Effects of Shading on the Internode Critical for Soybean (Glycine Max) Lodging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Comparison of Soybean Internode Lengths Growing under Different Planting Densities
2.1.2. Effects of Shading on Anatomical Structure and Fiber Composition of Soybean Stems
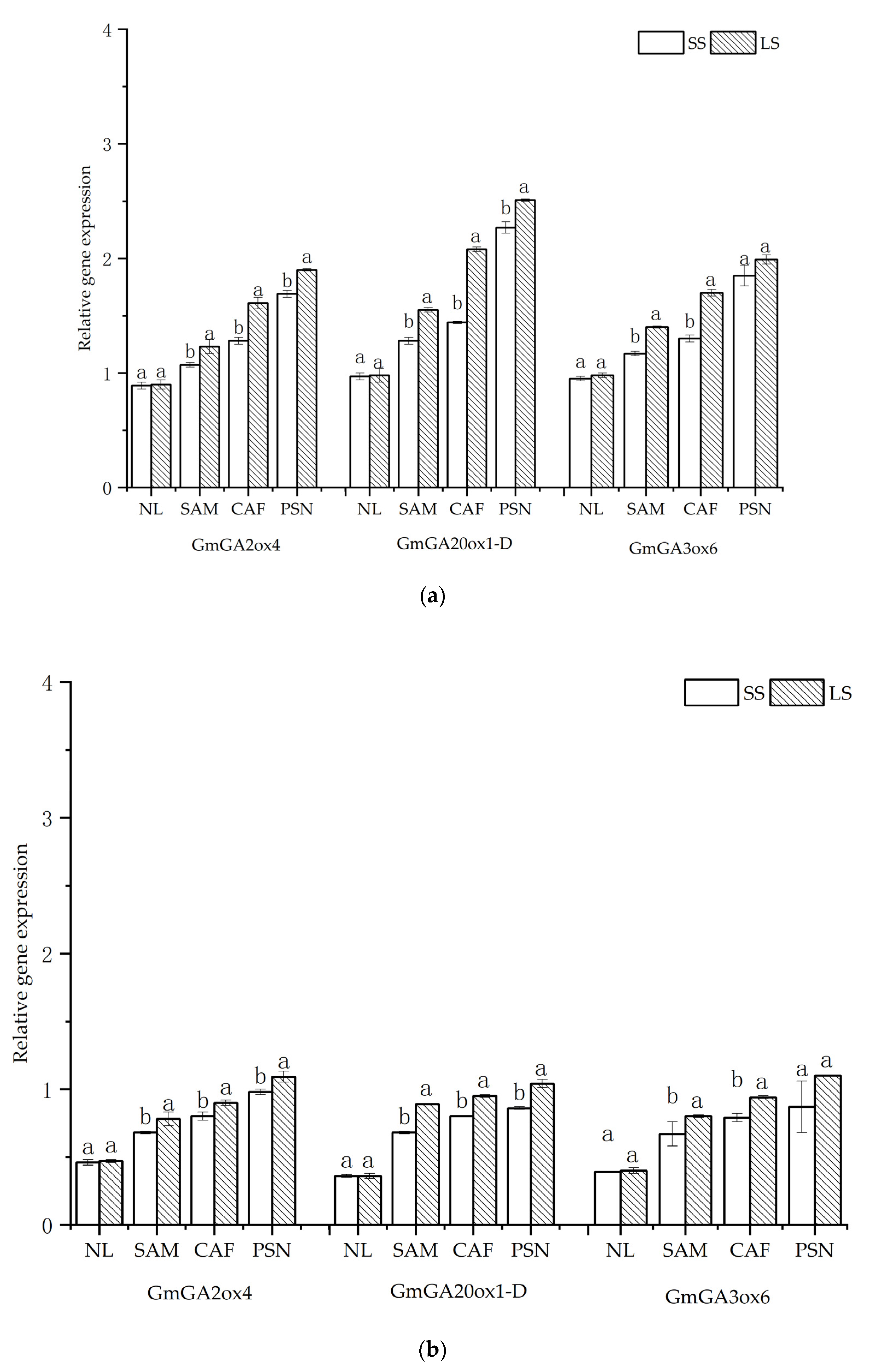
2.1.3. Effects of Shading on GAs in Soybean Stem and the Relative Expression of GA-Related Enzyme Genes
2.2. Measurements and Analyses
2.3. Statistical Analysis
3. Results
3.1. Comparison of Soybean Internode Length at Harvest under Different Planting Densities
3.2. Effects of Shading on Morphological Indexes of Soybean Internodes
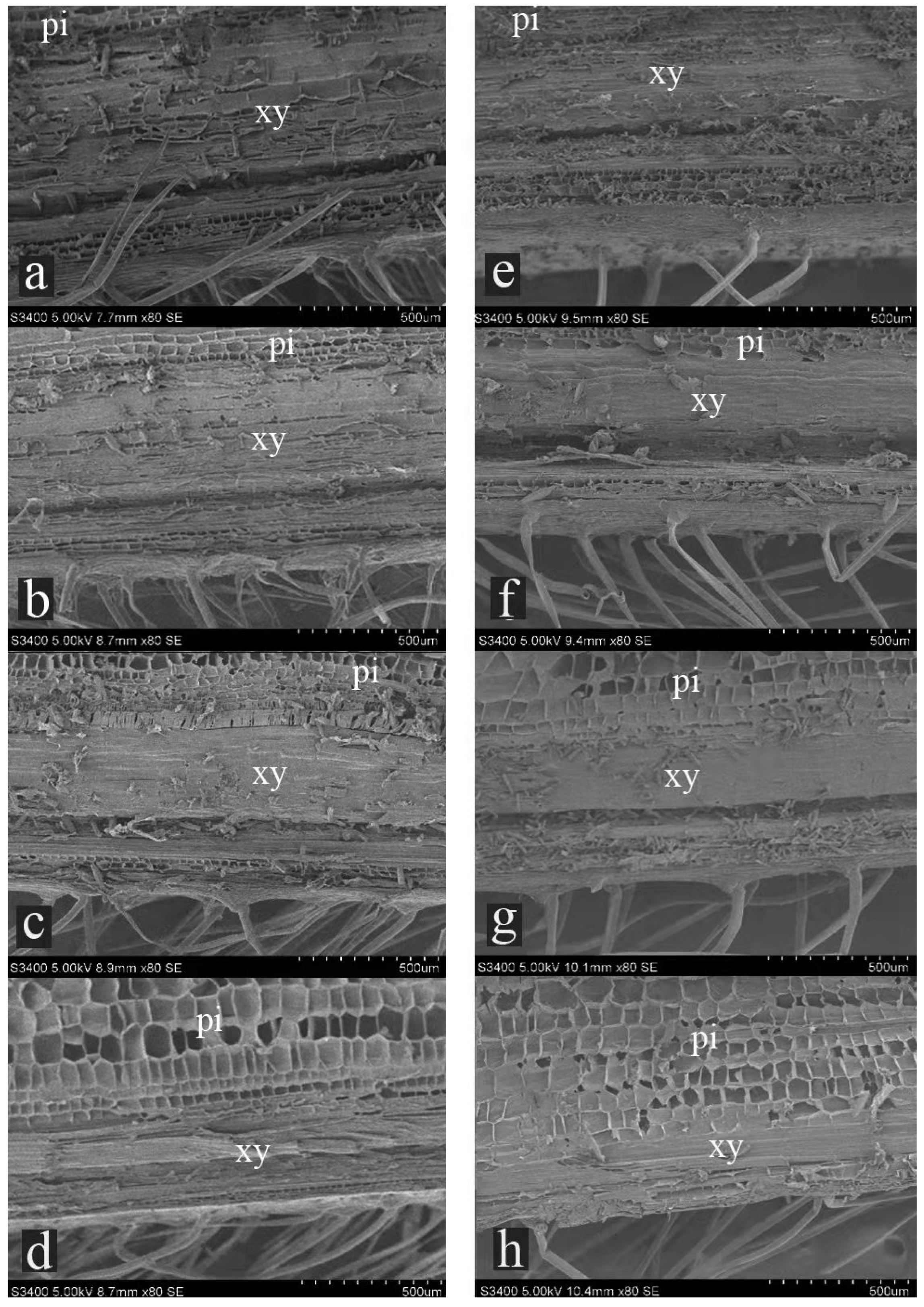
3.3. Effects of Shading on the Anatomical Structure of Internodes
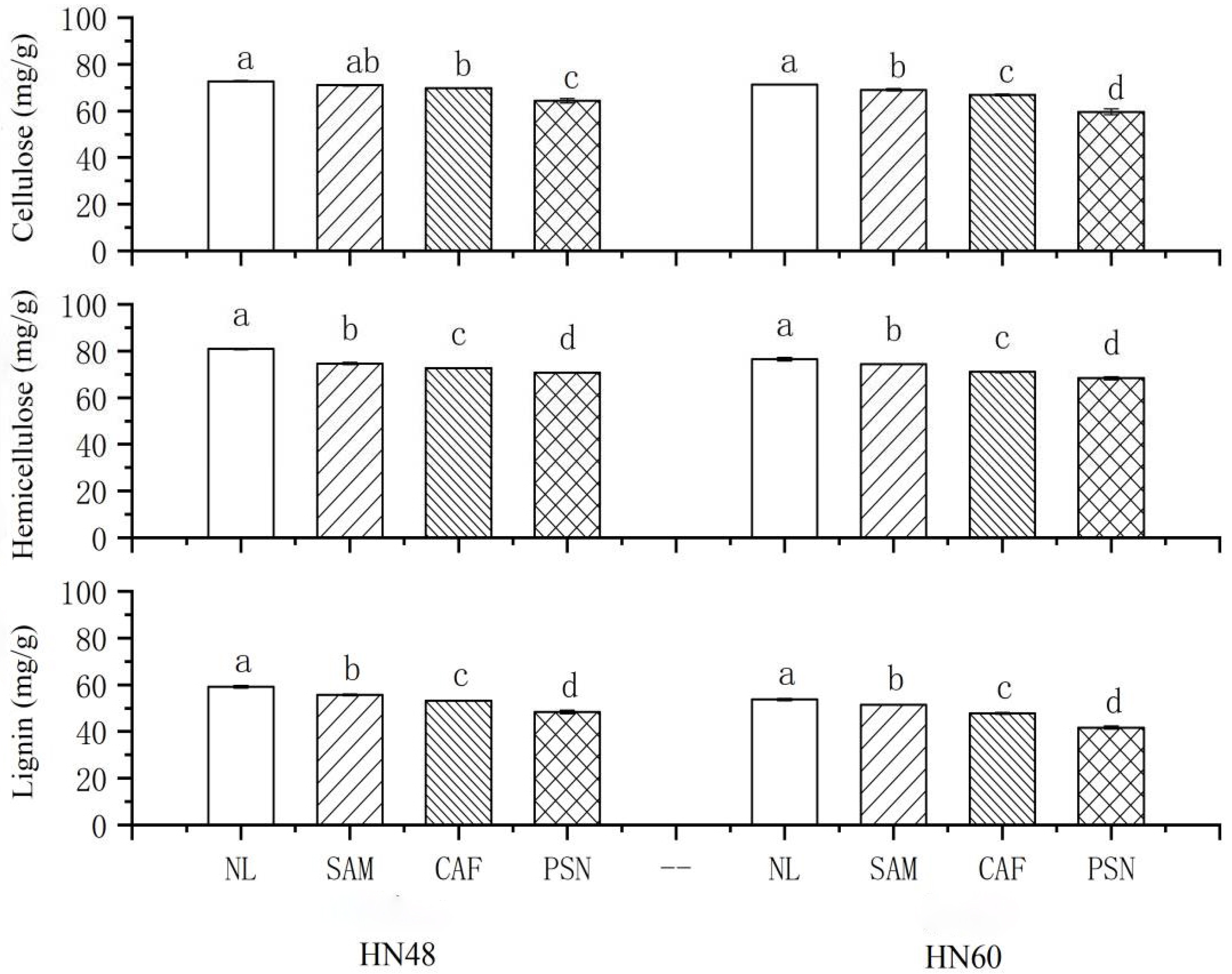
3.4. Effects of Shading on Soybean Internode Fiber Composition
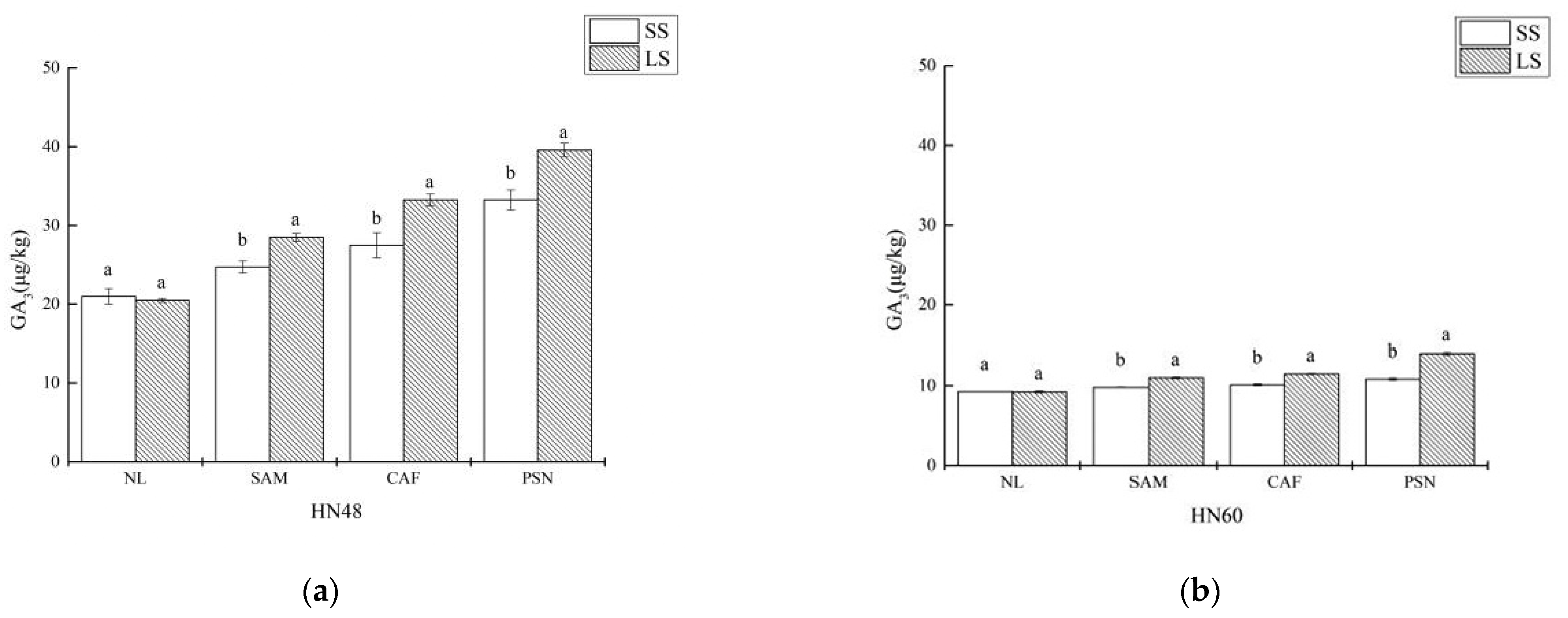
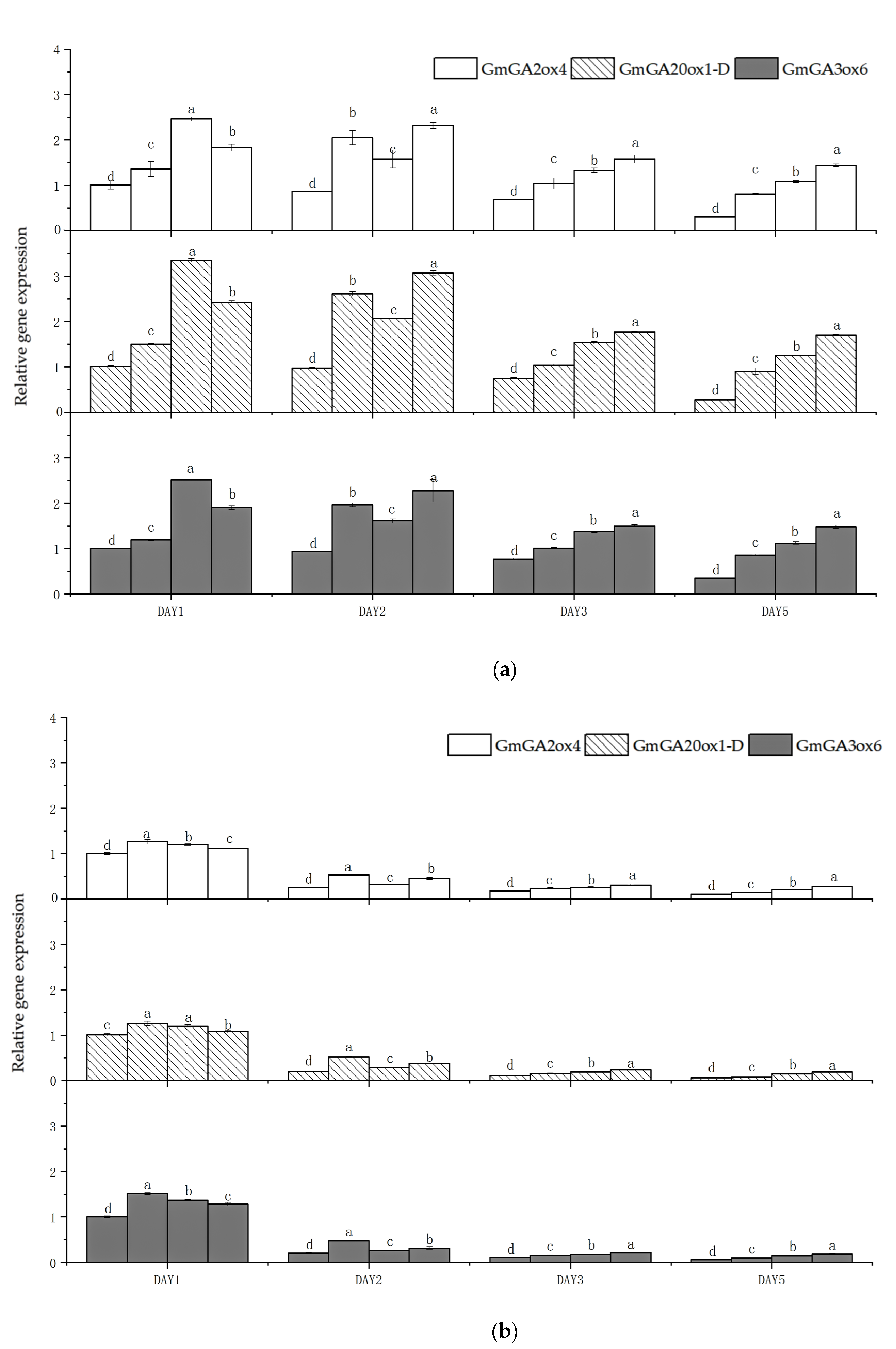
3.5. Effects of Shading on Soybean Internode GA3 Content and Relative Expression Levels of GA-Related Enzyme Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashiwagi, T.; Sasaki, H.; Ishimaru, K. Factors Responsible for Decreasing Sturdiness of the Lower Part in Lodging of Rice (Oryza sativa L.). Plant Prod. Sci. 2005, 8, 166–172. [Google Scholar] [CrossRef]
- Hiltbrunner, J.; Streit, B.; Liedgens, M. Are seeding densities an opportunity to increase grain yield of winter wheat in a living mulch of white clover? Field Crop. Res. 2007, 102, 163–171. