Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Temperature Experiment
2.2. Water Amount Experiment
2.3. Seed Number Experiment
2.4. Antifungal Experiment
2.5. Statistical Analysis
3. Results
3.1. Temperature Experiment
3.1.1. Germination Duration
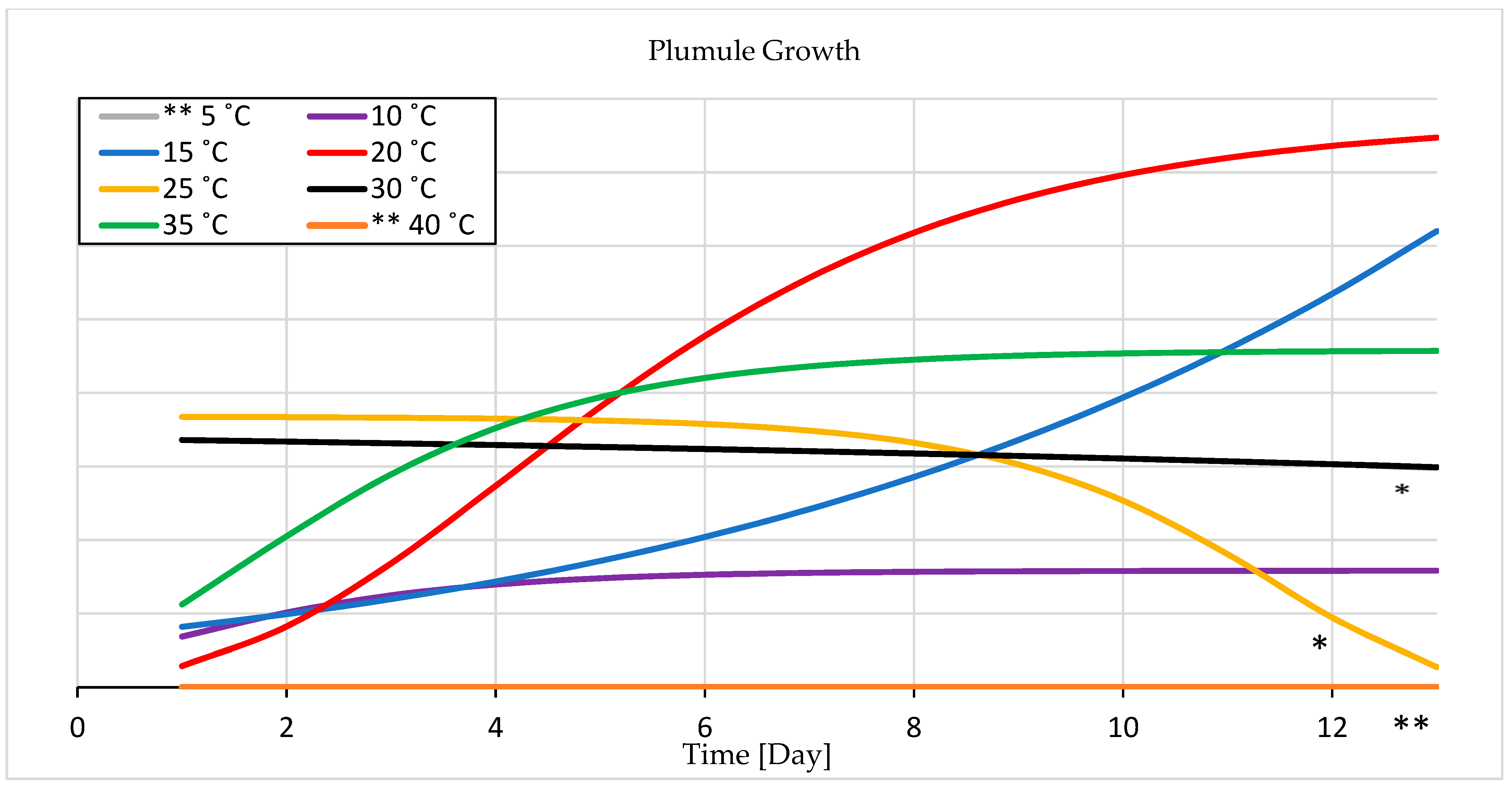
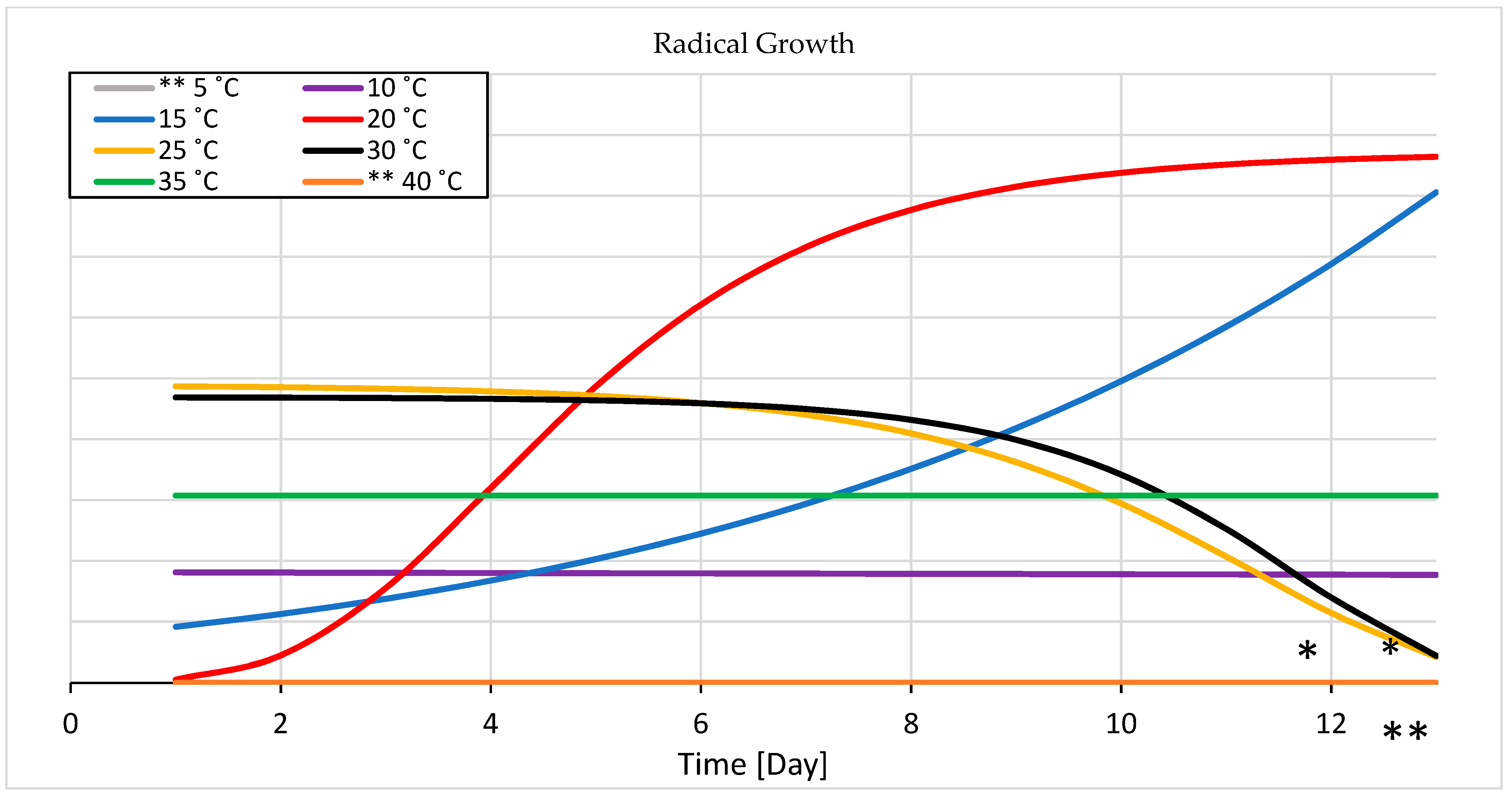
3.1.2. Seedling Growth
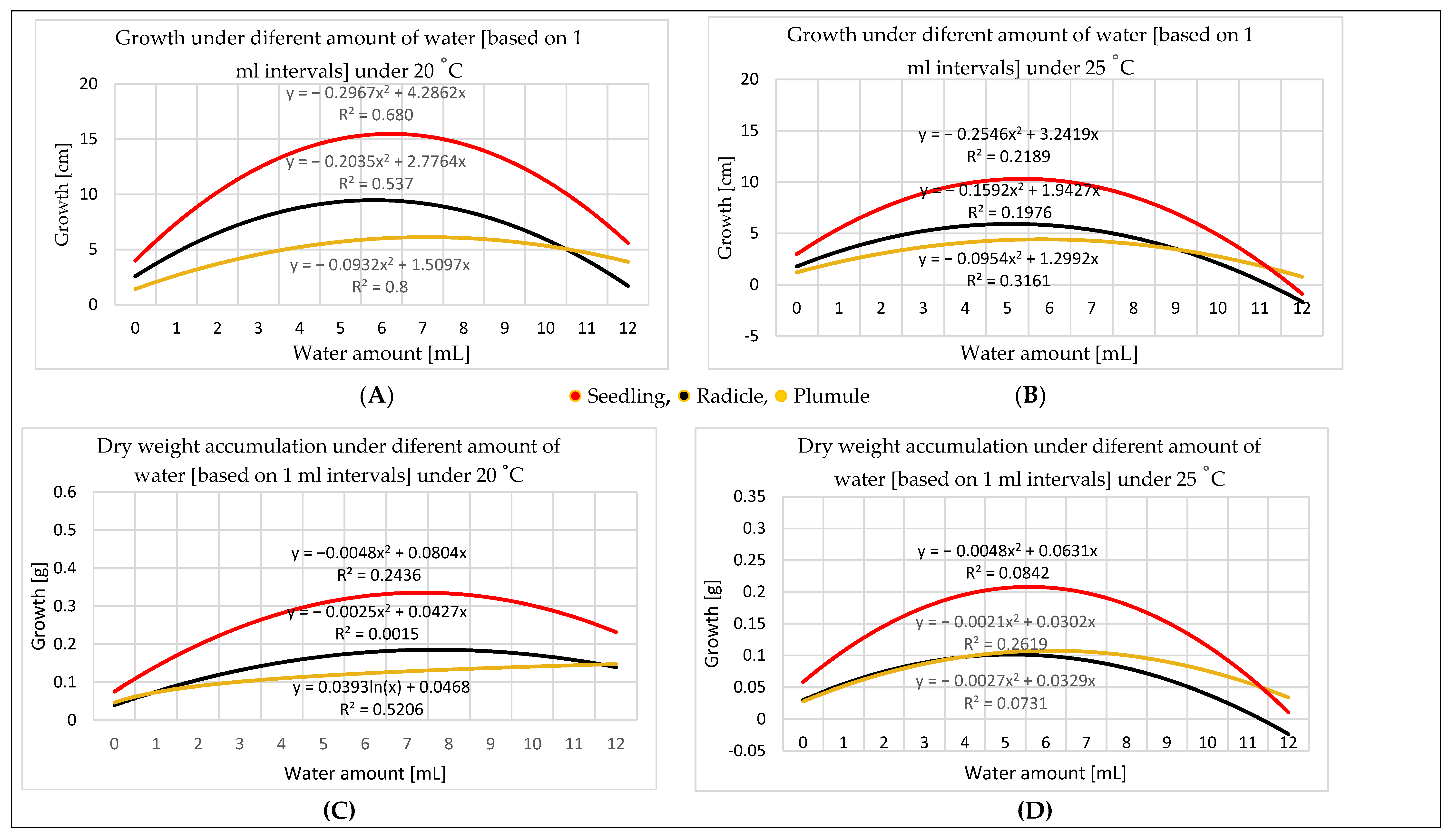
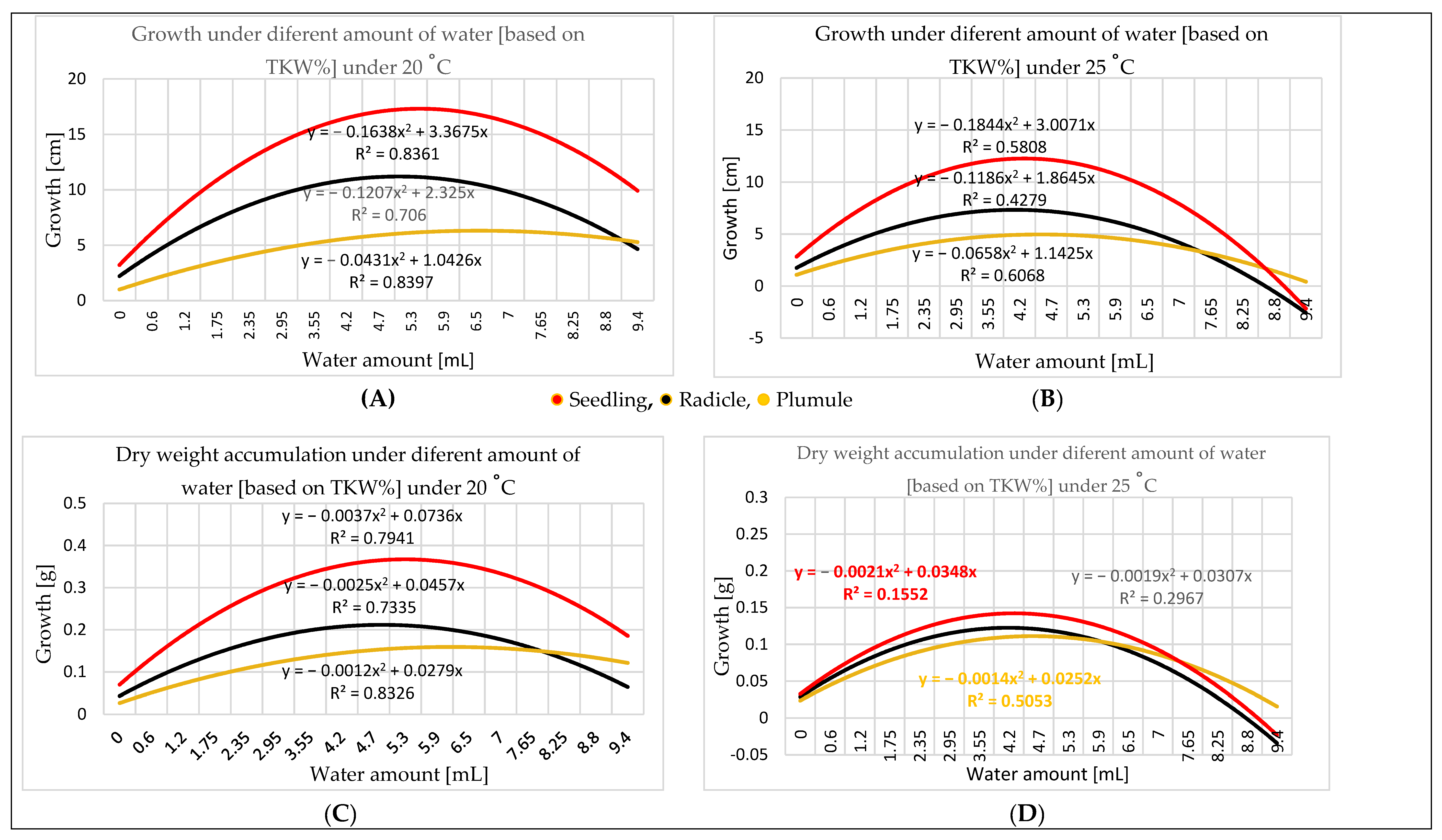
3.2. Water Amount Experiment
3.3. Seed Number Experiment
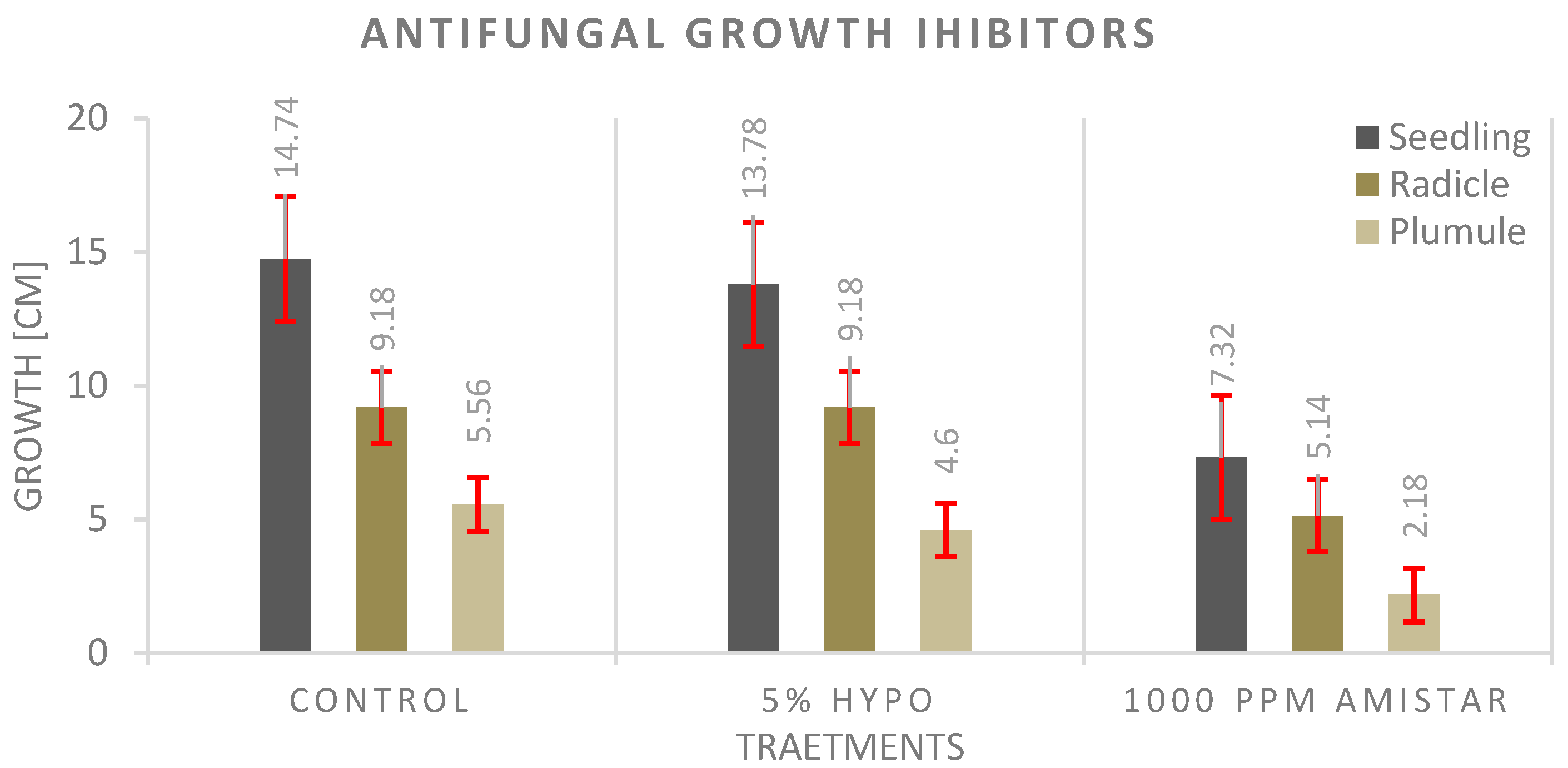
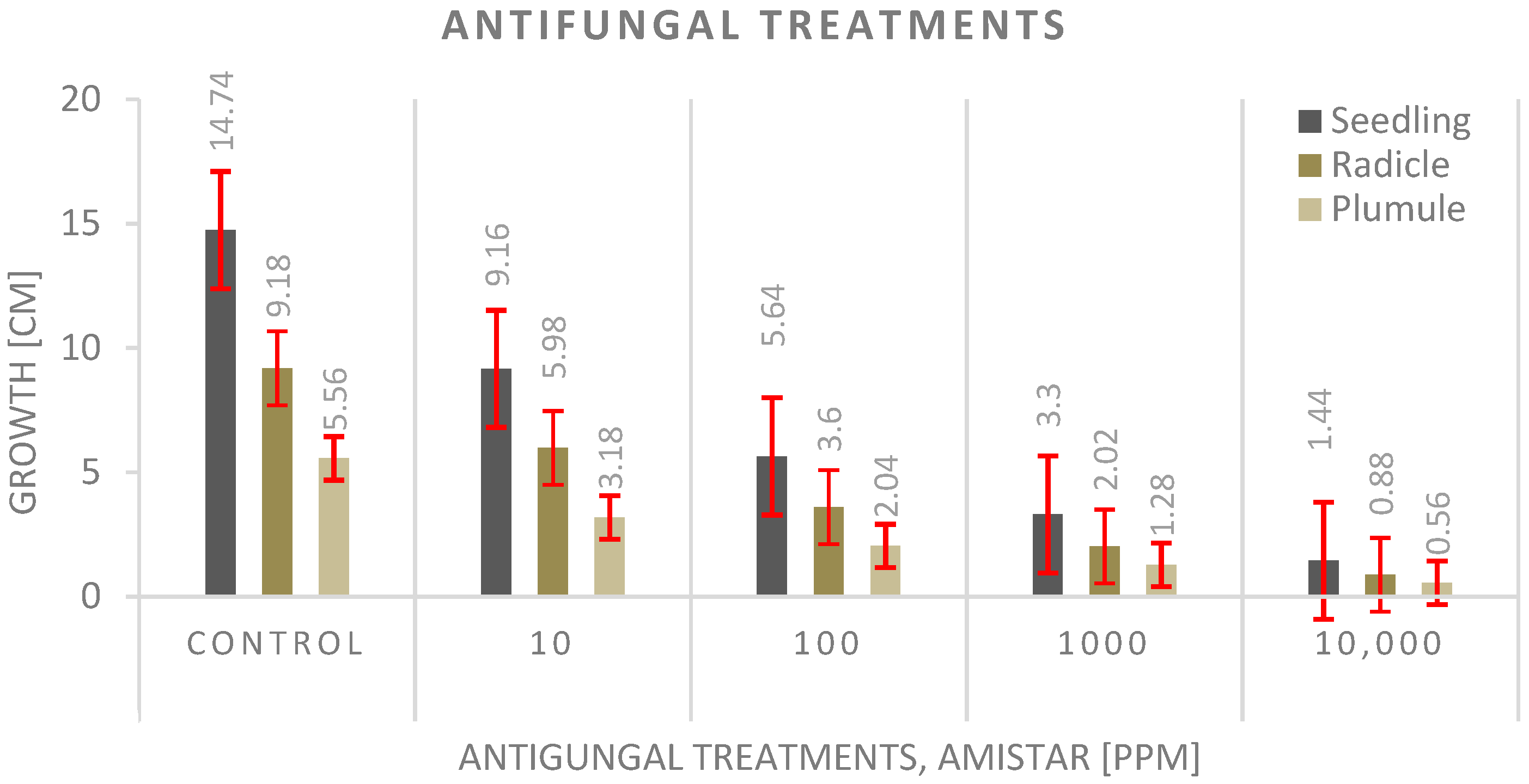
3.4. Antifungal Experiment
4. Discussion
4.1. Temperature Experiment
4.1.1. Germination Duration
4.1.2. Seedling Growth
4.2. Water Amount Experiment
4.3. Seed Number Experiment
4.4. Antifungal Experiment
5. Conclusions
- The sigmoid curves have a solid fit for the experimental data of germination and seedling growth temperatures. The optimal temperature for seedling growth is 20 °C, and a more comprehensive range for germination is from 20 to 35 °C. A temperature lower than the optimal range decreases the germination rate, and a higher temperature increases fungal growth.
- Seed size influences the quantity of water needed for germination. Therefore, TKW provides a more accurate perspective for water amount application. Germination in different percentages can occur in a wide range of water amounts starting at 0.60 mL, representing 25% of the maize TKW, but the optimal range for germination is 0.06–5.30 mL, representing 25–225% of the TKW. The optimal range for seedling growth is 2.35–7.75 mL. As the temperature increases, the optimal range for the water amount narrows; e.g., at 20 °C, it is broader than at 25 °C.
- Dry weight can indicate seedling development, because dry matter accumulation is consistent with the physical measurement of seedling growth.
- Different seed and seedling densities present no significant difference; thus, using a lower seed density is recommended for lab examination: six maize seeds per 9 cm PD.
