Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planting Material and Conditions
2.2. Measurement of Growth Characteristics
2.3. Estimation of Leaf Chlorophyll Pigments and Color Intensity Value
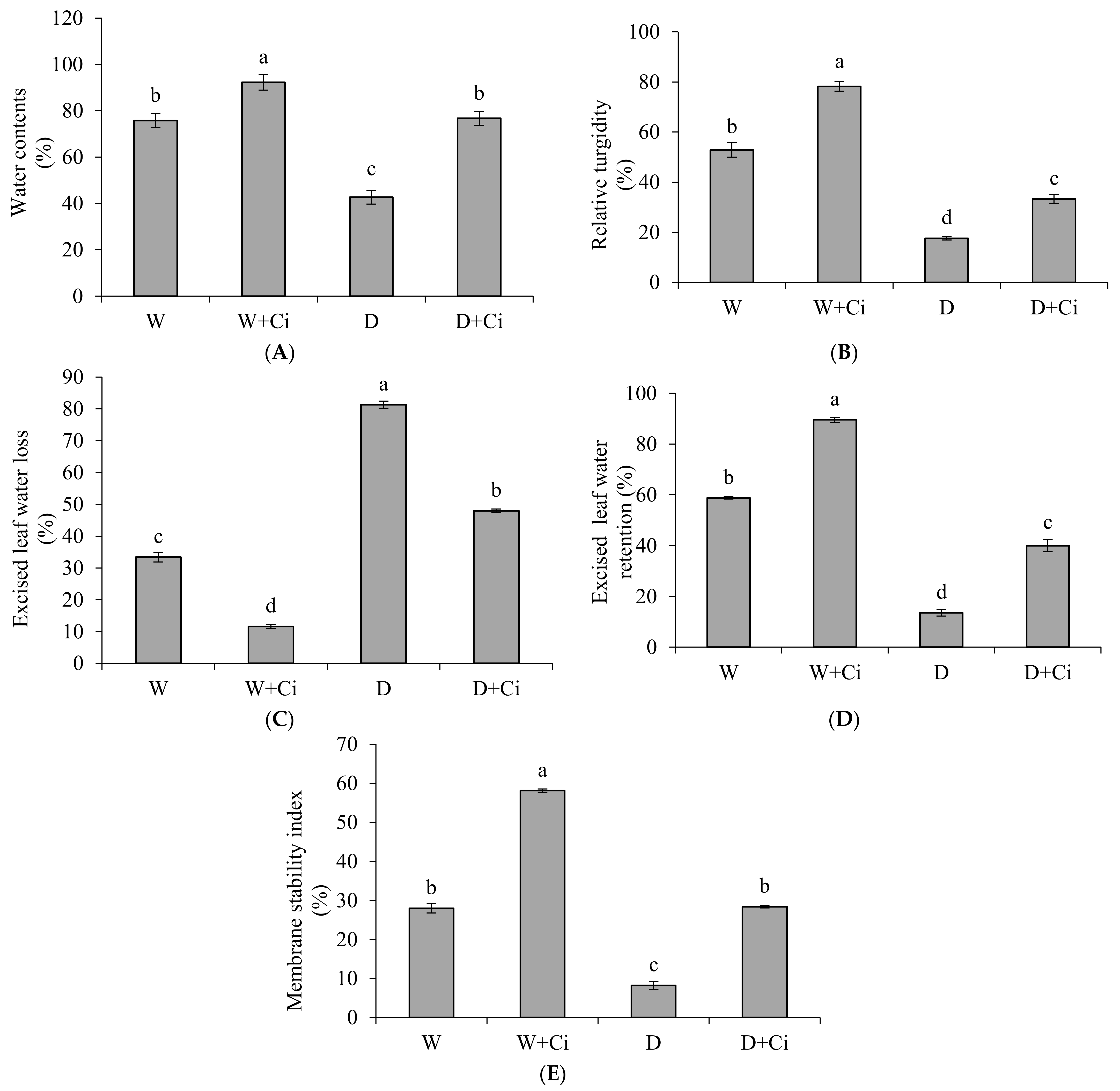
2.4. Estimation of Leaf Water Status and Membrane Stability Index
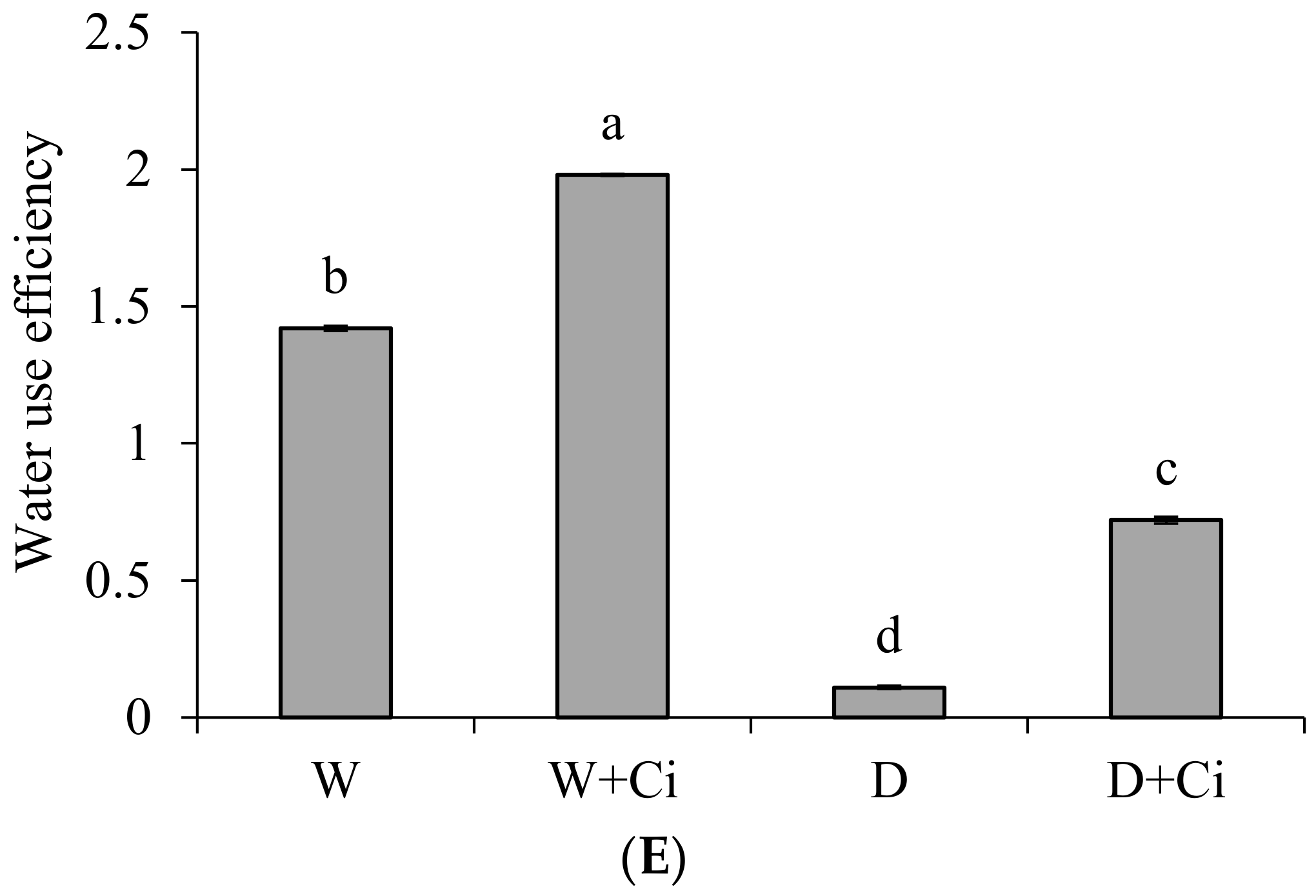
2.5. Determination of Gas Exchange Parameters
2.6. Determination of Antioxidant Enzymes Activity
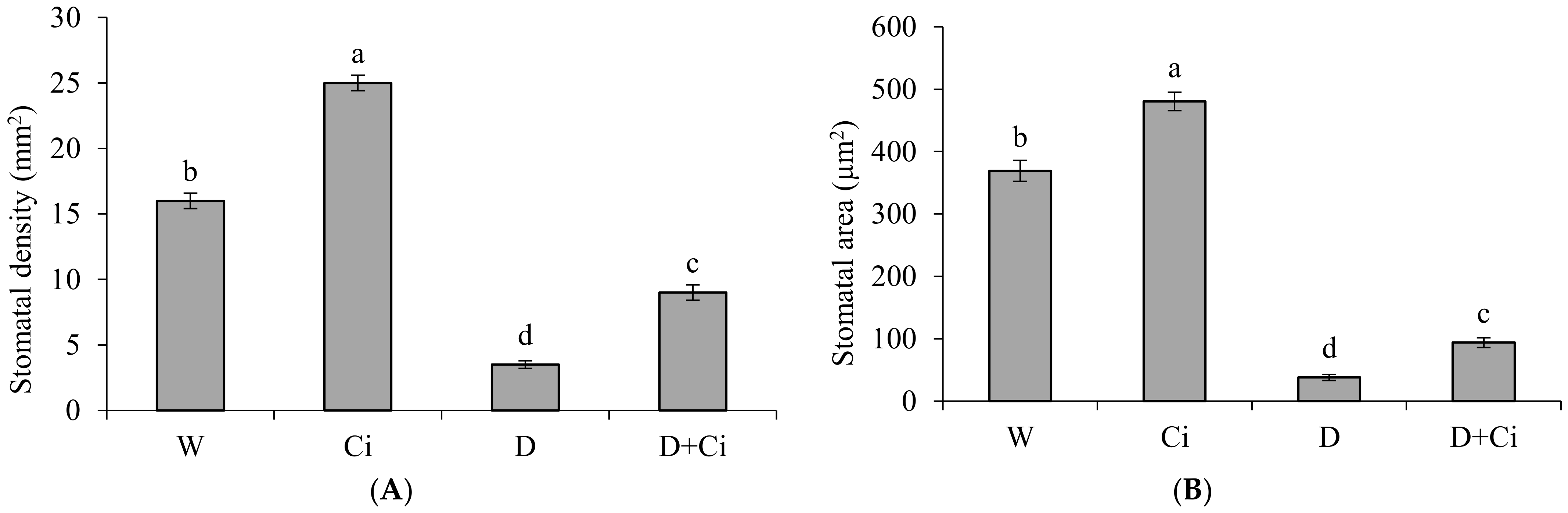
2.7. Measurement of Stomatal Density and Area
2.8. Statistical Analysis
3. Results
3.1. Biomass Attributes
3.2. Chlorophyll and Leaf Color Parameters
3.3. Water Status and Membrane Stability Index
3.4. Gas Exchange Parameters
3.5. Antioxidant Enzymes
3.6. Anatomical Parameters
3.7. Pearson Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cromack, H.T.H.; Smith, J.M. Calendula officinalis—Production Potential and Crop Agronomy in Southern England. Ind. Crop. Prod. 1998, 7, 223–2296. [Google Scholar] [CrossRef]
- Joly, R.; Forcella, F.; Peterson, D.; Eklund, J. Planting depth for oilseed calendula. Ind. Crop. Prod. 2013, 42, 133–136. [Google Scholar] [CrossRef]
- Raal, A.; Kirsipuu, K.; Must, R.; Tenno, S. Content of total carotenoids in Calendula officinalis L. From different countries cultivated in Estonia. Nat. Prod. Commun. 2009, 4, 35–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, S.M.; Kashani, H.H. Pot marigold (Calendula officinalis) medicinal usage and cultivation. Sci. Res. Essays 2012, 7, 1468–1472. [Google Scholar]
- Matic, I.Z.; Juranic, Z.; Savikin, K.; Zdunic, G.; Nadvinski, N.; Godevac, D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytother. Res. 2013, 27, 852–858. [Google Scholar] [CrossRef]
- Biermann, U.; Butte, W.; Holtgrefe, R.; Feder, W.; Metzger, J.O. Esters of calendula oil and tung oil as reactive diluents for alkyd resins. Eur. J. Lipid Sci. Technol. 2010, 112, 103–109. [Google Scholar] [CrossRef]
- Rezaei Nejad, A.; Khosravi Shakib, A. Ornamental value of Calendula officinalis “Yellow Gitana” as a result of nitrogen fertilizer and plant density. Int. J. Agric. Crop Sci. 2013, 5, 362–365. [Google Scholar]
