Effects of CO2 Enrichment on Yield, Photosynthetic Rate, Translocation and Distribution of Photoassimilates in Strawberry ‘Sagahonoka’
Abstract
:1. Introduction
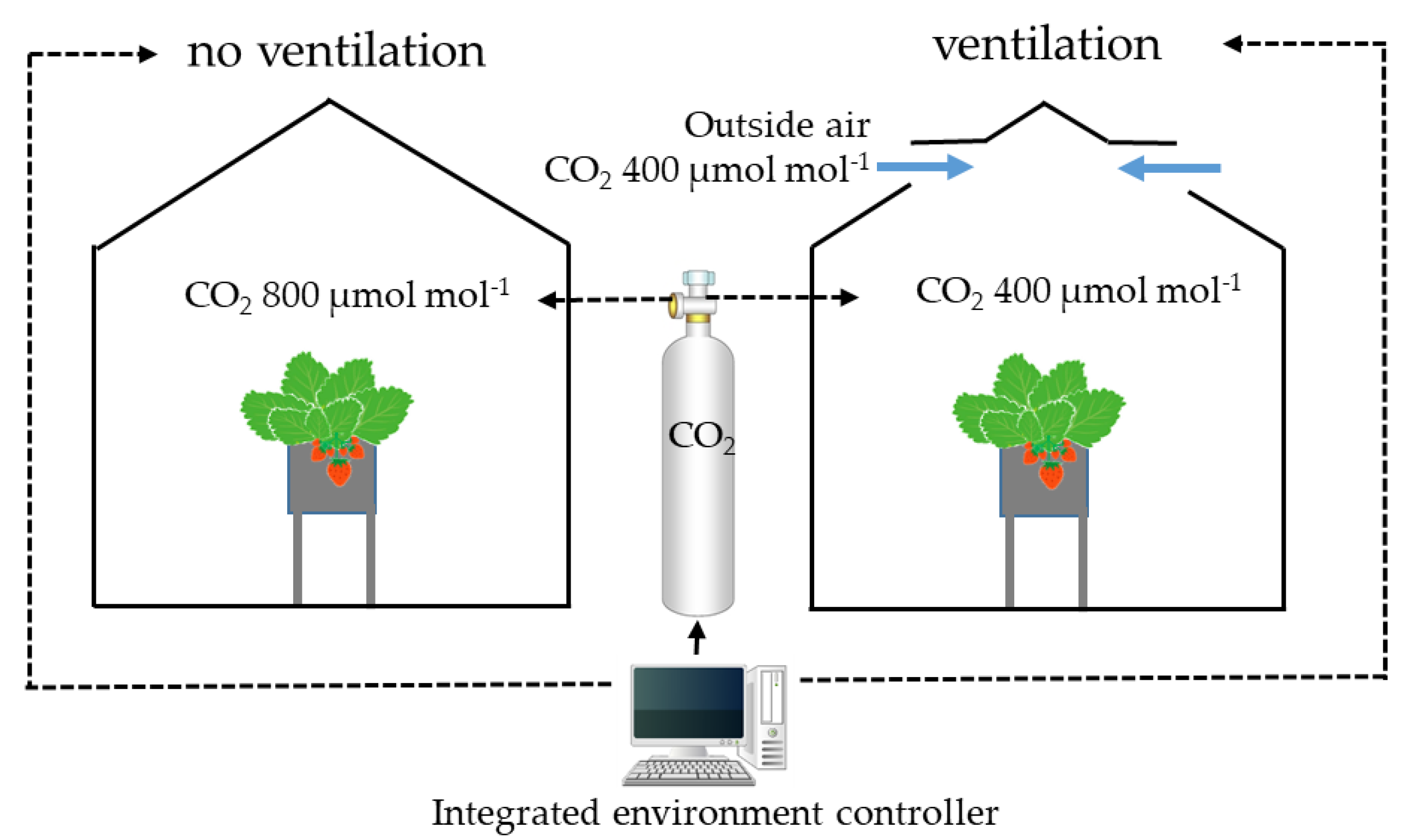
2. Materials and Methods