- The seed priming technique before planting shows a significantly much better effect on seedling growth than adding the antifungal to the growth media, and the highest values were recorded with the control.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, X.; Du, S.; Jiao, F.; Xi, M.; Wang, A.; Xu, H.; Jiao, Q.; Zhang, X.; Jiang, H.; Chen, J.; et al. The regulatory network behind maize seed germination: Effects of temperature, water, phytohormones, and nutrients. Crop J. 2021, 9, 901–914. [Google Scholar] [CrossRef]
- Sakina; Khan, A.S.; Nasrullah, A.; Ullah, F.; Muhammad, N.; Kubra, S.; Din, I.U.; Mutahir, Z. Effect of imidazolium’s ionic liquids with different anions and alkyl chain length on phytotoxicity and biochemical analysis of maize seedling. J. Mol. Liq. 2021, 321, 114491. [Google Scholar] [CrossRef]
- Yang, B.; Yin, Y.; Liu, C.; Zhao, Z.; Guo, M. Effect of germination time on the compositional, functional and antioxidant properties of whole wheat malt and its end-use evaluation in cookie-making. Food Chem. 2021, 349, 129125. [Google Scholar] [CrossRef] [PubMed]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Sarwar, N.; Atique-ur-Rehman; Farooq, O.; Wasaya, A.; Hussain, M.; El-Shehawi, A.M.; Ahmad, S.; Brestic, M.; Mahmoud, S.F.; Zivcak, M.; et al. Integrated nitrogen management improves productivity and economic returns of wheat-maize cropping system. J. King Saud Univ.-Sci. 2021, 33, 101475. [Google Scholar] [CrossRef]
- Cakmak, I.; Prom-u-thai, C.; Guilherme, L.R.G.; Rashid, A.; Hora, K.H.; Yazici, A.; Savasli, E.; Kalayci, M.; Tutus, Y.; Phuphong, P.; et al. Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant Soil 2017, 418, 319–335. [Google Scholar] [CrossRef]
- Tollenaar, M.; Lee, E.A. Yield potential, yield stability and stress tolerance in maize. Field Crops Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef]
- Wang, T.; Zang, Z.; Wang, S.; Liu, Y.; Wang, H.; Wang, W.; Hu, X.; Sun, J.; Tai, F.; He, R. Quaternary ammonium iminofullerenes promote root growth and osmotic-stress tolerance in maize via ROS neutralization and improved energy status. Plant Physiol. Biochem. 2021, 164, 122–131. [Google Scholar] [CrossRef]
- Li, Y.; Shan, X.; Jiang, Z.; Zhao, L.; Jin, F. Genome-wide identification and expression analysis of the GA2ox gene family in maize (Zea mays L.) under various abiotic stress conditions. Plant Physiol. Biochem. 2021, 166, 621–633. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E.; Horgan-Kobelski, T.; Riggs, C. Adaptive transgenerational plasticity in an annual plant: Grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 2012, 52, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.; Finnie, S.; Rose, D.J. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J. Cereal Sci. 2019, 86, 33–40. [Google Scholar] [CrossRef]
- Pangapanga-Phiri, I.; Mungatana, E.D. Adoption of climate-smart agricultural practices and their influence on the technical efficiency of maize production under extreme weather events. Int. J. Disaster Risk Reduct. 2021, 61, 102322. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef]
- MEI, Y.Q.; Song, S.Q. Early Morphological and Physiological Events Occurring During Germination of Maize Seeds. Agric. Sci. China 2008, 7, 950–957. [Google Scholar] [CrossRef]
- FU, F.F.; Peng, Y.S.; Wang, G.B.; EL-kassaby, Y.A.; Cao, F.L. Integrative analysis of the metabolome and transcriptome reveals seed germination mechanism in Punica granatum L. J. Integr. Agric. 2021, 20, 132–146. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed Dormancy and Germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds.Physiology of Development and Germination, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 1994; p. 421. [Google Scholar]
- Masubelele, N.H.; Dewitte, W.; Menges, M.; Maughan, S.; Collins, C.; Huntley, R.; Nieuwland, J.; Scofield, S.; Murray, J.A.H. D-type cyclins activate division in the root apex to promote seed germination in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 15694–15699. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Kasivelu, G.; Raguraman, V.; Malaichamy, K.; Sevathapandian, S.K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal. Agric. Biotechnol. 2020, 29, 101778. [Google Scholar] [CrossRef]
- Nciizah, A.D.; Rapetsoa, M.C.; Wakindiki, I.I.; Zerizghy, M.G. Micronutrient seed priming improves maize (Zea mays) early seedling growth in a micronutrient deficient soil. Heliyon 2020, 6, e04766. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic acid priming enhances seed germination and seedling growth of winter wheat under low temperature due to late sowing in Pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Lara-Núñez, A.; Romero-Sánchez, D.I.; Axosco-Marín, J.; Garza-Aguilar, S.M.; Gómez-Martínez, A.E.; Ayub-Miranda, M.F.; Bravo-Alberto, C.E.; Vázquez-Santana, S.; Vázquez-Ramos, J.M. Two cyclin Bs are differentially modulated by glucose and sucrose during maize germination. Biochimie 2021, 182, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.O.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, S256–S265. [Google Scholar] [CrossRef]