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef] [Green Version]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and Shade-avoidance Responses in Plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, W.Z.; Jiang, C.D.; Wu, Y.S.; Chen, H.H.; Liu, W.Y.; Yang, W.Y. Tolerance vs. avoidance: Two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica 2015, 53, 259–268. [Google Scholar] [CrossRef]
- Liu, W.G.; Deng, Y.C.; Hussain, S.; Zou, J.L.; Yuan, J.; Luo, L.; Yang, C.Y.; Yuan, X.Q.; Yang, W.Y. Relationship between cellulose accumulation and lodging resistance in the stem of relay intercropped soybean [Glycine max (L.) Merr.]. Field Crop. Res. 2016, 196, 261–267. [Google Scholar] [CrossRef]
- Liu, W.G.; Hussain, S.; Liu, T.; Zou, J.L.; Ren, M.L.; Zhou, T.; Liu, J.; Yang, F.; Yang, W.Y. Shade stress decreases stem strength of soybean through restraining lignin biosynthesis. J. Integr. Agric. 2019, 18, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Iqbal, N.; Pang, T.; Khan, M.N.; Liu, W.G.; Yang, W.Y. Weak stem under shade reveals the lignin reduction behavior. J. Integr. Agric. 2019, 18, 496–505. [Google Scholar] [CrossRef]
- Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Lu, J.; Gao, Y.; Qin, S.; Zhang, Y.; Ahmad, I.; Zhou, T.; et al. Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem. Agronomy 2020, 10, 1177. [Google Scholar] [CrossRef]
- Zhang, J.; Smith, D.L.; Liu, W.G.; Chen, X.F.; Yang, W.Y. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. Afr. J. Biotechnol. 2011, 10, 14392–14398. