Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars under Water Limitations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Site
2.2. Irrigation Water Treatments, Experimental Design, and Sampling for Measurements
2.3. Physiological Traits and Antioxidant Capacity
2.4. Growth, Yield, and Fiber Quality Traits
2.5. Statistical Analysis
3. Results
3.1. Physiological Traits
3.2. Antioxidant Capacity
3.3. Growth, and Yield Attributes
3.4. Fiber Quality Properties
3.5. Correlation Study among Seed Cotton Yield, Physiological, and Antioxidant Capacity
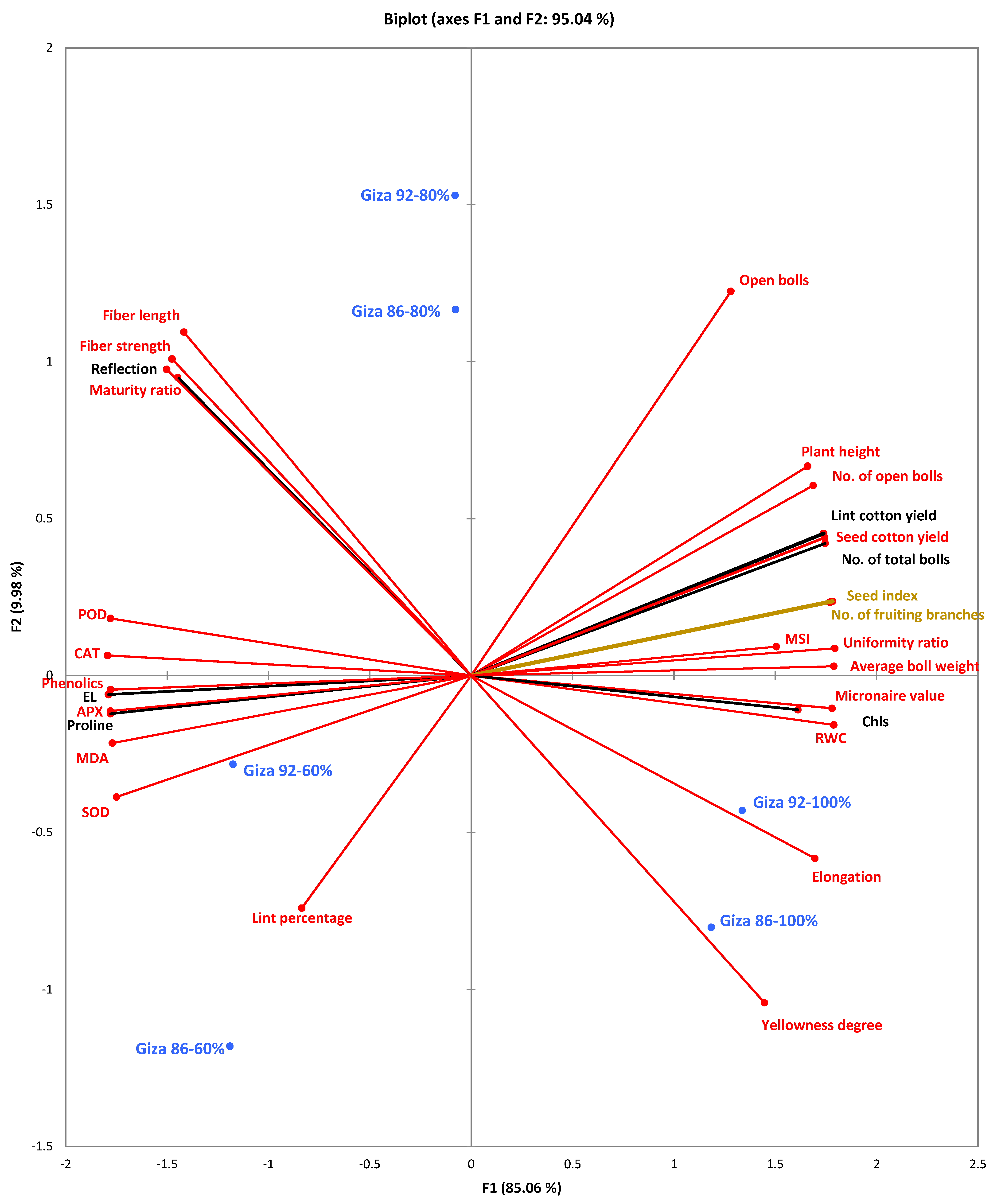
3.6. Princial Components Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; El-Mageed, T.A.A.; El-Yazal, M.A.S.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 2014, 89, 338–344. [Google Scholar] [CrossRef]
- Rady, M.M.; Abd El-Mageed, T.A.; Abdurrahman, H.A.; Mahdi, A.H. Humic acid application improves field performance of cotton (Gossypium barbadense L.) under saline conditions. J. Anim. Plant Sci. 2016, 26, 487–493. [Google Scholar]
- Rady, M.M.; Desoky, E.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Desoky, E.M.; Merwad, A.M.A.; ElSayed, A.I.; Rady, M.M. Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abdelaziz, S.A.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- ur Rehman, H.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, M.A.; Rady, M.M.; Belal, H.E.E.; Belal, E.E.; Al-Qthanin, R.; Al-Yasi, H.M.; Ali, E.F. Revitalizing fertility of nutrient-deficient virgin sandy soil using leguminous biocompost boosts Phaseolus vulgaris performance. Plants 2021, 10, 1637. [Google Scholar] [CrossRef]
- Gardner, W.R.; Gardner, H.R. Principles of water management under drought conditions. Agric. Water Manag. 1983, 7, 143–155. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Moisture deficit effects on cotton lint yield, yield components, and boll distribution. Agron. J. 2004, 96, 377–383. [Google Scholar] [CrossRef]
- Dağdelen, N.; Yılmaz, E.; Sezgin, F.; Gürbüz, T. Water-yield relation and water use efficiency of cotton (Gossypium hirsutum L.) and second crop corn (Zea mays L.) in western Turkey. Agric. Water Manag. 2006, 82, 63–85. [Google Scholar] [CrossRef]
- Basal, H.; Dagdelen, N.; Unay, A.; Yilmaz, E. Effects of deficit drip irrigation ratios on cotton (Gossypium hirsutum L.) yield and fibre quality. J. Agron. Crop Sci. 2009, 195, 19–29. [Google Scholar] [CrossRef]
- Yazar, A.; Sezen, S.M.; Sesveren, S. LEPA and trickle irrigation of cotton in the southeast Anatolia project (GAP) area in Turkey. Agric. Water Manag. 2002, 54, 189–203. [Google Scholar] [CrossRef]
- Aujla, M.S.; Thind, H.S.; Buttar, G.S. Cotton yield and water use efficiency at various levels of water and N through drip irrigation under two methods of planting. Agric. Water Manag. 2005, 71, 167–179. [Google Scholar] [CrossRef]
