [Google Scholar] [CrossRef] [Green Version]
- Proulx, R.A.; Naeve, S.L. Pod Removal, Shade, and Defoliation Effects on Soybean Yield, Protein, and Oil. Agron. J. 2009, 101, 971–978. [Google Scholar] [CrossRef]
- Zhang, H.; Flottmann, S. Source-Sink Manipulations Indicate Seed Yield in Canola Is Limited by Source Availability. Eur. J. Agron. 2018, 96, 70–76. [Google Scholar] [CrossRef]
- Daniells, J.W.; Lisle, A.T.; Bryde, N.J. Effect of Bunch Trimming and Leaf Removal at Flowering on Maturity Bronzing, Yield, and Other Aspects of Fruit Quality of Bananas in North Queensland. Aust. J. Exp. Agric. 1994, 34, 259–265. [Google Scholar] [CrossRef]
- Meyer, J.-P. Influence de l’ablation de mains sur le rendement en poids des régimes de bananes par catégories de conditionnement aux Antilles. Fruits 1975, 30, 663–668. [Google Scholar]
- Ney, B.; Bancal, M.O.; Bancal, P.; Bingham, I.J.; Foulkes, J.; Gouache, D.; Paveley, N.; Smith, J. Crop Architecture and Crop Tolerance to Fungal Diseases and Insect Herbivory. Mechanisms to Limit Crop Losses. Eur. J. Plant Pathol. 2013, 135, 561–580. [Google Scholar] [CrossRef]
- Robinson, J.C.; Anderson, T.; Eckstein, K. The Influence of Functional Leaf Removal at Flower Emergence on Components of Yield and Photosynthetic Compensation in Banana. J. Hortic. Sci. 1992, 67, 403–410. [Google Scholar] [CrossRef]
- Turner, D.W.; Fortescue, J.A.; Thomas, D.S. Environmental Physiology of the Bananas (Musa spp.). Braz. J. Plant Physiol. 2007, 19, 463–484. [Google Scholar] [CrossRef]
- Jullien, A.; Malézieux, E.; Michaux-Ferrière, N.; Chillet, M.; Ney, B. Within-Bunch Variability in Banana Fruit Weight: Importance of Developmental Lag Between Fruits. Ann. Bot. 2001, 87, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.L.; Savin, R.; Slafer, G.A. Fruiting Efficiency Differences between Cereal Species. Field Crops Res. 2019, 231, 68–80. [Google Scholar] [CrossRef]
- Elía, M.; Savin, R.; Slafer, G.A. Fruiting Efficiency in Wheat: Physiological Aspects and Genetic Variation among Modern Cultivars. Field Crops Res. 2016, 191, 83–90. [Google Scholar] [CrossRef]
- Van Oosterom, E.J.; Hammer, G.L. Determination of Grain Number in Sorghum. Field Crops Res. 2008, 108, 259–268. [Google Scholar] [CrossRef]


| Cultivars | Fruit Number | Indfw (cm3) | Bunch Weight (kg) | Cycle Duration (days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |||||||
| Ame-01 | 162 | cd | 241 | cd | 257 | 127 | ab | 35.9 | bcd | 28.1 | ab | 311 | bc | 195 |
| Cot-01 | 168 | c | 260 | abc | 253 | 117 | ab | 36.5 | bcd | 27.2 | ab | 321 | ab | 198 |
| DC-01 | 220 | a | 262 | abc | 285 | 89 | b | 46.4 | a | 23.3 | b | 335 | a | 216 |
| Gua-01 | 166 | c | 270 | abc | 255 | 130 | ab | 36.0 | bcd | 30.8 | a | 312 | abc | 206 |
| Gua-02 | 163 | c | 236 | cd | 269 | 117 | ab | 37.1 | bcd | 26.1 | ab | 321 | ab | 196 |
| Mat-01 | 156 | cd | 251 | abcd | 273 | 115 | ab | 37.4 | bcd | 26.3 | ab | 317 | abc | 221 |