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.S.; Gong, W.Z.; Yang, W.Y. Shade Inhibits Leaf Size by Controlling Cell Proliferation and Enlargement in Soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Fan, Y.F.; Wu, X.L.; Cheng, Y.J.; Liu, Q.L.; Feng, L.Y.; Chen, J.X.; Wang, Z.L.; Wang, X.C.; Yong, T.W.; et al. Auxin-to-Gibberellin Ratio as a Signal for Light Intensity and Quality in Regulating Soybean Growth and Matter Partitioning. Front. Plant Sci. 2018, 9, 56–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Yang, W.Y. Effects of Shading on Endogenous Hormone of Cardiocrinum Giganteum. North. Hortic. 2007, 8, 123–125. [Google Scholar]
- Gawronska, H.; Yang, Y.Y.; Furukawa, K.; Kendrick, R.E.; Takahashi, N.; Kamiya, Y. Effects of Low Irradiance Stress on Gibberellin Levels in Pea Seedlings. Plant Cell Physiol. 1995, 36, 1361–1367. [Google Scholar] [CrossRef]
- Suo, H.C.; Ma, Q.B.; Ye, K.X.; Yang, C.Y.; Tang, Y.J.; Hao, J.; Zhang, Z.Y.; Chen, M.L.; Feng, Y.Q.; Nian, H. Overexpression of AtDREB1A causes a severe dwarf phenotype by decreasing endogenous gibberellin levels in soybean [Glycine max (L.) Merr.]. PLoS ONE 2012, 7, e1515. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2001, 5, 523–530. [Google Scholar] [CrossRef]
- Coles, J.P.; Phillips, A.L.; Croker, S.J.; García-Lepe, R.; Lewis, M.J.; Hedden, P. Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J. 1999, 17, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Shan, F.X.; Wang, C.; Yan, C.; Dong, S.K.; Xu, Y.; Gong, Z.P.; Ma, C.M. Internode elongation pattern, internode diameter and hormone changes in soybean (Glycine max) under different shading conditions. Crop Pasture Sci. 2020, 71, 679–688. [Google Scholar] [CrossRef]