- Abdel-Kader, M.A.; Esmail, A.M.; El Shouny, K.A.; Ahmed, M.F. Evaluation of the Drought Stress Effects on Cotton Genotypes by Using Physiological and Morphological Traits. IJSR 2015, 4, 2319–7064. [Google Scholar]
- DeTar, W.R. Yield and growth characteristics for cotton under various irrigation regimes on sandy soil. Agric. Water Manag. 2008, 95, 69–76. [Google Scholar] [CrossRef]
- Mert, M. Irrigation of cotton cultivars improves seed cotton yield, yield components and fiber properties in the Hatay region, Turkey. Acta Agric. Scand. 2005, 55, 44–50. [Google Scholar]
- Onder, D.; Akiscan, Y.; Onder, S.; Mert, M. Effect of different irrigation water level on cotton yield and yield components. Afr. J. Biotech. 2009, 8, 1536–1544. [Google Scholar]
- Daud, M.K.; Ali, S.; Variath, M.T.; Zhu, S.J. Antioxidative enzymes status in upland cotton callus culture under osmotic stresses. In Proceedings of the International Conference on Computational Techniques and Artificial Intelligence (ICCTAI’), Penang, Malaysia, 11–12 February 2012. [Google Scholar]
- Aboeldhab, A.A.; Amany, M.A.; Hamoda, S.A.F. Response of some Egyptian cotton cultivars to different drip irrigation durations under sandy soil conditions in Wadi El-natron. J. Plant Prod. Mansoura Univ. 2012, 3, 369–384. [Google Scholar]
- Hamoda, S.A.F. Response of Giza 90 cotton cultivar to foliar application of some drought tolerance inducers under water stress and high temperature conditions in upper Egypt. J. Plant Prod. Mansoura Univ. 2012, 3, 493–507. [Google Scholar] [CrossRef][Green Version]
- Page, A.I.; Miller, R.H.; Keeny, D.R. Part II: Chemical and Microbiological Methods. In Methods of Soil Analysis, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Klute, A. Part I: Physical and Mineralogical Methods. In Methods of Soil Analysis, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- Allen, R.G.; Pruitt, W.O.; Businger, J.A.; Fritschen, L.J.; Jensen, M.E.; Quinn, F.H. Evaporation and Transpiration. In Handbook of Hydrology; ASCE: New York, NY, USA, 1996; pp. 125–252. [Google Scholar]
- Osman, A.S.; Rady, M.M. Exogenously-applied sulphur and ascorbic acid positively altered their endogenous concentrations, and increased growth and yield in Cucurbita pepo L. plants grown on a newly- reclaimed saline soil. J. Biotech. Sci. 2014, 2, 1–9. [Google Scholar]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Food Phenolics: Sources, Chemistry, Effects, Applications; Technomic Publishing Company Inc.: Lancaster, PA, USA, 1995; pp. 231–245. [Google Scholar]