- Rizzardi, M.A.; Luiz, A.R.; Roman, E.S.; Vargas, L. Temperatura cardeal e potencial hídrico na germinação de sementes de corda-de-viola (Ipomoea triloba). Planta Daninha 2009, 27, 13–21. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Whigham, D.F.; Burnett, R.K. Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. J. Ecol. 2016, 104, 744–754. [Google Scholar] [CrossRef]
- Cone, J.W.; Spruit, C.J.P. Imbibition conditions and seed dormancy of Arabidopsis thaliana. Physiol. Plant. 1983, 59, 416–420. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Ozden, E.; Light, M.E.; Demir, I. Alternating temperatures increase germination and emergence in relation to endogenous hormones and enzyme activities in aubergine seeds. S. Afr. J. Bot. 2021, 139, 130–139. [Google Scholar] [CrossRef]
- Norgrove, L. Trade-offs in maize seedling losses in African grasslands. Crop Prot. 2021, 146, 105676. [Google Scholar] [CrossRef]
- Focks, N.; Benning, C. Wrinkled1: A Novel, Low-Seed-Oil Mutant of Arabidopsis with a Deficiency in the Seed-Specific Regulation of Carbohydrate Metabolism 1. Plant Physiol. 1998, 118, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Prerna, D.I.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Vasantharaja, R.; Chaturvedi, S.; Shkolnik, D. Influence of nanoscale micro-nutrient α-Fe2O3 on seed germination, seedling growth, translocation, physiological effects and yield of rice (Oryza sativa) and maize (Zea mays). Plant Physiol. Biochem. 2021, 162, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.J.; Nonogaki, H. Annual Plant Reviews, Seed Development, Dormancy and Germination; Wiley-Blackwell: Oxford, UK, 2007; pp. 1–367. [Google Scholar] [CrossRef]
- Díaz-Granados, V.H.; López-López, J.M.; Flores-Sánchez, J.; Olguin-Alor, R.; Bedoya-López, A.; Dinkova, T.D.; Salazar-Díaz, K.; Vázquez-Santana, S.; Vázquez-Ramos, J.M.; Lara-Núñez, A. Glucose modulates proliferation in root apical meristems via TOR in maize during germination. Plant Physiol. Biochem. 2020, 155, 126–135. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Deng, B.; Yang, K.; Zhang, Y.; Li, Z. Can heavy metal pollution defend seed germination against heat stress? Effect of heavy metals (Cu2+, Cd2+ and Hg2+) on maize seed germination under high temperature. Environ. Pollut. 2016, 216, 46–52. [Google Scholar] [CrossRef]
- Khalid, N.; Tarnawa, Á.; Kende, Z.; Kassai, K.M.; Jolánkai, M. Viability of maize (Zea mays L.) seeds influenced by water, temperature, and salinity stress. Acta Hydrol. Slovaca 2021, 22, 113–117. [Google Scholar] [CrossRef]
- Riley, G.J.P. Effects of High Temperature on the Germination of Maize (Zea mays L.). Planta 1981, 151, 68–74. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Gong, M.; Chen, B.O.; Li, Z.G.; Guo, L.H. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J. Plant Physiol. 2001, 158, 1125–1130. [Google Scholar] [CrossRef]
- Ostadian Bidgoly, R.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of temperature and water potential on Carthamus tinctorius L. seed germination: Quantification of the cardinal temperatures and modeling using hydrothermal time. Ind. Crops Prod. 2018, 113, 121–127. [Google Scholar] [CrossRef]
- Dadach, M.; Mehdadi, Z.; Latreche, A. Effect of water stress on seed germination of Thymus serpyllum L. from Tessala mount. J. Plant Sci. 2015, 10, 151–158. [Google Scholar] [CrossRef][Green Version]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and seedling growth responses of zygophyllum fabago, salsola kali l. And atriplex canescens to peg-induced drought stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Andronis, E.A.; Moschou, P.N.; Toumi, I.; Roubelakis-Angelakis, K.A. Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 132. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, D.; Zhang, H.; Tian, Y.; Japhet, W.; Wang, P. Germination responses of Medicago ruthenica seeds to salinity, alkalinity, and temperature. J. Arid Environ. 2009, 73, 135–138. [Google Scholar] [CrossRef]
- Yan, M.; Xue, C.; Xiong, Y.; Meng, X.; Li, B.; Shen, R.; Lan, P. Proteomic dissection of the similar and different responses of wheat to drought, salinity and submergence during seed germination. J. Proteom. 2020, 220, 103756. [Google Scholar] [CrossRef] [PubMed]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Deng, X.; Liu, D.; Liu, N.; Yan, Y. Integrated physiology and proteome analysis of embryo and endosperm highlights complex metabolic networks involved in seed germination in wheat (Triticum aestivum L.). J. Plant Physiol. 2018, 229, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Abido, W.A.E.; Zsombik, L. Effect of water stress on germination of some Hungarian wheat landraces varieties. Shengtai Xuebao/Acta Ecol. Sin. 2020, 38, 422–428. [Google Scholar] [CrossRef]
- Aderounmu, A.F.; Nkemnkeng, F.J.; Anjah, G.M. Effects of seed provenance and growth media on the growth performance of Vitellaria paradoxa C.F. Gaertn. Int. J. Biol. Chem. Sci. 2020, 14, 2659–2669. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive ability effects of datura stramonium l. And xanthium strumarium l. on the development of maize (Zea mays) seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Fenotiana, R.V. In Vitro Experiments to Study the Effect of Stress Factors on the Germination of Maize; Hungarian University of Agriculture and Life Sciences: Gödöllő, Hungary, 2017. [Google Scholar]