- Sauter, M.; Kende, H. Gibberellin-induced growth and regulation of the cell division cycle in deepwater rice. Planta 1992, 188, 362–368. [Google Scholar] [CrossRef]
- Reza, Y.; Morteza, S.; Hamidreza, M.; Dastan, S.; Alireza, N. Effect of Plant Density on Morphologic Characteristics Related to Lodging and Yield Components in Different Rice Varieties (Oriza Sativa L.). J. Agric. Sci. 2011, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.J.; Chen, J.; Shi, Y.H.; Li, Y.X.; Yin, Y.P.; Yang, D.Q.; Luo, Y.L.; Pang, D.W.; Xu, X.; Li, W.Q.; et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 2016, 7, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Echezona, B.C. Corn-stalk lodging and borer damage as influenced by varying corn densities and planting geometry with soybean (Glycinemax. L. Merrill). Int. Agrophys. 2007, 21, 133–143. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Ullah, S.; Fahad, S.; Zhou, G.S. Optimization of Nitrogen Rate and Planting Density for Improving Yield, Nitrogen Use Efficiency, and Lodging Resistance in Oilseed Rape. Front. Plant Sci. 2017, 8, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhang, R.; Hou, Z.F.; Yan, C.; Xia, X.; Ma, C.M.; Dong, S.K.; Gong, Z.P. Mechanical properties of soybean plants under various plant densities. Crop Pasture Sci. 2020, 71, 249–259. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 1–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohnen, M.V.; Schmid-Siegert, E.; Trevisan, M.; Petrolati, L.A.; Sénéchal, F.; Müller-Moulé, P.; Maloof, J.; Xenarios, I.; Fankhauser, C. Neighbor detection induces organ-specific transcriptomes, revealing patterns underlying hypocotyl-specific growth. Plant Cell 2016, 28, 2889–2904. [Google Scholar] [CrossRef] [Green Version]