2.1. Effects of CO2 Concentration on Yield, Fruit Brix, and Economic Value
2.2. Effect of CO2 Concentration on Photosynthetic Rate
2.3. Effect of CO2 Concentration on Translocation and Distribution Rate of 13CO2-Photoassimilates
3. Results
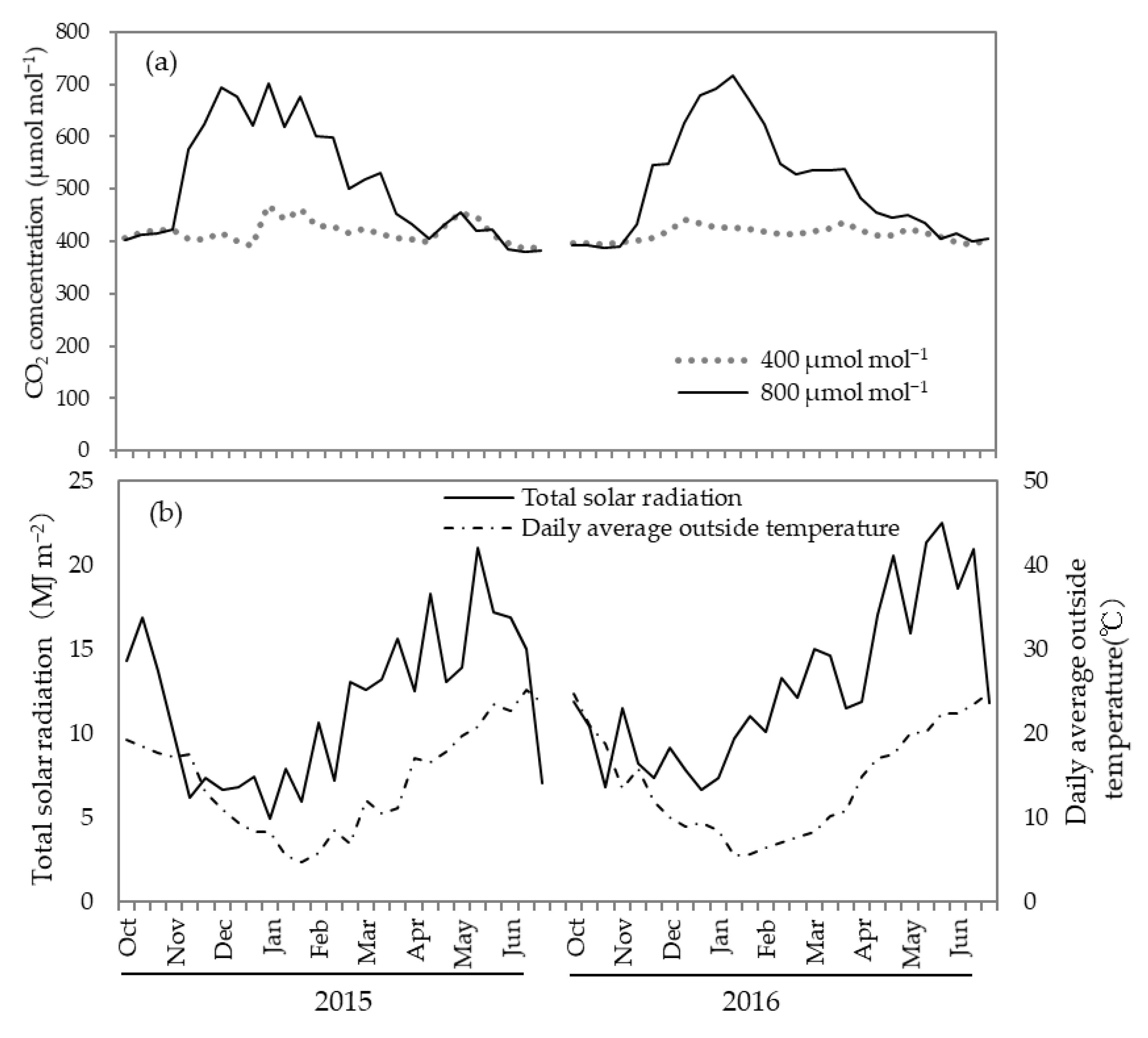
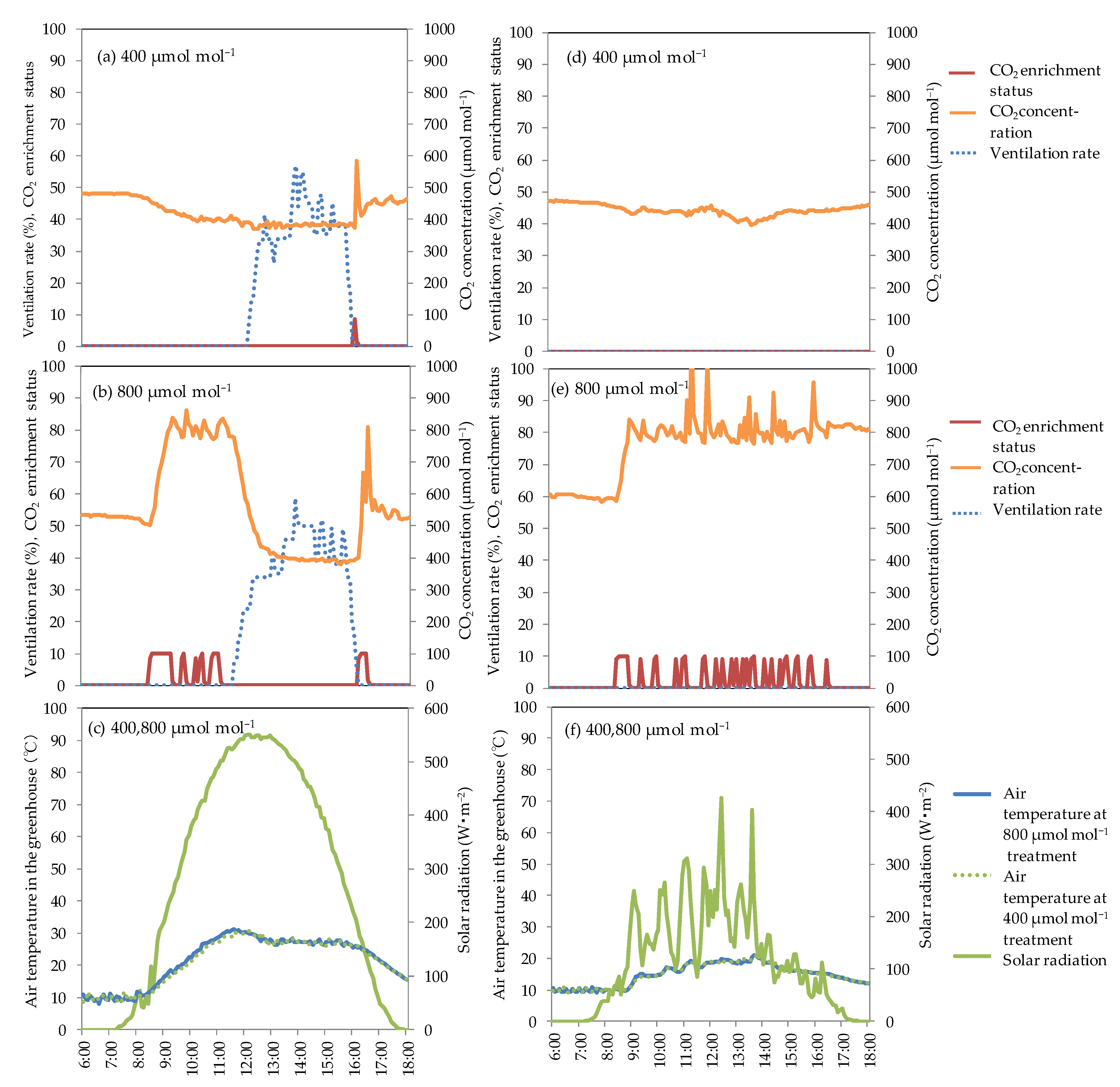
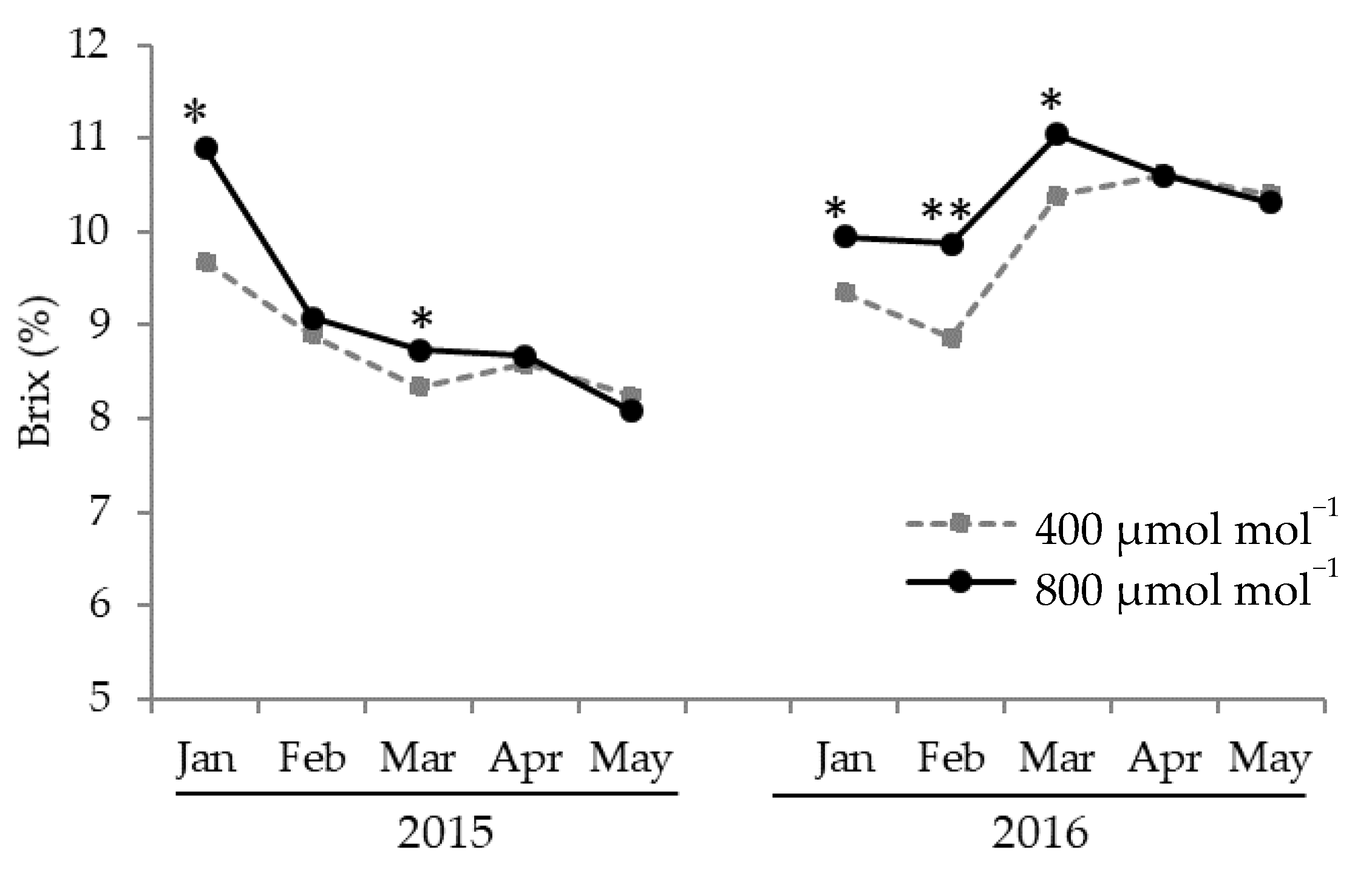
3.1. Effect of CO2 Concentration on Yield, Fruit Brix, and Economic Value the Average Daytime
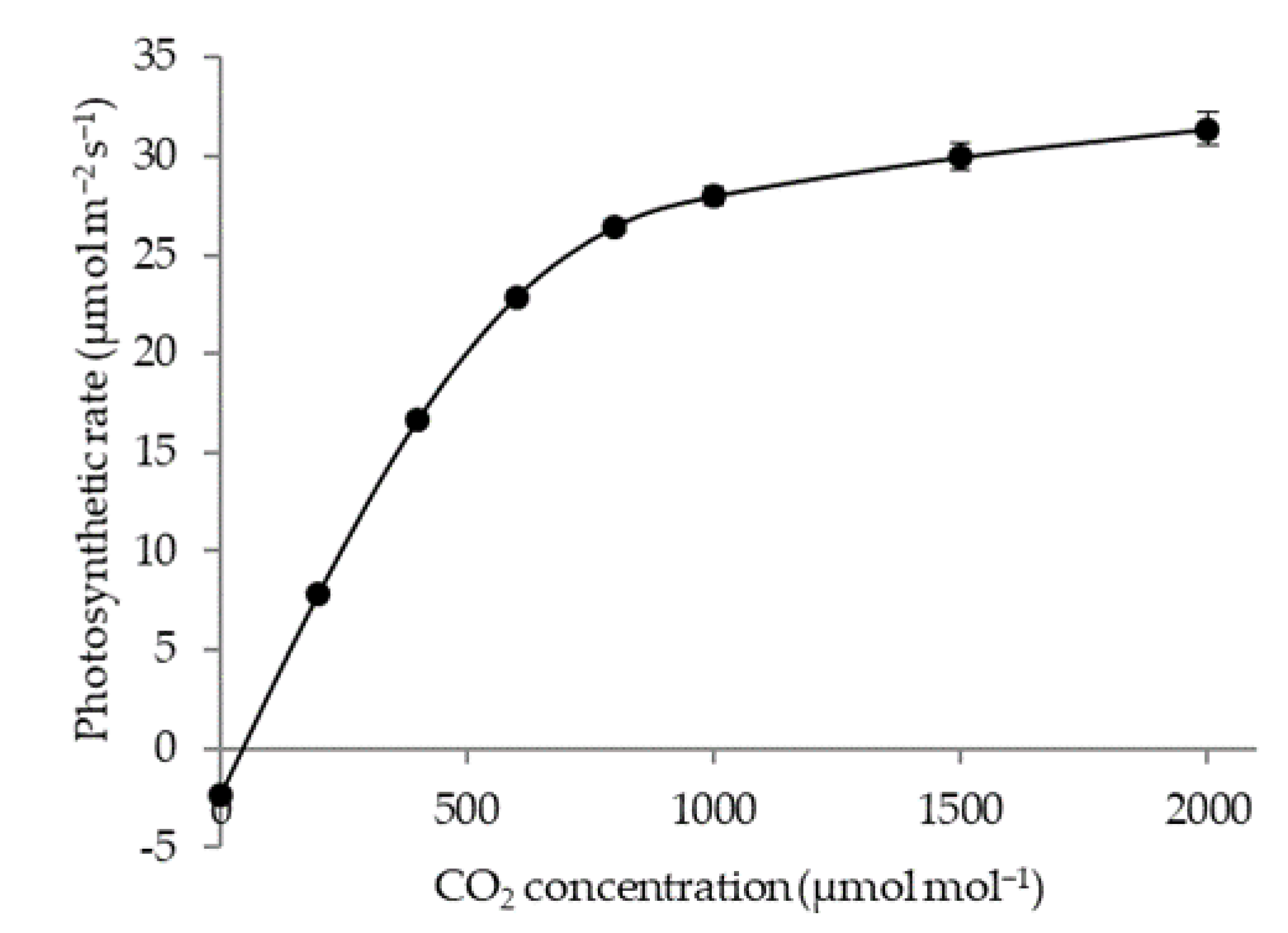