- Grant, O.M.; Davies, M.J.; Longbottom, H.; Harrison-Murray, R. Evapotranspiration of container ornamental shrubs: Modelling crop-specific factors for a diverse range of crops. Irrig. Sci. 2012, 30, 1–12. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Ren, B.; Ding, L.; Gao, C.; Shen, Q.; Guo, S. Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol. 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Nawaz, F.; Ahmad, F.; Ahmad, N.; Masood, S. Protective effect of potassium and chitosan supply on growth, physiological processes and antioxidative machinery in sunflower (Helianthus annuus L.) under drought stress. Ecotoxicol. Environ. Saf. 2020, 187, 109841. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Mphande, W.; Kettlewell, P.S.; Grove, I.G.; Farrell, A.D. The potential of antitranspirants in drought management of arable crops: A review. Agric. Water Manag. 2020, 236, 106143. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Tourian, N.; Sinaki, J.M.; Hasani, N.; Madani, H. Change in photosynthetic pigment concentration of wheat grass (Agropyron repens) cultivars response to drought stress and foliar application with chitosan. Int. J. Agron. Plant Prod. 2013, 4, 1084–1091. [Google Scholar]
- El-Serafy, R.S. Phenotypic Plasticity, Biomass Allocation, and Biochemical Analysis of Cordyline Seedlings in Response to Oligo-Chitosan Foliar Spray. J. Soil Sci. Plant Nutr. 2020, 20, 1503–1514. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Salachna, P.; Zawadzi ńska, A. Effect of chitosan on plant growth, flowering and corms yield of potted freesia. Ecol. Eng. 2014, 15, 97–102. [Google Scholar]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdel-Hamid, A.M.; Yessoufou, K.; Al-Mana, F.A.; El-Ansary, D.O.; Mahmoud, E.A.; Al-Yafrasi, M.A. Physiological and molecular characterization of water-stressed Chrysanthemum under robinin and chitosan treatment. Acta Physiol. Plant. 2020, 42, 31. [Google Scholar] [CrossRef]