- Garrison, R.; Briggs, W.R. Internodal Growth in Localized Darkness. Bot. Gaz. 1972, 133, 270–276. [Google Scholar] [CrossRef]
- Keuskamp, D.H.; Sasidharan, R.; Pierik, R. Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal. Behav. 2010, 5, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Wen, B.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.; Yang, J.; Xu, M.; Qin, S.; Yang, W.; Liu, W. Slight shading stress at seedling stage does not reduce lignin biosynthesis or affect lodging resistance of soybean stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef] [Green Version]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [Green Version]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishijima, T.; Katsura, N.; Koshioka, M.; Yamazaki, H.; Nakayama, M.; Yamane, H.; Yamaguchi, I.; Yokota, T.; Murofushi, N.; Takahashi, N.; et al. Effects of Gibberellins and Gibberellin-biosynthesis Inhibitors on Stem Elongation and Flowering of Raphanus sativus L. J. Jpn. Soc. Hortic. Sci. 2008, 67, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Plackett, A.R.G.; Powers, S.J.; Fernandez-Garcia, N.; Urbanova, T.; Takebayashi, Y.; Seo, M.; Jikumaru, Y.; Benlloch, R.; Nilsson, O.; Ruiz-Rivero, O.; et al. Analysis of the developmental roles of the arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 2012, 24, 941–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef]