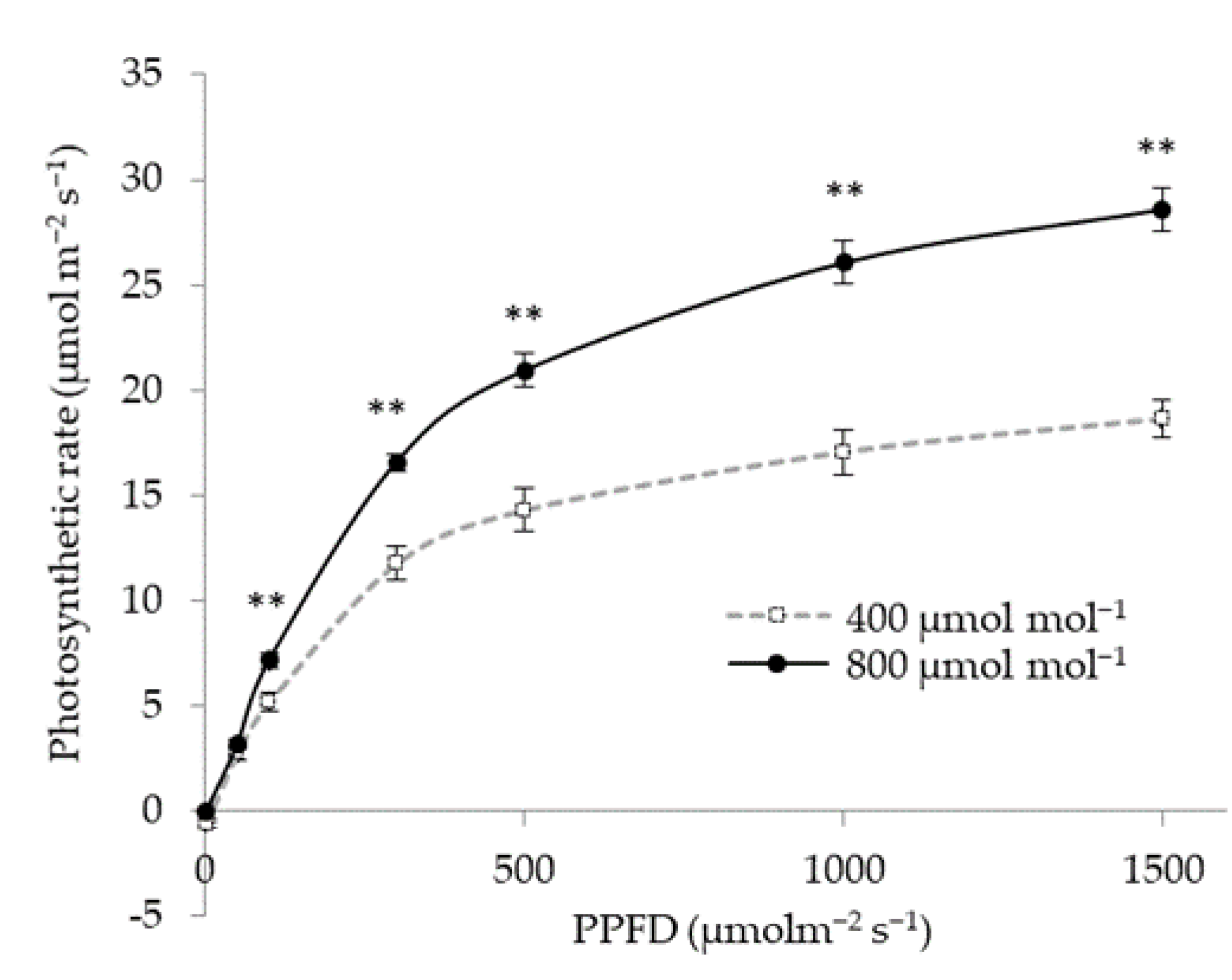
3.2. Effect of CO2 Concentration on Photosynthetic Rate
3.3. Effect of CO2 Concentration on Translocation and Distribution Rate of 13CO2-Photoassimilates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture. Forestry and Fisheries in Japan. e-Stat. Portal Site of Official Statistics of Japan. 2019. Available online: http://www.maff.go.jp/j/tokei/kouhyou/sakumotu/index.html (accessed on 7 December 2021).