- Ahmad Samir, A. Comparative Study on Wheat Production in Afghanistan and Hungary; Hungariun University of Agriculture and Life Sciences: Gödöllő, Hungary, 2016. [Google Scholar]
- Bulmer, M.G.; Harrison, T.K. Journal of the Royal Statistical Society. Series D. Statistician 1966, 16, 217. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Kidwell, K.K.; Waller, J.E. Base growth temperatures, germination rates and growth response of contemporary spring wheat (Triticum aestivum L.) cultivars from the US Pacific Northwest. Field Crops Res. 2002, 75, 47–52. [Google Scholar] [CrossRef]
- Boyce, D.S. Heat and moisture transfer in ventilated grain. J. Agric. Eng. Res. 1966, 11, 255–265. [Google Scholar] [CrossRef]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.J.; de Medeiros, A.D.; Oliveira, A.M.S. SeedCalc, a new automated R software tool for germination and seedling length data processing. J. Seed Sci. 2019, 41, 250–257. [Google Scholar] [CrossRef]
- Sabouri, A.; Azizi, H.; Nonavar, M. Hydrotime model analysis of lemon balm (melissa officinalis L.) using different distribution functions. S. Afr. J. Bot. 2020, 135, 158–163. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Shaban, M. Effect of water and temperature on seed germination and emergence as a seed hydrothermal time model. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1686–1691. [Google Scholar]
- Gao, C.; Liu, F.; Zhang, C.; Feng, D.; Li, K.; Cui, K. Germination responses to water potential and temperature variation among provenances of Pinus yunnanensis. Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 276–277, 151786. [Google Scholar] [CrossRef]
- Zhang, K.; Ji, Y.; Fu, G.; Yao, L.; Liu, H.; Tao, J. Dormancy-breaking and germination requirements of Thalictrum squarrosum Stephan ex Willd. seeds with underdeveloped embryos. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100311. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Morton, T.C.; Romeo, J.T. Solution volume and seed number: Often overlooked factors in allelopathic bioassays. J. Chem. Ecol. 1987, 13, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Aldars-García, L.; Marín, S.; Sanchis, V.; Magan, N.; Medina, A. Assessment of intraspecies variability in fungal growth initiation of Aspergillus flavus and aflatoxin B1 production under static and changing temperature levels using different initial conidial inoculum levels. Int. J. Food Microbiol. 2018, 272, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef]




| Amount of Water Based on 1 mL Interval | Amount of Water Based on the TKW % | ||||
|---|---|---|---|---|---|
| a Treatment Number | b Water Amount (mL) | a Treatment Number | c Proposed % of Water Amount | d Amount of Water (mL) | e Rounded Amount of Water (mL) |
| 1 | 0 | 14 | 25% | 0.588 | 0.6 |
| 2 | 1 | 15 | 50% | 1.176 | 1.2 |
| 3 | 2 | 16 | 75% | 1.765 | 1.75 |
| 4 | 3 | 17 | 100% | 2.352 | 2.35 |
| 5 | 4 | 18 | 125% | 2.94 | 2.95 |
| 6 | 5 | 19 | 150% | 3.528 | 3.55 |
| 7 | 6 | 20 | 175% | 4.116 | 4.20 |
| 8 | 7 | 21 | 200% | 4.704 | 4.70 |
| 9 | 8 | 22 | 225% | 5.292 | 5.30 |
| 10 | 9 | 23 | 250% | 5.88 | 5.90 |
| 11 | 10 | 24 | 275% | 6.468 | 6.50 |
| 12 | 11 | 25 | 300% | 7.056 | 7.00 |
| 13 | 12 | 27 | 325% | 7.644 | 7.65 |
| 28 | 350% | 8.232 | 8.25 | ||
| 29 | 375% | 8.82 | 8.80 | ||
| 30 | 400% | 9.408 | 9.40 | ||
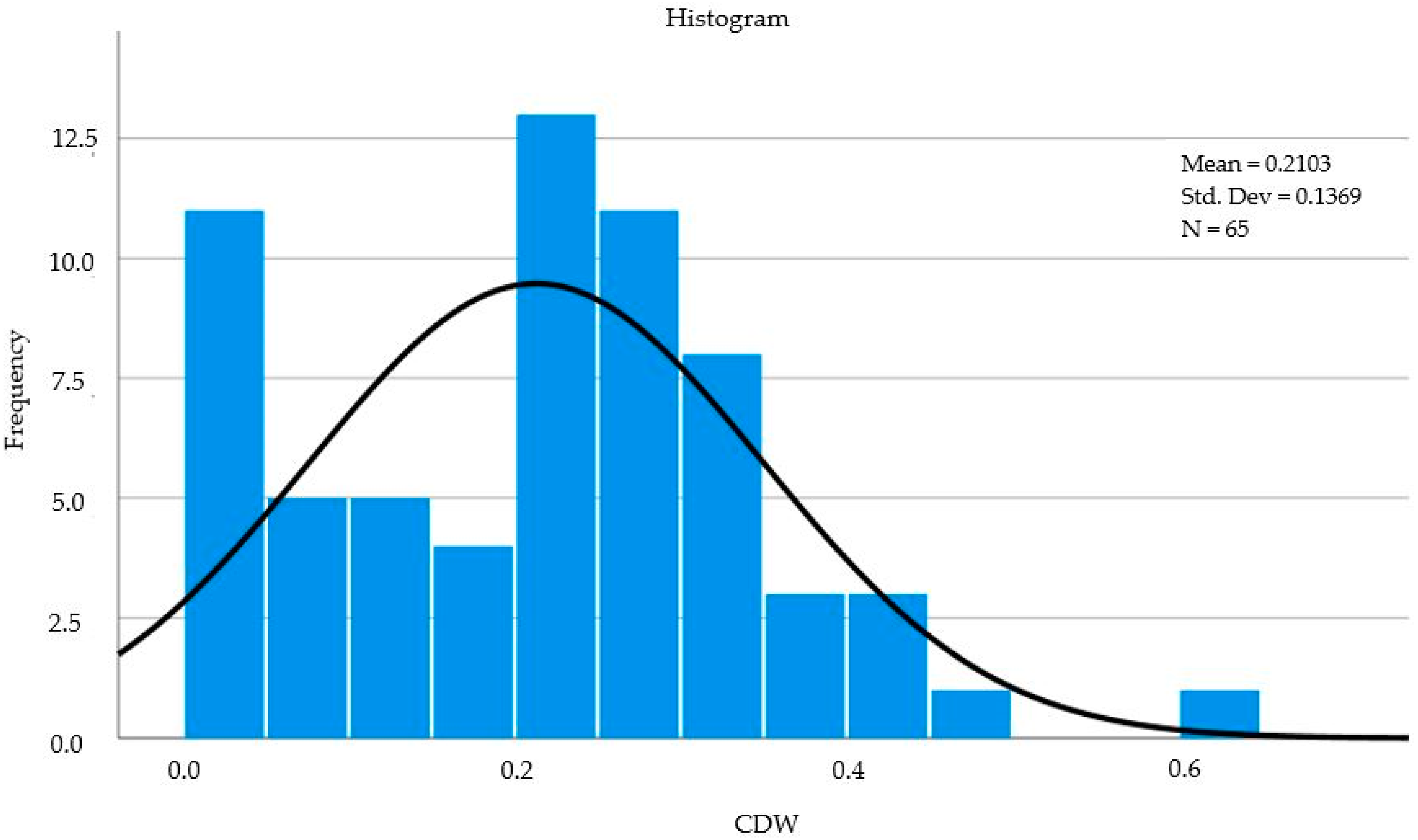
| a N | Valid | 65 |
|---|---|---|
| Missing | 1 | |
| Mean | 0.212966 | |
| Skewness | 0.292 | |
| Std. error of Skewness | 0.297 | |
| Kurtosis | 0.232 | |
| b Std. error of Kurtosis | 0.586 | |
| 1 Water (mL) | 2 Germinated Seeds | 3 Radicle (cm) | 4 Plumule (cm) | 5 Seedling (cm) | 6 Radicle DW (g) | 7 Plumule DW (g) | 8 Seedling DW (g) | 9 Corrected DW (g) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.00 ± 0.00 f | 0.00 ± 0.00 e | 0.00 ± 0.00 c | 0.00 ± 0.00 f | 0.00 ± 0.00 c | 0.00 ± 0.00 d | 0.00 ± 0.00 c | 0.00 ± 0.00 f |
| 1 | 10.0 ± 0.00 a | 8.04 ± 0.96 abc | 1.35 ± 0.39 c | 4.70 ± 0.57 cde | 0.17 ± 0.02 bc | 0.07 ± 0.02 c | 0.24 ± 0.19 b | 0.237 ± 0.02 b |
| 2 | 9.80 ± 0.45 a | 8.66 ± 0.88 ab | 4.22 ± 0.56 b | 6.44 ± 0.65 abcde | 0.18 ± 0.03 b | 0.12 ± 0.01 abc | 0.29 ± 0.03 b | 0.284 ± 0.02 a |
| 3 | 9.20 ± 0.84 ab | 8.92 ± 1.21 ab | 5.46 ± 0.48 ab | 7.19 ± 0.68 abcd | 0.18 ± 0.05 b | 0.14 ± 0.02 ab | 0.29 ± 0.07 b | 0.291 ± 0.08 b |
| 4 | 9.60 ± 0.55 a | 10.90 ± 2.08 a | 6.16 ± 0.73 ab | 8.53 ± 1.33 a | 0.19 ± 0.04 b | 0.14 ± 0.03 ab | 0.31 ± 010 b | 0.321 ± 0.08 b |
| 5 | 8.80 ± 1.30 ab | 9.18 ± 4.22 ab | 5.56 ± 1.88 ab | 7.37 ± 2.95 abc | 0.15 ± 0.06 bc | 0.13 ± 0.03 ab | 0.33 ± 0.08 ab | 0.255 ± 0.12 c |
| 6 | 8.00 ± 1.41 abc | 9.14 ± 3.32 ab | 5.88 ± 1.21 ab | 7.51 ± 2.20 ab | 0.15 ± 0.06 bc | 0.14 ± 0.03 ab | 0.28 ± 0.10 b | 0.244 ± 0.10 def |
| 7 | 7.20 ± 2.49 bcde | 6.84 ± 3.70 bcd | 5.24 ± 2.63 ab | 6.04 ± 3.14 abcde | 0.14 ± 0.09 bc | 0.12 ± 0.06 ab | 0.29 ± 0.08 b | 0.213 ± 0.13 def |
| 8 | 7.40 ± 1.67 bcd | 7.90 ± 2.58 abc | 7.16 ± 1.48 a | 7.53 ± 1.99 ab | 0.14 ± 0.04 bc | 0.16 ± 0.03 a | 0.26 ± 0.14 b | 0.232 ± 0.10 ef |