- Liu, W.G.; Jiang, T.; She, Y.H.; Yang, F.; Yang, W.Y. Preliminary study on physiological response mechanism of soybean (Glycinemax) stem to shade stress at seedling stage. Chin. J. Oil Crop Sci. 2011, 33, 141–146. [Google Scholar]

| Target Name | Primer Sequences |
|---|---|
| GmGA3ox6—F | 5′-AACTTCTACCCTCGTTGTCCTGA-3′ |
| GmGA3ox6—R | 5′-CACTGAGTATCTTTCCCTTGCG-3′ |
| GmGA20ox1-D—F | 5′-CTCAAAACACATCCGCAGAAAA-3′ |
| GmGA20ox1-D—R | 5′-GGAGGGTATGTTGGACTGGT-3′ |
| GmGA2ox4—F | 5′-AACAGTTTGCTACAACAGTTTCGAG-3′ |
| GmGA2ox4—R | 5′-CATGAGACCGTGAATCCCAAA-3′ |
| Actin—F | 5′-CCATAAACGATGCCGACCAG-3′ |
| Actin—R | 5′-AGCCTTGCGACCATACTCCC-3′ |
| Cultivar | Internode Position | D10 | D20 | D30 | D40 |
|---|---|---|---|---|---|
| HN48 | 1 | 3.80 ± 0.06 a | 3.83 ± 0.04 a | 3.97 ± 0.08 a | 4.03 ± 0.11 a |
| 2 | 2.40 ± 0.05 b | 2.42 ± 0.05 b | 2.53 ± 0.02 ab | 2.57 ± 0.04 a | |
| 3 | 2.07 ± 0.03 b | 2.10 ± 0.04 b | 2.52 ± 0.06 a | 2.53 ± 0.04 a | |
| 4 | 2.52 ± 0.09 c | 2.57 ± 0.06 c | 3.62 ± 0.05 b | 4.00 ± 0.05 a | |
| 5 | 3.07 ± 0.49 d | 3.47 ± 0.05 c | 4.40 ± 0.06 b | 5.17 ± 0.08 a | |
| 6 | 2.92 ± 0.05 d | 3.53 ± 0.02 c | 3.87 ± 0.04 b | 4.43 ± 0.04 a | |
| 7 | 3.30 ± 0.03 d | 3.85 ± 0.07 c | 4.58 ± 0.07 b | 5.10 ± 0.08 a | |
| 8 | 4.17 ± 0.06 d | 4.65 ± 0.05 c | 5.35 ± 0.11 b | 6.35 ± 0.06 a | |
| 9 | 3.70 ± 0.06 d | 5.07 ± 0.07 c | 5.53 ± 0.03 b | 6.18 ± 0.08 a | |
| 10 | 3.95 ± 0.03 d | 5.17 ± 0.06 c | 5.85 ± 0.08 b | 6.17 ± 0.08 a | |
| 11 | 4.25 ± 0.08 d | 4.77 ± 0.08 c | 6.22 ± 0.05 b | 6.43 ± 0.03 a | |