- Carleton, A.E.; Foote, W.H. A comparison of methods for estimating total leaf area of barley plants. Crop Sci. 1965, 5, 602–603. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, J.; Song, T.; Li, J.; Tian, J.; Jin, K.; Yao, Y. Low medium pH value enhances anthocyanin accumulation in Malus crabapple leaves. PLoS ONE 2014, 9, 97904. [Google Scholar] [CrossRef]
- Redondo-Gomez, S.; Andrades-Moreno, L.; Mateos-Naranjo, E.; Parra, R.; Valera-Burgos, J.; Aroca, R. Synergic effect of salinity and zinc stress on growth and photosynthetic responses of the cordgrass, Spartina densiflora. J. Exp. Bot. 2011, 62, 5521–5530. [Google Scholar] [CrossRef]
- Clausen, J.J.; Kozlowski, T.T. Use of the relative turgidity technique for measurement of water stresses in gymnosperm leaves. Can. J. Bot. 1965, 43, 305–316. [Google Scholar] [CrossRef]
- Lonbani, M.; Arzani, A. Morpho-physiological traits associated with terminal drought-stress tolerance in triticale and wheat. Agron. Res. 2011, 9, 315–329. [Google Scholar]
- Clarke, J.M.; McCaig, T.N. Excised-leaf water retention capability as an indicator of drought resistance of Triticum genotypes. Can. J. Plant Sci. 1982, 62, 571–578. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Plant Biol. 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Urbanek, H.; Kuzniak-Gebarowska, E.; Herka, K. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plant. 1991, 13, 43–50. [Google Scholar]
- Van Rossum, M.W.P.C.; Alberda, M.; van der Plas, L.H.W. Role of oxidative damage in tulip bulb scale micro propagation. Plant Sci. 1997, 130, 207–216. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Yavari, S.; Hassani, M.E.; Yavari, S. Induction of autotetraploidy in dragonhead (Dracocephalum moldavica L.) by colchicine treatment. J. Fruit Ornam. Plant Res. 2010, 18, 23–35. [Google Scholar]
- Lopez-Moya, F.; Escudero, N.; Zavala-Gonzalez, E.A.; Esteve-Bruna, D.; Blázquez, M.A.; Alabadí, D.; Lopez-Llorca, L.V. Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosan. Sci. Rep. 2017, 7, 16813. [Google Scholar] [CrossRef] [Green Version]