| Mat-02 | 162 | cd | 258 | abcd | 232 | 112 | ab | 31.1 | d | 26.7 | ab | 295 | c | 215 |
| Mat-03 | 163 | cd | 249 | bcd | 245 | 134 | ab | 34.9 | cd | 29.5 | ab | 306 | bc | 199 |
| Mat-11 | 175 | bc | 250 | abcd | 277 | 130 | ab | 41.0 | abc | 28.0 | ab | 313 | abc | 224 |
| Mat-12 | 177 | bc | 295 | a | 266 | 99 | b | 42.2 | ab | 32.5 | a | 320 | abc | 193 |
| Poyo | 143 | d | 218 | d | 240 | 153 | a | 32.2 | d | 31.1 | a | 309 | bc | 191 |
| Ruby | 190 | bc | 283 | ab | 221 | 105 | b | 37.3 | bcd | 27.9 | ab | 321 | ab | 198 |
| p-value | *** | *** | n.s. | ** | *** | ** | *** | n.s | ||||||
| Cultivars | Fruit Number/ALAfi (Fruit Number/m2) | |||
|---|---|---|---|---|
| Cycle 1 | Cycle 2 | |||
| Ame-01 | 24.0 | ab | 16.0 | c |
| Cot-01 | 23.0 | ab | 19.2 | bc |
| DC-01 | 28.0 | a | 25.6 | a |
| Gua-01 | 26.7 | ab | 18.6 | bc |
| Gua-02 | 23.5 | ab | 19.2 | bc |
| Mat-01 | 23.2 | ab | 21.2 | ab |
| Mat-02 | 28.4 | a | 18.9 | bc |
| Mat-03 | 21.7 | b | 15.8 | c |
| Mat-11 | 23.7 | ab | 19.9 | bc |
| Mat-12 | 25.5 | ab | 19.6 | bc |
| Poyo | 21.2 | b | 17.0 | bc |
| Ruby | 26.3 | ab | 20.0 | bc |
| p-value | ** | *** | ||
| Cultivars | Vps (dm3) | Vpsr (dm3) | Bunch Weight/Vpsr (kg/dm3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |||||||
| Ame-01 | 93.9 | ab | 169.6 | a | 40.5 | ab | 80.1 | ab | 0.93 | cd | 0.41 | abc |
| Cot-01 | 81.3 | ab | 142.5 | abc | 34.4 | ab | 53.3 | bc | 1.19 | abcd | 0.59 | ab |
| DC-01 | 88.6 | ab | 109.7 | c | 24.6 | b | 44.6 | c | 2.04 | ab | 0.64 | a |
| Gua-01 | 92.1 | ab | 148.6 | abc | 38.7 | ab | 83.3 | ab | 1.01 | bcd | 0.39 | abc |
| Gua-02 | 86.1 | ab | 143.1 | abc | 35.5 | ab | 64.2 | abc | 1.16 | bcd | 0.45 | abc |
| Mat-01 | 89.7 | ab | 131.7 | bc | 23.2 | b | 62.8 | abc | 2.22 | a | 0.44 | abc |
| Mat-02 | 80.2 | b | 149.2 | ab | 31.3 | ab | 71.1 | abc | 1.14 | bcd | 0.41 | abc |
| Mat-03 | 97.0 | ab | 168.8 | a | 47.1 | a | 92.2 | a | 0.80 | d | 0.37 | bc |
| Mat-11 | 91.3 | ab | 131.2 | bc | 26.8 | b | 59.0 | abc | 1.88 | ab | 0.52 | abc |
| Mat-12 | 102.7 | a | 171.8 | a | 45.3 | ab | 87.5 | ab | 1.03 | bcd | 0.38 | abc |
| Poyo | 97.5 | ab | 151.9 | ab | 39.6 | ab | 91.6 | a | 0.99 | bcd | 0.36 | c |
| Ruby | 91.4 | ab | 136.8 | abc | 49.7 | a | 75.6 | abc | 0.86 | d | 0.41 | abc |
| p-value | * | *** | *** | *** | *** | ** | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapetti, M.; Dorel, M. Bunch Weight Determination in Relation to the Source-Sink Balance in 12 Cavendish Banana Cultivars. Agronomy 2022, 12, 333. https://doi.org/10.3390/agronomy12020333
Rapetti M, Dorel M. Bunch Weight Determination in Relation to the Source-Sink Balance in 12 Cavendish Banana Cultivars. Agronomy. 2022; 12(2):333. https://doi.org/10.3390/agronomy12020333
Chicago/Turabian StyleRapetti, Manon, and Marc Dorel. 2022. "Bunch Weight Determination in Relation to the Source-Sink Balance in 12 Cavendish Banana Cultivars" Agronomy 12, no. 2: 333. https://doi.org/10.3390/agronomy12020333