| 12 | 4.43 ± 0.03 d | 5.23 ± 0.09 c | 7.05 ± 0.03 b | 7.97 ± 0.08 a | |
| 13 | 5.13 ± 0.09 b | 7.42 ± 0.06 a | 7.45 ± 0.07 a | 7.50 ± 0.04 a | |
| HN60 | 1 | 2.82 ± 0.03 a | 2.85 ± 0.08 a | 2.92 ± 0.04 a | 2.93 ± 0.03 a |
| 2 | 1.80 ± 0.04 a | 1.82 ± 0.04 a | 1.90 ± 0.03 a | 1.92 ± 0.04 a | |
| 3 | 1.47 ± 0.02 b | 1.68 ± 0.04 a | 1.70 ± 0.03 a | 1.72 ± 0.04 a | |
| 4 | 1.58 ± 0.03 b | 1.60 ± 0.04 b | 1.63 ± 0.03 ab | 1.72 ± 0.03 a | |
| 5 | 2.05 ± 0.03 d | 2.40 ± 0.04 c | 3.23 ± 0.09 b | 3.77 ± 0.12 a | |
| 6 | 2.02 ± 0.02 d | 2.43 ± 0.06 c | 3.17 ± 0.08 b | 3.50 ± 0.04 a | |
| 7 | 2.07 ± 0.04 d | 2.48 ± 0.04 c | 3.17 ± 0.10 b | 3.57 ± 0.03 a | |
| 8 | 2.68 ± 0.05 d | 3.73 ± 0.15 c | 4.70 ± 0.14 b | 5.47 ± 0.07 a | |
| 9 | 3.32 ± 0.04 d | 3.78 ± 0.08 c | 4.35 ± 0.09 b | 5.53 ± 0.11 a | |
| 10 | 3.45 ± 0.02 d | 4.42 ± 0.03 c | 4.83 ± 0.08 b | 5.50 ± 0.04 a | |
| 11 | 3.08 ± 0.04 d | 4.02 ± 0.05 c | 5.15 ± 0.15 b | 6.88 ± 0.15 a | |
| 12 | 3.77 ± 0.14 d | 4.53 ± 0.08 c | 6.28 ± 0.10 b | 7.27 ± 0.08 a | |
| 13 | 3.17 ± 0.10 b | 4.48 ± 0.09 b | 4.58 ± 0.23 ab | 4.95 ± 0.10 a |
| Cultivar | Fifth Internode | NL | SAM | CAF | PSN |
|---|---|---|---|---|---|
| HN48 | Internode length (cm) | 5.66 ± 0.06 d | 6.68 ± 0.12 c | 8.56 ± 0.09 b | 11.42 ± 0.17 a |
| Internode diameter (mm) | 6.65 ± 0.07 a | 6.17 ± 0.04 b | 5.99 ± 0.09 b | 4.09 ± 0.07 c | |
| Internode diameter/internode length (%) | 11.75 ± 0.58 a | 9.24 ± 0.06 b | 7.02 ± 0.02 c | 3.58 ± 0.01 d | |
| HN60 | Internode length (cm) | 3.78 ± 0.07 d | 4.26 ± 0.04 c | 5.56 ± 0.11 b | 9.16 ± 0.20 a |