- Tantasawat, P.; Wannajindaporn, A.; Chantawaree, C.; Wangpunga, C.; Poomsom, K.; Sorntip, A. Chitosan stimulates growth of micropropagated Dendrobium plantlets. Acta Hortic. 2010, 878, 205–212. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A. Improving seed germination of Althaea rosea L. under salt stress by seed soaking with silicon and nano silicon. Egypt. J. Plant Breed. 2017, 21, 764–777. [Google Scholar]
- Para đikovi ć, N.; Zeljkovi ć, S.; Tkalec, M.; Vinkovi ć, T.; Maksimovi ć, I.; Haramija, J. Influence of biostimulant application on growth, nutrient status and proline concentration of begonia transplants. Biol. Agric. Hortic. 2017, 33, 89–96. [Google Scholar] [CrossRef]
- Uthairatanakij, A.; Teixeira da Silva, J.A.; Obsuwan, K. Chitosan for improving orchid production and quality. Orchid Sci. Biotechnol. 2007, 1, 1–5. [Google Scholar]
- Usmani, M.M.; Nawaz, F.; Majeed, S.; Shehzad, M.A.; Ahmad, K.S.; Akhtar, G.; Aqib, M.; Shabbir, R.N. Sulfate-mediated drought tolerance in maize involves regulation at physiological and biochemical levels. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Muley, A.B.; Shingote, P.R.; Patila, A.P.; Dalvi, S.G.; Suprasanna, P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.). Carbohydr. Polym. 2019, 210, 289–301. [Google Scholar] [CrossRef]
- Nahar, S.J.; Kazuhiko, S.; Haque, S.M. Effect of polysaccharides including elicitors on organogenesis in protocorm-like body (PLB) of Cymbidium insigne in vitro. J. Agric. Sci. Technol. 2012, 2, 1029–1033. [Google Scholar]
- Khayatnezhad, M.; Gholamin, R.; Jamaati, S.; Zabihi-E-Mahmoodabad, R. The leaf chlorophyll content and stress resistance relationship considering in Corn cultivars (Zea mays). Adv. Environ. Biol. 2011, 5, 118–122. [Google Scholar]
- Oraee, A.; Tehranifar, A. Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Sci. Hortic. 2020, 265, 109225. [Google Scholar] [CrossRef]
- Hajiboland, R.; Farhanghi, F. Effect of low boron supply in turnip plants under drought stress. Biol. Plant. 2011, 55, 775–778. [Google Scholar] [CrossRef]
- Hussain, R.A.; Ahmad, R.; Nawaz, F.; Ashraf, M.Y.; Warraich, E.A. Foliar NK application mitigates drought effects in sunflower (Helianthus annuus L.). Acta Physiol. Plant. 2016, 38, 83. [Google Scholar] [CrossRef]