- Tanaka, M.; Tanaka, M. Research on high level carnation production technique in northern Kyushu. Bull. Saga. Agri. Expt. Sta. 1987, 24, 1–82. [Google Scholar]
- Tagawa, A.; Ito, Y.; Ehara, M.; Araki, T.; Ozaki, Y.; Shishido, Y. Effect of daytime heating on fruit yield, photosynthesis, translocation and distribution of 13C-photoassimilates of strawberry ‘Sagahonoka’ during winter season. J. Jpn. Soc. Hort. Sci. 2022, in press. [Google Scholar]
- Mortensen, L.M. Review: CO2 enrichment in greenhouses. Crop response. Sci. Hortic. 1987, 33, 1–25. [Google Scholar] [CrossRef]
- Poorter, H. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 1993, 104/105, 77–97. [Google Scholar] [CrossRef]
- Kawashiro, H.; Tsuchiya, K.; Sakiyama, H.; Udagawa, Y. Effects of low-concentration carbon dioxide supplementation on fruit yield and economic value of cucumber on forced culture. J. Jpn. Soc. Hort. Sci. 2009, 8, 445–449. [Google Scholar] [CrossRef]
- Kawashima, N.; Yamamoto, H.; Kurozumi, T.; Tanigawa, K.; Tanaka, Y. Studies on the CO2 enrichment in a greenhouse (4) Effect on the growth of some fruit vegetables. Bull. Nara. Agri. Expt. Sta. 1993, 24, 25–30. [Google Scholar]
- Balasooriya, H.N.; Dassanayake, K.B.; Seneweera, S.; Ajlouni, S. Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria × ananassa) growth and fruit yield. Int. J. Agric. Biosyst. Eng. 2018, 12, 279–287. [Google Scholar]
- Kawashima, N. Studies on the CO2 enrichment in a greenhouse (3) Effect on the growth of strawberry. Bull. Nara. Agri. Expt. Sta. 1991, 22, 65–72. [Google Scholar]
- Sánchez-Guerrero, M.C.; Lorenzo, P.; Medrano, E.; Castilla, N.; Soriano, T.; Baille, A. Effect of variable CO2 enrichment on greenhouse production in mild winter climates. Agric. Meteorol. 2005, 132, 244–252. [Google Scholar] [CrossRef]
- Tagawa, A.; Mizoguchi, C.; Ehara, M.; Nakashima, T. The method of spread and partitioning of CO2 in the strawberry cultivation greenhouse. Environ. Cont. Biol. Abst. 2015, 164–165. [Google Scholar]
- Shishido, Y. Translocation and Distribution of Photoassimilates: Consider Vegetable Productivity; Yokendo: Tokyo, Japan, 2016; pp. 3–4. [Google Scholar]
- Kuroyanagi, T. History of carbon dioxide application in greenhouses and engineering approach to efficient application. Agric. Hortic. 2014, 89, 143–148. [Google Scholar]
- Kawashima, N. Present situation and problems of CO2 enrichment in a greenhouse. J. Agr. Met. 1991, 47, 177–182. [Google Scholar] [CrossRef]
- Chen, K.; Hu, G.Q.; Lenz, F. Effects of CO2 concentration on strawberry. I. Plant growth analysis. Angew. Bot. 1997, 71, 168–172. [Google Scholar]
- Nishizawa, T.; Hori, Y. Translocation and distribution of 14C-photoassimilates in strawberry plants varying in developmental stages of the inflorescence. J. Jpn. Soc. Hort. Sci. 1988, 57, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Hu, G.Q.; Lenz, F. Effects of CO2 concentration on strawberry. VI. Fruits yield and quality. Angew. Bot. 1997, 71, 195–200. [Google Scholar]
- Deng, X.; Woodward, F.I. The growth and yield responses of Fragaria ananassa to elevated CO2 and N supply. Ann. Bot. 1998, 81, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Mantri, N.; Lou, H.; Hu, Y.; Sun, D.; Zhu, Y.; Dongand, T.; Lu, H. Effects of elevated CO2 and temperature on yield and fruit quality of strawberry (Fragaria 6ananassa Duch.) at two levels of nitrogen application. PLoS ONE 2012, 7, e41000. [Google Scholar] [CrossRef]