| Internode diameter (mm) | 5.95 ± 0.03 a | 5.74 ± 0.05 b | 5.64 ± 0.09 b | 3.67 ± 0.05 c | |
| Internode diameter/internode length (%) | 16.08 ± 0.35 a | 13.81 ± 0.33 b | 10.34 ± 0.18 c | 4.01 ± 0.02 d |
| Cultivar | Days/Treatment | NL | SAM | CAF | PSN |
|---|---|---|---|---|---|
| HN48 | 1 | 21.87 ± 0.98 c | 24.51 ± 0.13 c | 58.25 ± 4.80 a | 36.15 ± 0.81 b |
| 2 | 19.33 ± 1.08 d | 41.28 ± 2.75 b | 32.14 ± 1.61 c | 56.61 ± 1.24 a | |
| 3 | 16.46 ± 1.26 c | 22.43 ± 2.48 bc | 28.97 ± 1.40 b | 30.08 ± 2.86 a | |
| 5 | 7.75 ± 1.52 d | 18.07 ± 0.97 c | 23.65 ± 0.78 b | 29.34 ± 0.79 a | |
| HN60 | 1 | 11.45 ± 0.21 c | 19.09 ± 0.86 a | 17.38 ± 0.07 b | 13.08 ± 0.48 c |
| 2 | 5.85 ± 0.13 d | 9.79 ± 0.23 a | 7.39 ± 0.24 c | 8.23 ± 0.17 b | |
| 3 | 2.64 ± 0.06 c | 2.94 ± 0.03 bc | 3.40 ± 0.05 b | 5.39 ± 0.35 a | |
| 5 | 1.82 ± 0.07 c | 2.14 ± 0.15 c | 2.91 ± 0.04 b | 3.50 ± 0.24 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, F.; Sun, K.; Gong, S.; Wang, C.; Ma, C.; Zhang, R.; Yan, C. Effects of Shading on the Internode Critical for Soybean (Glycine Max) Lodging. Agronomy 2022, 12, 492. https://doi.org/10.3390/agronomy12020492
Shan F, Sun K, Gong S, Wang C, Ma C, Zhang R, Yan C. Effects of Shading on the Internode Critical for Soybean (Glycine Max) Lodging. Agronomy. 2022; 12(2):492. https://doi.org/10.3390/agronomy12020492
Chicago/Turabian StyleShan, Fuxin, Kexin Sun, Shengdan Gong, Chang Wang, Chunmei Ma, Rui Zhang, and Chao Yan. 2022. "Effects of Shading on the Internode Critical for Soybean (Glycine Max) Lodging" Agronomy 12, no. 2: 492. https://doi.org/10.3390/agronomy12020492