- Yagmur, M.; Kaydan, D. Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. Afr. J. Biotechnol. 2008, 7, 2156–2162. [Google Scholar]
- Zivcak, M.; Repková, J.; Olšovská, K.; Brestič, M. Osmotic adjustment in winter wheat varieties and its importance as a mechanism of drought tolerance. Cereal Res. Commun. 2009, 37, 569–572. [Google Scholar]
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2007, 31, 11–38. [Google Scholar] [CrossRef]
- Lee, B.R.; Jin, Y.L.; Park, S.H.; Zaman, R.; Zhang, Q.; Avice, J.C.; Ourry, A.; Kim, T.H. Genotypic variation in N uptake and assimilation estimated by 15N tracing water deficit-stressed Brassica napus. Environ. Exp. Bot. 2015, 109, 73–79. [Google Scholar] [CrossRef]
- González Gómez, H.; Ramírez Godina, F.; Ortega Ortiz, H.; Benavides Mendoza, A.; Robledo Torres, V.; Cabrera De la Fuente, M. Use of chitosan-PVA hydrogels with copper nanoparticles to improve the growth of grafted watermelon. Molecules 2017, 22, 1031. [Google Scholar] [CrossRef] [Green Version]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor. Environ. Exp. Bot. 2007, 60, 105–111. [Google Scholar] [CrossRef]
- Alvarez, S.; Navarro, A.; Nicolás, E.; Sánchez-Blanco, M.J. Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions. Sci. Hortic. 2011, 129, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Riaz, A.; Younis, A.; Taj, A.R.; Riaz, S. Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.). Pak. J. Bot. 2013, 45, 123–131. [Google Scholar]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of Wheat (Triticum aestivum L.) Genotypes for Drought Tolerance through Agronomic and Physiological Response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Naz, M.; Hussain, S.; Javed, T.; Aslam, S.; Raza, A.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.; et al. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE 2021, 16, e0260556. [Google Scholar] [CrossRef]
- Bahar, A.A.; Faried, H.N.; Razzaq, K.; Ullah, S.; Akhtar, G.; Amin, M.; Bashir, M.; Ahmed, N.; Masoud, F.; Ahmar, S.; et al. Potassium-induced drought tolerance of potato by improving morpho-physiological and biochemical attributes. Agronomy 2021, 11, 2573. [Google Scholar] [CrossRef]