- Kuroyanagi, T.; Yasuba, K.; Higashide, T.; Iwasaki, Y.; Takaichi, M. Efficiency of carbon dioxide enrichment in an unventilated greenhouse. Biosyst. Eng. 2014, 119, 58–68. [Google Scholar] [CrossRef]
- Yasutake, D.; Tanioka, H.; Ino, A.; Takahashi, A.; Yokoyama, T.; Mori, M.; Kitano, M.; Miyauchi, K. Dynamic evaluation of natural ventilation characteristics of a greenhouse with CO2 enrichment. Acad. J. Agric. Res. 2017, 5, 312–319. [Google Scholar] [CrossRef]
- Iwasaki, Y. Chapter 5. Carbon Dioxide control. In Greenhouse Horticulture Plant Factory Handbook; Japan Greenhouse Horticulture Association, Ed.; Rural Culture Association: Tokyo, Japan, 2015; p. 186. [Google Scholar]
- Wada, Y.; Soeno, T.; Inaba, Y. Effects of light and temperature on photosynthetic enhancement by high CO2 concentration of strawberry cultivar ‘Tochiotome’ leaves under forcing or half-forcing culture. Jpn. J. Crop. Sci. 2010, 79, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Forney, C.F.; Breen, P.J. Growth of strawberry fruit and sugar uptake of fruit discs at different inflorescence positions. Sci. Hortic. 1985, 27, 55–62. [Google Scholar] [CrossRef]
- Tagawa, A.; Ito, Y.; Ehara, M.; Araki, T.; Ozaki, Y.; Shishido, Y. Effect of Day and Night Temperature on Translocation and Distribution of 13C- Photosynthetic Assimilates in Strawberry ‘Sagahonoka’. J. Japan. Soc. Hort. Sci. 2021, 20, 95–100. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Hidaka, K.; Okayasu, T.; Yasutake, D.; Kitano, M. Effects of local CO2 enrichment on strawberry cultivation during the winter season. Environ. Control. Biol. 2017, 55, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Ishii, S.; Fujimaki, S.; Suzui, N.; Matsuhashi, S.; Honda, I.; Shishido, Y.; Kawachi, N. Real-time analysis of photoassimilate translocation in intact eggplant fruit using 11CO2 and a positron-emitting tracer imaging system. J. Jpn. Soc. Hort. Sci. 2008, 77, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, K.; Miyoshi, Y.; Ishii, S.; Suzui, N.; Yin, Y.; Kurita, K.; Nagao, K.; Araki, T.; Yasutake, D.; Kitano, M.; et al. Dynamic analysis of photosynthate translocation into strawberry fruits using non-invasive 11C-labeling supported with conventional destructive measurements using 13C-labeling. Front. Plant Sci. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Hidaka, K.; Yin, Y.; Suzui, N.; Kurita, K.; Kawachi, N. Non-invasive 11C-imaging revealed the spatiotemporal variability in the translocation of photosynthates into strawberry fruits in response to increasing daylight integrals at leaf surface. Front. Plant Sci. 2021, 12, 1–14. [Google Scholar] [CrossRef]


| Year | CO2 Treatment (µmol mol−1) | Number of Marketable Fruits per Plant | Average Fruit Weight (g) | Marketable Fruit Rate (%) | Marketable Fruits Yield per Plant (g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Month | Total | ||||||||
| 11–12 | 1–2 | 3–4 | 5–6 | ||||||
| 2015 | 400 | 38.6 | 13.4 | 81.4 | 74.6 | 126.2 | 179.0 | 135.6 | 515.4 |
| 800 | 48.0 | 14.4 | 87.6 | 96.5 | 171.4 | 215.3 | 193.4 | 676.6 | |
| 2016 | 400 | 35.4 | 13.5 | 78.8 | 50.3 | 124.6 | 158.1 | 143.5 | 476.4 |
| 800 | 41.4 | 13.9 | 80.8 | 67.1 | 129.0 | 186.7 | 193.0 | 575.8 | |