- Petters, W.; Piepenbrock, M.; Lenz, B.; Schmitt, J.M. Cytokinine as a negative effecter of phosphoenolpyruvate carboxylase induction in Mesembryanthemum crystallinum. J. Plant Physiol. 1997, 151, 362–367. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Annual. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aeby, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Kumar, K.B.; Khan, P.A. Peroxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Ind. J. Exp. Bot. 1982, 20, 412–416. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- American Society for Testing Materials (ASTM). Standard test method for length and length uniformity of cotton fibers by fibrograph measurement (D1447-89). In Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1998; Volume 07.01, pp. 391–395. [Google Scholar]
- American Society for Testing Materials (ASTM). Standard test method for breaking strength and elongation of cotton fibers (flat bundle method) (D1445-95). In Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1998; Volume 07.01, pp. 383–390. [Google Scholar]
- American Society for Testing Materials (ASTM). Standard test method micronaire reading of cotton fibers (D1448-97). In Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1998; Volume 07.01, pp. 396–398. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons, Inc.: London, UK, 1984; pp. 13–175. [Google Scholar]
- Waller, R.A.; Duncan, D.B. A Bayes rule for the symmetric multiple comparison problem. J. Am. Stat. Assoc. 1969, 64, 1484–1504. [Google Scholar]
- Afiah, S.A.N.; Ghoneim, E.M. Correlation, stepwise and path coefficient analysis in Egyptian cotton under saline conditions. Arab Univ. J. Agric. Sci. 2000, 8, 607–618. [Google Scholar]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability. 2021, 13, 1318. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic Practices to Increase the Yield and Quality of Common Bean (Phaseolus vulgaris L.): A Systematic Review. Agronomy 2022, 12, 271. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Kanaya, M.; Ogata, S. Cell membrane stability and leaf surface wax content as affected by increasing water deficits in maize. J. Exp. Bot. 1991, 42, 167–171. [Google Scholar] [CrossRef]
- Sibet, T.; Birol, T. Some physiological responses of drought stress in wheat genotypes with different ploidity in turkey. World, J. Agric. Sci. 2007, 3, 178–183. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Saxena, D.C. Oxidative stress and antioxidants in wheat genotypes: Possible mechanism of water stress tolerance. J. Agron. Crop Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Sanchez-Blanco, J.; Fernandez, T.; Morales, A.; Morte, A.; Alarcon, J.J. Variation in water stress, gas exchange, and growth in Rasmanrins officinalis plants infected with Glamus deserticola under drought condition. J. Plant Physiol. 2006, 161, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Patil, M.D.; Biradar, D.P.; Patil, V.C.; Janagoudar, B.S. Response of cotton genotypes to drought mitigation practices. Am.-Euras. J. Agric. Environ. Sci. 2011, 11, 360–364. [Google Scholar]