- Islam, M.R.; Sarker, B.C.; Alam, M.A.; Javed, T.; Alam, M.J.; Zaman, M.S.U.; Azam, M.G.; Shabbir, R.; Raza, A.; Habib-ur-Rahman, M.; et al. Yield Stability and Genotype Environment Interaction of Water Deficit Stress Tolerant Mung Bean (Vigna radiata L. Wilczak) Genotypes of Bangladesh. Agronomy 2021, 11, 2136. [Google Scholar] [CrossRef]
- Mahmood, T.; Wang, X.; Ahmar, S.; Abdullah, M.; Iqbal, M.S.; Rana, R.M.; Yasir, M.; Khalid, S.; Javed, T.; Mora-Poblete, F.; et al. Genetic potential and inheritance pattern of phenological growth and drought tolerance in cotton (Gossypium hirsutum L.). Front. Plant Sci. 2021, 12, 705392. [Google Scholar]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.; Hakim, M.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Afzal, I.; Saleem, S.; Skalicky, M.; Javed, T.; Bakhtavar, M.A.; Kamran, M.; Shahid, M.; Sohail Saddiq, M.; Afzal, A.; Shafqat, N.; et al. Magnetic field treatments improves sunflower yield by inducing physiological and biochemical modulations in seeds. Molecules 2021, 26, 2022. [Google Scholar] [CrossRef]
- Afzal, I.; Imran, S.; Javed, T.; Basra, S.M.A. Evaluating the integrative response of moringa leaf extract with synthetic growth promoting substances in maize under early spring conditions. S. Afr. J. Bot. 2020, 132, 378–387. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Afzal, I.; Sabagh, A.E.; Ali, A.; Vicente, O.; Chen, P. Modern biotechnologies: Innovative and sustainable approaches for the improvement of sugarcane tolerance to environmental stresses. Agronomy 2021, 11, 1042. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress. Acta Physiol. Plant. 2018, 40, 206. [Google Scholar] [CrossRef]
- Yin, H.; Frette, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought: From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Doares, S.H.; Syrovets, T.; Wieler, E.W.; Ryan, A. Oligogalacturonides and chitosan activate plant defensive gene through the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098. [Google Scholar] [CrossRef] [Green Version]