| Year | * | ns | ns | ** | * | * | ns | * | |
| CO2 treatment | ** | * | ns | * | * | ** | ** | ** | |
| Interaction | ns | ns | ns | ns | * | ns | ns | ns | |
| CO2 Treatment | CO2 Amount Used 1 | Returns 2 | Shipping Cost 4 | CO2 Application Cost (Yen a−1) | Difference of Returns 7 | |
|---|---|---|---|---|---|---|
| (µmol mol−1) | (kg a−1) | (Yen a−1) 3 | (Yen a−1) | Equipment Cost 5 | LPG Fuel Cost 6 | (Yen a−1) |
| 400 | 57.1 | 484,783 | 105,683 | 1500 | 2997 | - |
| 800 | 288.1 | 610,693 | 133,131 | 4400 | 15,123 | - |
| Difference | 231.0 | 125,910 | 27,448 | 2900 | 12,126 | 83,436 |
| Rate of 800/400(%) | 505 | 126 | 126 | 293 | 505 | - |
| Experimental Plot | Leaves (3rd–7th Leaf) | New Leaves (First–Second Leaf) | Total Leaves 2 | Crown | Aerial Part 3 | Roots | |
|---|---|---|---|---|---|---|---|
| Period | CO2 Concentration 1 (μmol mol−1) | ||||||
| Flowering time | - | 6.90 ± 0.25 a | 3.36 ± 0.24 a | 10.26 ± 0.19 ab | 2.42 ± 0.12 | 13.48 ± 0.24 c | 6.86 ± 0.41 cd |
| 12days After flowering | 400 | 7.52 ± 0.25 a | 2.21 ± 0.23 c | 9.73 ± 0.46 ab | 2.79 ± 0.17 | 14.50 ± 0.31 c | 8.62 ± 0.53 bc |
| 800 | 7.53 ± 0.17 a | 1.96 ± 0.05 c | 9.49 ± 0.19 ab | 2.63 ± 0.16 | 14.38 ± 0.20 c | 9.74 ± 0.88 ab | |
| 24days After flowering | 400 | 6.60 ± 0.20 a | 2.23 ± 0.15 bc | 8.83 ± 0.35 b | 2.75 ± 0.06 | 17.47 ± 0.75 b | 10.77 ± 0.26 ab |
| 800 | 7.66 ± 0.39 a | 2.99 ± 0.15 ab | 10.66 ± 0.47 a | 2.88 ± 0.13 | 20.18 ± 0.66 a | 11.07 ± 0.64 a | |
| Experimental Plot | Primary Fruit | Secondary Fruits | Tertiary Fruits | Peduncle | Fruit Bunch 4 | Total 5 | |
| Period | CO2 Concentration (μmol mol−1) | ||||||
| Flowering time | - | - | - | - | - | 0.81 ± 0.03 c | 20.34 ± 0.62 cd |
| 12days After flowering | 400 | 0.56 ± 0.06 c | 0.39 ± 0.05 b | 0.27 ± 0.04 b | 0.75 ± 0.08 b | 1.97 ± 0.20 bc | 23.12 ± 0.36 bc |
| 800 | 0.63 ± 0.04 c | 0.47 ± 0.04 b | 0.34 ± 0.02 b | 0.82 ± 0.05 b | 2.27 ± 0.15 b | 24.12 ± 0.90 b | |
| 24days After flowering | 400 | 2.35 ± 0.14 b | 1.44 ± 0.19 a | 0.93 ± 0.14 a | 1.18 ± 0.13 a | 5.90 ± 0.46 a | 28.69 ± 0.82 a |
| 800 | 2.87 ± 0.12 a | 1.56 ± 0.18 a | 1.06 ± 0.10 a | 1.16 ± 0.03 a | 6.65 ± 0.36 a | 31.25 ± 0.95 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagawa, A.; Ehara, M.; Ito, Y.; Araki, T.; Ozaki, Y.; Shishido, Y. Effects of CO2 Enrichment on Yield, Photosynthetic Rate, Translocation and Distribution of Photoassimilates in Strawberry ‘Sagahonoka’. Agronomy 2022, 12, 473. https://doi.org/10.3390/agronomy12020473
Tagawa A, Ehara M, Ito Y, Araki T, Ozaki Y, Shishido Y. Effects of CO2 Enrichment on Yield, Photosynthetic Rate, Translocation and Distribution of Photoassimilates in Strawberry ‘Sagahonoka’. Agronomy. 2022; 12(2):473. https://doi.org/10.3390/agronomy12020473
Chicago/Turabian StyleTagawa, Ai, Megumi Ehara, Yuusuke Ito, Takuya Araki, Yukio Ozaki, and Yoshihiro Shishido. 2022. "Effects of CO2 Enrichment on Yield, Photosynthetic Rate, Translocation and Distribution of Photoassimilates in Strawberry ‘Sagahonoka’" Agronomy 12, no. 2: 473. https://doi.org/10.3390/agronomy12020473
APA StyleTagawa, A., Ehara, M., Ito, Y., Araki, T., Ozaki, Y., & Shishido, Y. (2022). Effects of CO2 Enrichment on Yield, Photosynthetic Rate, Translocation and Distribution of Photoassimilates in Strawberry ‘Sagahonoka’. Agronomy, 12(2), 473. https://doi.org/10.3390/agronomy12020473