| 9 | 6.60 ± 2.07 cde | 4.86 ± 2.66 cd | 4.84 ± 1.49 b | 4.85 ± 2.05 bcde | 0.10 ± 0.05 bc | 0.13 ± 0.05 ab | 0.30 ± 0.07 b | 0.158 ± 0.09 cd |
| 10 | 5.60 ± 2.30 de | 4.72 ± 2.78 cd | 5.18 ± 2.40 ab | 4.95 ± 2.57 bcde | 0.09 ± 0.04 bc | 0.13 ± 0.05 ab | 0.22 ± 0.09 b | 0.136 ± 0.10 ef |
| 11 | 5.20 ± 2.17 e | 4.72 ± 4.28 cd | 4.44 ± 3.02 b | 4.58 ± 3.61 de | 0.41 ± 0.46 a | 0.10 ± 0.08 bc | 0.51 ± 0.09 a | 0.302 ± 0.28 de |
| 12 | 6.00 ± 2.55 cde | 4.16 ± 2.93 d | 4.42 ± 2.95 b | 4.29 ± 2.87 e | 0.07 ± 0.04 c | 0.11 ± 0.06 abc | 0.18 ± 0.10 bc | 0.096 ± 0.09 def |
| LSD * | 2.07 | 3.49 | 2.26 | 2.78 | 0.172 | 0.054 | 0.20 | 0.053 |
| 1 Water (mL) | 2 Water (TKW) | 3 Germinated Seeds | 4 Radicle (cm) | 5 Plumule (cm) | 6 Seedling (cm) | 7 Radicle DW (g) | 8 Plumule DW (g) | 9 Seedling DW (g) | 10 Corrected DW (g) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 25% | 0.00 ± 0.00 f | 0.00 ± 0.00 h | 0.00 ± 0.00 e | 0.00 ± 0.00 h | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 g | 0.00 ± 0.00 g |
| 0.60 | 50% | 10.0 ± 0.00 a | 6.74 ± 0.92 fg | 0.28 ± 0.15 e | 3.51 ± 0.41 g | 0.07 ± 0.01 e | 0.03 ± 0.02 ef | 0.10 ± 0.01 f | 0.10 ± 0.01 fg |
| 1.20 | 75% | 10.0 ± 0.00 a | 7.64 ± 0.80 defg | 1.36 ± 0.15 de | 4.50 ± 0.36 fg | 0.15 ± 0.01 cd | 0.05 ± 0.01 e | 0.19 ± 0.01 e | 0.19 ± 0.01 cdef |
| 1.75 | 100% | 9.80 ± 0.45 a | 8.12 ± 0.80 cdef | 2.24 ± 0.67d | 5.18 ± 0.36 efg | 0.18 ± 0.01 abc | 0.09 ± 0.02 d | 0.23 ± 0.02 cde | 0.27 ± 0.01 abcd |
| 2.35 | 125% | 9.60 ± 0.55 a | 9.36 ± 0.97 abcde | 4.82 ± 1.61 c | 7.09 ± 1.25 bcde | 0.19 ± 0.02 abc | 0.13 ± 0.03 bcd | 0.32 ± 0.03 abcd | 0.31 ± 0.04 ab |
| 2.95 | 150% | 9.00 ± 0.00 abc | 8.74 ± 1.00 bcdef | 5.14 ± 0.38 abc | 6.94 ± 0.57 bcde | 0.18 ± 0.02 abc | 0.14 ± 0.00 abc | 0.32 ± 0.02 abcd | 0.29 ± 0.02 abc |
| 3.55 | 175% | 9.20 ± 0.84 ab | 10.28 ± 0.87 abcde | 5.94 ± 0.70 abc | 8.11 ± 0.63 abcd | 0.21 ± 0.04 ab | 0.17 ± 0.01 a | 0.38 ± 0.05 a | 0.35 ± 0.05 a |
| 4.20 | 200% | 8.80 ± 1.30 abcd | 10.30 ± 0.70 abcd | 5.60 ± 0.66 abc | 7.95 ± 0.64 abcd | 0.22 ± 0.40 ab | 0.16 ± 0.01 ab | 0.38 ± 0.05 ab | 0.34 ± 0.08 a |
| 4.70 | 225% | 9.20 ± 0.45 ab | 11.40 ± 0.72 ab | 6.76 ± 0.53 a | 9.08 ± 0.46 ab | 0.21 ± 0.02 ab | 0.16 ± 0.01 ab | 0.37 ± 0.02 ab | 0.34 ± 0.01 a |
| 5.30 | 250% | 8.80 ± 1.30 abcd | 12.18 ± 2.44 a | 6.68 ± 1.47 ab | 9.43 ± 1.89 a | 0.24 ± 0.11 a | 0.15 ± 0.02 abc | 0.38 ± 0.12 a | 0.34 ± 0.10 a |
| 5.90 | 275% | 7.60 ± 1.80 bcde | 10.38 ± 3.50 abcd | 6.76 ± 1.96 a | 8.57 ± 2.65 abc | 0.15 ± 0.04cd | 0.14 ± 0.02 abc | 0.29 ± 0.05 bcd | 0.23 ± 0.10 bcde |
| 6.50 | 300% | 7.00 ± 2.92 de | 10.24 ± 4.72 abcde | 6.36 ± 2.37 abc | 8.30 ± 3.53 abc | 0.16 ± 0.08 bcd | 0.15 ± 0.05 ab | 0.31 ± 0.13 abcd | 0.25 ± 0.16 abcde |
| 7.65 | 325% | 7.20 ± 1.92 cde | 10.86 ± 4.01 abc | 6.62 ± 1.36 ab | 8.74 ± 2.64 abc | 0.17 ± 0.07 bc ± d | 0.17 ± 0.05 a | 0.34 ± 0.11 abc | 0.26 ± 0.13 abcde |
| 8.25 | 350% | 5.80 ± 2.95 e | 6.560 ± 4.84 fg | 5.02 ± 2.40 bc | 5.79 ± 3.59 defg | 0.12 ± 0.06 de | 0.14 ± 0.06 abc | 0.25 ± 0.12 de | 0.17 ± 0.15 def |
| 8.80 | 375% | 5.80 ± 1.48 e | 4.960 ± 2.51 g | 4.86 ± 1.70 c | 4.91 ± 2.06 efg | 0.08 ± 0.04 e | 0.11 ± 0.03 cd | 0.19 ± 0.06 e | 0.12 ± 0.06 f |
| 9.40 | 400% | 6.00 ± 1.58 e | 7.160 ± 2.46 efg | 6.20 ± 1.53 abc | 6.68 ± 1.87 cdef | 0.12 ± 0.03 de | 0.15 ± 0.02 abc | 0.27 ± 0.05 cde | 0.17 ± 0.07 ef |
| LSD * | 1.834 | 3.13 | 1.70 | 2.32 | 0.058 | 0.036 | 0.086 | 0.102 |
| 1 Water (mL) | 2 Germinated Seeds | 3 Radicle (cm) | 4 Plumule (cm) | 5 Seedling (cm) | 6 Radicle DW (g) | 7 Plumule DW (g) | 8 Seedling DW (g) | 9 Corrected DW (g) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.0 ± 0.00 g | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 h | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 g | 0.00 ± 0.00 f |
| 1 | 9.8 ± 0.45 a | 8.04 ± 0.93 a | 2.34 ± 0.26 de | 5.19 ± 0.44 bcd | 0.15 ± 0.03 ab | 0.06 ± 0.01 cde | 0.11 ± 0.01 bc | 0.21 ± 0.03 b |
| 2 | 10 ± 0.00 a | 8.76 ± 0.75 3 | 4.82 ± 0.48 abc | 6.79 ± 0.54 ab | 0.18 ± 0.01 a | 0.11 ± 0.01 ab | 0.15 ± 0.01 a | 0.29 ± 0.01 a |
| 3 | 8.2 ± 0.45 bc | 7.50 ± 1.07 ab | 5.58 ± 0.82 ab | 6.54 ± 0.86 abc | 0.15 ± 0.02 ab | 0.13 ± 0.02 a | 0.14 ± 0.02 ab | 0.23 ± 0.04 b |
| 4 | 9.4 ± 0.89 ab | 9.16 ± 1.92 a | 6.02 ± 0.65 a | 7.59 ± 1.13 a | 0.12 ± 0.03 b | 0.13 ± 0.02 ab | 0.13 ± 0.02 ab | 0.22 ± 0.08 b |
| 5 | 7.2 ± 1.79 c | 4.90 ± 4.39 bc | 4.00 ± 2.39 bcd | 4.45 ± 3.31 cde | 0.06 ± 0.05 c | 0.10 ± 0.05 abcd | 0.08 ± 0.02 cd | 0.11 ± 0.07 c |
| 6 | 5.2 ± 2.28 d | 3.54 ± 2.95 cd | 4.06 ± 2.74 abcd | 3.80 ± 2.81 def | 0.06 ± 0.05 c | 0.09 ± 0.05 abcd | 0.08 ± 0.04 cde | 0.04 ± 0.01 def |
| 7 | 4.8 ± 1.48 de | 2.08 ± 1.21 def | 2.96 ± 1.99 cde | 2.52 ± 1.58 efg | 0.04 ± 0.02 cde | 0.07 ± 0.04 bcd | 0.05 ± 0.03 def | 0.05 ± 0.03 def |
| 8 | 3.2 ± 1.30 de | 0.68 ± 0.36 ef | 1.00 ± 0.66 ef | 0.84 ± 0.51 gh | 0.01 ± 0.01 ef | 0.04 ± 0.03 ef | 0.02 ± 0.02 fg | 0.02 ± 0.02 ef |
| 9 | 4.8 ± 1.64 de | 3.28 ± 3.78 cde | 3.72 ± 2.67 bcd | 3.50 ± 3.14 def | 0.05 ± 0.06 cd | 0.11 ± 0.07 abc | 0.08 ± 0.06 cd | 0.09 ± 0.07 cd |
| 10 | 3.8 ± 0.84 ef | 0.90 ± 0.78 ef | 2.38 ± 1.20 de | 1.64 ± 0.94 fgh | 0.02 ± 0.01 df | 0.07 ± 0.03 bcde | 0.04 ± 0.02 ef | 0.04 ± 0.02 ef |