| Treatments | NOL | LA (cm2) | SL (cm) | RL (cm) | SDW (g) | RDW(g) | FDW |
|---|---|---|---|---|---|---|---|
| W | 35 c ± 1.76 | 3910 b ± 30.41 | 14.00 c ± 0.34 | 23 c ± 1.18 | 12.98 c ± 0.19 | 9.00 c ± 0.39 | 0.84 b ±0.04 |
| W + Ci | 53 a ± 0.34 | 5195 a ± 58.87 | 23.67 a ± 0.34 | 39 a ± 0.59 | 46.88 a ± 0.40 | 27.33 a ± 0.39 | 1.77 a ± 0.07 |
| D | 28 d ± 1.48 | 3420 c ± 183.42 | 12.67 c ± 0.90 | 17 d ± 1.18 | 12.77 c ± 0.69 | 5.78 d ± 0.41 | 0.64 c ± 0.02 |
| D + Ci | 40 b ± 0.90 | 4097 b ± 46.78 | 17.67 b ± 0.90 | 27 b ± 0.90 | 29.33 b ± 0.52 | 11.33 b ± 0.71 | 0.95 b ± 0.04 |
| p-value | |||||||
| D | <0.0001 | <0.0001 | 0.0004 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Ci | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D × Ci | 0.0755 | 0.0149 | 0.0117 | 0.0116 | <0.0001 | <0.0001 | 0.0001 |
| CV | 5.45 | 4.10 | 6.76 | 6.37 | 3.25 | 6.27 | 7.42 |
| Treatments | Chl a (mg g−1) | Chl b (mg g−1) | Car (mg g−1) | L* | a* | b* |
|---|---|---|---|---|---|---|
| W | 0.57 c ± 0.03 | 1.27 c ± 0.04 | 0.38 c ± 0.04 | 34.78 c ± 0.42 | 7.39 c ± 0.15 | 10.40 c ± 0.03 |
| W + Ci | 0.8 a ± 0.07 | 1.87 a ± 0.05 | 0.56 a ± 0.07 | 41.78 a ± 1.02 | 9.62 a ± 0.02 | 10.85 a ± 0.02 |
| D | 0.26 d ± 0.08 | 1 d ± 0.06 | 0.11 d ± 0.05 | 26.65 d ± 0.96 | 4.79 d ± 0.06 | 9.93 d ± 0.06 |
| D + Ci | 0.6 b ± 0.06 | 1.68 b ± 0.01 | 0.39 b ± 0.04 | 36.98 b ± 0.19 | 7.72 b ± 0.03 | 10.58 b ± 0.04 |
| p-value | ||||||
| D | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Ci | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| D × Ci | 0.0004 | 0.0052 | 0.0006 | 0.0243 | 0.0046 | 0.0120 |
| CV | 2.80 | 1.20 | 4.35 | 2.75 | 1.87 | 0.49 |
| LA | SDW | RDW | SL | RL | WC | RT | SD | FDW | Chl a | Chl b | Car | SOD | GPX | CAT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | |||||||||||||||
| SDW | 0.928 ** | ||||||||||||||
| RDW | 0.956 ** | 0.943 ** | |||||||||||||
| SL | 0.916 ** | 0.960 ** | 0.959 ** | ||||||||||||
| RL | 0.953 ** | 0.938 ** | 0.956 ** | 0.954 ** | |||||||||||