- Massacci, A.; Nabiev, S.M.; Pietrosanti, L.; Nematov, S.K.; Chernikova, T.N.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Shinwari, Z.K.; Khan, A.L.; Ahmad, N.; Lee, I.J. Effect of polyethylene glycol induced drought stress on physio-hormonal attributes of soybean. Pakistan J. Bot. 2010, 42, 977–986. [Google Scholar]
- Castillo, J.P.M. Resistance to drought in crops. In Abiotic Stress and Plant Responses; Khan, N.A., Singh, S., Eds.; International Publishing House Pvt. Ltd.: New Delhi, India, 2008; Volume 10, pp. 198–204. [Google Scholar]
- Yildiz-Aktas, L.; Dagnon, S.; Gurel, A.; Gesheva, E.; Edreva, A. Drought Tolerance in Cotton: Involvement of Non-enzymatic ROS Scavenging Compounds. J. Agron. Crop Sci. 2009, 195, 247–253. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food. Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Sundaresan, S.; Sudhakaran, P.R. Water stress-induced alterations in proline metabolism of drought-susceptible and -tolerant cassava (Manihot esculenta) cultivars. Physiol. Plant. 1995, 94, 635–642. [Google Scholar] [CrossRef]
- Lee, T.M.; Liu, C.H. Correlation of decreases calcium contents with proline accumulation in the marine green macroalga Ulva fasciata exposed to elevated NaCl contents in seawater. J. Exp. Bot. 1999, 50, 1855–1862. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Kamal, M.; El Sowfy, D.M.; Rady, M.M.; Mohamed, G.F.; Al-Dhumri, S.A.; AL-Harbi, M.S.; Abdou, N.M. Small-sized nanophosphorus has a positive impact on the performance of fenugreek plants under soil-water deficit stress: A case study under field conditions. Biology 2022, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizadeh, M.; Nori, A.; Shahbazi, H.; Habibpour, M. Effects of drought stress on some agronomic and morphological traits of durum wheat (Triticum durum Desf.) landraces under greenhouse condition. Afr. J. Biotechnol. 2011, 10, 14097–14107. [Google Scholar]
- Zhang, P.P.; Feng, B.L.; Wang, P.K.; Dai, H.P.; Song, H.; Gao, J.F.; Chen, J.; Chai, Y. Leaf senescence and activities of antioxidant enzymes in different broomcorn millet (Panicum miliaceum L.) cultivars under simulated drought condition. J. Food Agric. Environ. 2012, 10, 438–444. [Google Scholar]
- Krieg, D.R. Genetic and environmental factors affecting productivity of cotton. In Proceedings of the Beltwide Cotton Conference, New Orleans, LA, USA, 7–10 January 1997; Dugger, P., Richter, D.A., Eds.; National Cotton Council of America: Memphis, TN, USA; p. 1347. [Google Scholar]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
| Soil Depth (cm) | Sand (%) | Silt (%) | Clay (%) | Soil Texture | ||||
|---|---|---|---|---|---|---|---|---|
| 0–30 | 88.2 | 10.3 | 1.5 | Sandy | ||||
| 30–60 | 91.4 | 8.3 | 0.3 | Sandy | ||||
| Soil depth (cm) | pH | O.M | EC (dS m−1) | CaCO3 | ||||
| 0–30 | 7.41 | 0.43 | 0.48 | 0.28 | ||||