| 11 | 5.4 ± 1.14 f | 1.06 ± 0.88 ef | 2.18 ± 1.25 de | 1.62 ± 1.04 fgh | 0.03 ± 0.01 cdef | 0.07 ± 0.04 bcde | 0.05 ± 0.03 def | 0.05 ± 0.03 de |
| 12 | 4.6 ± 1.14 def | 1.68 ± 2.17 def | 2.16 ± 1.89 de | 1.92 ± 1.96 fgh | 0.03 ± 0.03 cdef | 0.06 ± 0.04 de | 0.04 ± 0.04 ef | 0.04 ± 0.05 def |
| LSD * | 1.55 | 2.64 | 2.01 | 2.22 | 0.038 | 0.047 | 0.035 | 0.053 |
| 1 Water (mL) | 2 Water (TKW) | 3 Germinated Seeds | 4 Radicle (cm) | 5 Plumule (cm) | 6 Seedling (cm) | 7 Radicle DW (g) | 8 Plumule DW (g) | 9 Seedling DW (g) | 10 Corrected DW (g) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 25% | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 h | 0.00 ± 0.00 e | 0.00 ± 0.00 f | 0.00 ± 0.00 i | 0.00 ± 0.00 h |
| 0.60 | 50% | 10.0 ± 0.00 a | 7.08 ± 0.88 bc | 0.32 ± 0.19 ef | 3.70 ± 0.38 de | 0.07 ± 0.01 cde | 0.03 ± 0.02 ef | 0.10 ± 0.01 ef | 0.10 ± 0.01 def |
| 1.20 | 75% | 10.0 ± 0.00 a | 8.46 ± 0.89 ab | 1.50 ± 0.22 def | 4.98 ± 0.39 bcd | 0.15 ± 0.01 abc | 0.05 ± 0.01 e | 0.19 ± 0.01 b | 0.19 ± 0.01 b |
| 1.75 | 100% | 9.80 ± 0.45 a | 8.50 ± 0.59 ab | 2.40 ± 0.71 cd | 5.45 ± 0.37 bcd | 0.18 ± 0.01 ab | 0.09 ± 0.02 cd | 0.28 ± 0.02 a | 0.27 ± 0.01 a |
| 2.35 | 125% | 9.60 ± 0.55 ab | 9.98 ± 0.90 a | 5.64 ± 0.51 a | 7.81 ± 0.38 a | 0.16 ± 0.03 abc | 0.15 ± 0.01 a | 0.16 ± 0.01 bcd | 0.15 ± 0.02 c |
| 2.95 | 150% | 9.20 ± 0.84 ab | 8.62 ± 2.47 ab | 6.58 ± 0.55 a | 7.60 ± 1.40 a | 0.11 ± 0.04 bcd | 0.13 ± 0.02 ab | 0.12 ± 0.03 cde | 0.11 ± 0.03 cde |
| 3.55 | 175% | 8.20 ± 1.79 abc | 6.04 ± 1.07 cd | 5.38 ± 0.93 a | 5.71 ± 0.99 bc | 0.09 ± 0.02 bcde | 0.12 ± 0.00 abc | 0.11 ± 0.01 def | 0.09 ± 0.02 ef |
| 4.20 | 200% | 7.80 ± 1.79 bc | 6.68 ± 3.45 bc | 5.26 ± 1.81 a | 5.97 ± 2.62 abc | 0.09 ± 0.04 bcde | 0.11 ± 0.03 bcd | 0.10 ± 0.04 ef | 0.08 ± 0.04 ef |
| 4.70 | 225% | 6.80 ± 2.86 cd | 5.62 ± 2.94 cd | 5.00 ± 2.22 ab | 5.31 ± 2.56 bcd | 0.07 ± 0.03 cde | 0.10 ± 0.03 bcd | 0.08 ± 0.03 efg | 0.06 ± 0.04 fg |
| 5.30 | 250% | 7.80 ± 2.05 bc | 7.16 ± 3.29 bc | 6.02 ± 2.16 a | 6.59 ± 2.71 ab | 0.23 ± 0.30 a | 0.12 ± 0.04 abcd | 0.17 ± 0.15 bc | 0.13 ± 0.11 cd |
| 5.90 | 275% | 5.00 ± 1.58 de | 4.10 ± 2.00 de | 5.12 ± 1.39 a | 4.61 ± 1.70 cd | 0.04 ± 0.02 de | 0.10 ± 0.04 bcd | 0.07 ± 0.03 efgh | 0.04 ± 0.02 gh |
| 6.50 | 300% | 3.80 ± 0.84 e | 1.20 ± 1.01 f | 1.84 ± 2.84 cde | 1.52 ± 1.40 fgh | 0.02 ± 0.01 de | 0.03 ± 0.03 ef | 0.02 ± 0.02 hi | 0.01 ± 0.01 h |
| 7.65 | 325% | 4.80 ± 1.79 e | 1.94 ± 1.74 ef | 2.46 ± 2.22 cd | 2.20 ± 1.98 efg | 0.03 ± 0.03 de | 0.05 ± 0.04 e | 0.04 ± 0.03 ghi | 0.02 ± 0.02 gh |
| 8.25 | 350% | 3.40 ± 1.67 e | 0.44 ± 0.31 f | 0.52 ± 0.40 ef | 0.48 ± 0.34 gh | 0.01 ± 0.01 e | 0.03 ± 0.03 ef | 0.02 ± 0.02 hi | 0.01 ± 0.01 h |
| 8.80 | 375% | 4.80 ± 0.45 e | 1.76 ± 0.84 f | 3.36 ± 1.48 dc | 2.56 ± 1.13 ef | 0.04 ± 0.02 de | 0.09 ± 0.03 d | 0.06 ± 0.03 fgh | 0.03 ± 0.01 gh |
| 9.40 | 400% | 5.20 ± 2.95 de | 0.60 ± 0.26 f | 1.14 ± 0.75 def | 0.87 ± 0.50 fgh | 0.02 ± 0.01 de | 0.04 ± 0.02 e | 0.03 ± 0.02 hi | 0.02 ± 0.02 h |
| LSD * | 1.950 | 2.25 | 1.67 | 1.86 | 0.098 | 0.034 | 0.055 | 0.043 |
| 1 Seeds (n) | 2 Non-Germinated seeds (%) | 3 Started germination (%) | 4 Seedlings with radicle only (%) | 5 Seedlings with a short plumule (%) | 6 Seedlings with normal plumule (%) | 7 Aggregated value (%) |
|---|---|---|---|---|---|---|
| 20 °C | ||||||
| 6 | 0.133 ± 0.11 | 0.017 ± 0.05 | 0.067 ± 0.14 | 0.033 ± 0.11 | 0.750 ± 0.26 | 0.790 ± 0.19 |
| 8 | 0.188 ± 0.21 | 0.013 ± 0.04 | 0.000 ± 0.00 | 0.025 ± 0.08 | 0.787 ± 0.17 | 0.805 ± 0.17 |
| 10 | 0.090 ± 0.13 | 0.020 ± 0.04 | 0.010 ± 0.03 | 0.130 ± 0.22 | 0.780 ± 0.33 | 0.869 ± 0.21 |
| 12 | 0.192 ± 0.18 | 0.008 ± 0.03 | 0.000 ± 0.00 | 0.167 ± 0.25 | 0.558 ± 0.38 | 0.668 ± 0.32 |
| LSD | * N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| 25 °C | ||||||
| 6 | 0.133 ± 0.15 | 0.017 ± 0.05 | 0.000 ± 0.00 | 0.033 ± 0.07 | 0.817 ± 0.21 a | 0.840 ± 0.18 |
| 8 | 0.300 ± 0.13 | 0.013 ± 0.04 | 0.038 ± 0.06 | 0.112 ± 0.14 | 0.613 ± 0.26 ab | 0.696 ± 0.19 |
| 10 | 0.290 ± 0.21 | 0.020 ± 0.06 | 0.030 ± 0.07 | 0.170 ± 0.28 | 0.490 ± 0.28 b | 0.610 ± 0.21 |
| 12 | 0.233 ± 0.17 | 0.058 ± 0.09 | 0.008 ± 0.03 | 0.108 ± 0.09 | 0.600 ± 0.22 ab | 0.678 ± 0.19 |
| LSD | N.S. | N.S. | N.S. | N.S. | 0.222 | N.S. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaeim, H.; Kende, Z.; Jolánkai, M.; Kovács, G.P.; Gyuricza, C.; Tarnawa, Á. Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.). Agronomy 2022, 12, 397. https://doi.org/10.3390/agronomy12020397
Khaeim H, Kende Z, Jolánkai M, Kovács GP, Gyuricza C, Tarnawa Á. Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.). Agronomy. 2022; 12(2):397. https://doi.org/10.3390/agronomy12020397
Chicago/Turabian StyleKhaeim, Hussein, Zoltán Kende, Márton Jolánkai, Gergő Péter Kovács, Csaba Gyuricza, and Ákos Tarnawa. 2022. "Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.)" Agronomy 12, no. 2: 397. https://doi.org/10.3390/agronomy12020397
APA StyleKhaeim, H., Kende, Z., Jolánkai, M., Kovács, G. P., Gyuricza, C., & Tarnawa, Á. (2022). Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.). Agronomy, 12(2), 397. https://doi.org/10.3390/agronomy12020397