| WC | 0.840 ** | 0.746 ** | 0.770 ** | 0.805 ** | 0.862 ** | ||||||||||
| RT | 0.882 ** | 0.713 ** | 0.871 ** | 0.804 ** | 0.879 ** | 0.851 ** | |||||||||
| SD | 0.890 ** | 0.717 ** | 0.876 ** | 0.792 ** | 0.862 ** | 0.863 ** | 0.986 ** | ||||||||
| FDW | 0.962 ** | 0.932 ** | 0.984 ** | 0.957 ** | 0.960 ** | 0.776 ** | 0.882 ** | 0.867 ** | |||||||
| Chl a | 0.915 ** | 0.828 ** | 0.853 ** | 0.869 ** | 0.925 ** | 0.961 ** | 0.893 ** | 0.893 ** | 0.865 ** | ||||||
| Chl b | 0.886 ** | 0.929 ** | 0.845 ** | 0.917 ** | 0.923 ** | 0.877 ** | 0.718 ** | 0.715 ** | 0.849 ** | 0.928 ** | |||||
| Car | 0.904 ** | 0.812 ** | 0.842 ** | 0.856 ** | 0.913 ** | 0.975 ** | 0.896 ** | 0.899 ** | 0.848 ** | 0.993 ** | 0.916 ** | ||||
| SOD | 0.280 ns | 0.551 ns | 0.254 ns | 0.439 ns | 0.351 ns | 0.254 ns | −0.095 ns | −0.103 ns | 0.252 ns | 0.295 ns | 0.613 * | 0.268 ns | |||
| GPX | 0.277 ns | 0.523 ns | 0.220 ns | 0.406 ns | 0.319 ns | 0.268 ns | −0.104 ns | −0.106 ns | 0.223 ns | 0.303 ns | 0.606 * | 0.276 ns | 0.991 ** | ||
| CAT | 0.166 ns | 0.455 ns | 0.148 ns | 0.339 ns | 0.252 ns | 0.194 ns | −0.193 ns | −0.193 ns | 0.133 ns | 0.209 ns | 0.535 ns | 0.193 ns | 0.986 ** | 0.976 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. https://doi.org/10.3390/agronomy12020474
Akhtar G, Faried HN, Razzaq K, Ullah S, Wattoo FM, Shehzad MA, Sajjad Y, Ahsan M, Javed T, Dessoky ES, et al. Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy. 2022; 12(2):474. https://doi.org/10.3390/agronomy12020474
Chicago/Turabian StyleAkhtar, Gulzar, Hafiz Nazar Faried, Kashif Razzaq, Sami Ullah, Fahad Masoud Wattoo, Muhammad Asif Shehzad, Yasar Sajjad, Muhammad Ahsan, Talha Javed, Eldessoky S. Dessoky, and et al. 2022. "Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.)" Agronomy 12, no. 2: 474. https://doi.org/10.3390/agronomy12020474
APA StyleAkhtar, G., Faried, H. N., Razzaq, K., Ullah, S., Wattoo, F. M., Shehzad, M. A., Sajjad, Y., Ahsan, M., Javed, T., Dessoky, E. S., Abdelsalam, N. R., & Chattha, M. S. (2022). Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy, 12(2), 474. https://doi.org/10.3390/agronomy12020474