| 30–60 | 7.40 | 0.41 | 0.35 | 0.25 | ||||
| Soil depth (cm) | Soluble cations (meq L−1) | Soluble anions (meq L−1) | ||||||
| Ca2+ | Mg2+ | K+ | Na+ | HCO3− | SO42− | Cl− | ||
| 0–30 | 1.83 | 1.02 | 0.38 | 0.80 | 2.60 | 0.17 | 0.93 | |
| 30–60 | 1.91 | 0.80 | 0.21 | 0.86 | 2.90 | 0.19 | 0.96 | |
| Source of Variation | RWC | MSI | EL | Chls (mg g−1 FW) |
|---|---|---|---|---|
| (%) | ||||
| Cultivar (Cv) | * | * | * | * |
| Giza 86 | 48.0 b ± 0.92 | 31.8 b ± 0.71 | 34.2 a ± 0.91 | 0.97 b ± 0.040 |
| Giza 92 | 49.7 a ± 1.05 | 34.0 a ± 0.92 | 31.4 b ± 0.81 | 1.04 a ± 0.048 |
| WRs | ** | * | ** | * |
| 100% ETc | 69.3 a ± 1.40 | 49.6 a ± 1.16 | 11.6 c ± 0.47 | 1.35 a ± 0.061 |
| 80% ETc | 45.4 b ± 0.93 | 32.5 b ± 0.77 | 33.8 b ± 0.86 | 0.95 b ± 0.042 |
| 60% ETc | 31.8 c ± 0.63 | 16.6 c ± 0.56 | 53.0 a ± 1.26 | 0.72 c ± 0.030 |
| Cv × WRs | * | * | * | * |
| G 86 × 100% ETc | 68.0 b ± 1.32 | 48.4 b ± 1.01 | 11.9 e ± 0.42 | 1.31 a ± 0.051 |
| G 86 × 80% ETc | 44.9 c ± 0.83 | 31.4 c ± 0.72 | 35.3 c ± 0.90 | 0.92 c ± 0.041 |
| G 86 × 60% ETc | 31.0 d ± 0.62 | 15.5 e ± 0.50 | 55.3 a ± 1.42 | 0.68 e ± 0.029 |
| G 92 × 100% ETc | 70.5 a ± 1.48 | 50.7 a ± 1.32 | 11.3 e ± 0.52 | 1.39 a ± 0.071 |
| G 92 × 80% ETc | 45.9 c ± 1.03 | 33.5 c ± 0.82 | 32.3 d ± 0.82 | 0.98 b ± 0.043 |
| G 92 × 60% ETc | 32.6 d ± 0.64 | 17.7 d ± 0.62 | 50.6 b ± 1.10 | 0.75 d ± 0.031 |
| Source of Variation | Phenolics | Proline | MDA (nmol g−1 FW) | SOD | CAT | POD | APX |
|---|---|---|---|---|---|---|---|
| (mg g−1 FW) | (Unit mg−1 Protein) | ||||||
| Cultivar (Cv) | * | * | * | ns | ns | * | * |
| Giza 86 | 13.0 b ± 0.17 | 0.34 b ± 0.014 | 56.6 a ± 1.10 | 266 ± 5.4 | 1013 ± 14.9 | 893 b ± 12.2 | 105 b ± 2.1 |
| Giza 92 | 13.9 a ± 0.15 | 0.35 a ± 0.015 | 51.7 b ± 0.87 | 264 ± 5.4 | 1037 ± 15.9 | 956 a ± 13.1 | 111 a ± 2.2 |
| WRs | * | * | ** | ** | * | * | ** |
| 100% ETc | 8.3 c ± 0.12 | 0.28 c ± 0.011 | 35.6 c ± 0.74 | 145 c ± 3.2 | 605 c ± 10.4 | 518 c ± 8.6 | 65 c ± 1.3 |
| 80% ETc | 13.4 b ± 0.15 | 0.34 b ± 0.012 | 53.6 b ± 0.90 | 239 b ± 5.0 | 1062 b ± 15.9 | 979 b ± 12.7 | 106 b ± 2.2 |
| 60% ETc | 18.7 a ± 0.21 | 0.42 a ± 0.021 | 73.3 a ± 1.31 | 412 a ± 8.1 | 1409 a ± 20.1 | 1277 a ± 16.7 | 154 a ± 3.0 |
| Cv × WRs | * | * | * | * | * | * | * |
| G 86 × 100% ETc | 7.9 d ± 0.13 | 0.27 c ± 0.011 | 36.9 e ± 0.90 | 143 c ± 3.1 | 603 d ± 10.2 | 505 e ± 8.3 | 63 c ± 1.2 |
| G 86 × 80% ETc | 13.1 c ± 0.17 | 0.34 b ± 0.012 | 56.2 c ± 0.89 | 237 b ± 5.2 | 1032 c ± 15.4 | 945 d ± 12.1 | 103 b ± 2.1 |
| G 86 × 60% ETc | 18.1 b ± 0.21 | 0.41 a ± 0.020 | 76.8 a ± 1.51 | 419 a ± 8.0 | 1404 a ± 19.2 | 1229 b ± 16.2 | 150 a ± 3.0 |
| G 92 × 100% ETc | 8.7 d ± 0.11 | 0.28 c ± 0.011 | 34.3 e ± 0.58 | 146 c ± 3.2 | 606 d ± 10.5 | 531 e ± 8.9 | 66 c ± 1.4 |
| G 92 × 80% ETc | 13.7 c ± 0.13 | 0.34 b ± 0.012 | 51.0 d ± 0.91 | 240 b ± 4.8 | 1091 b ± 16.3 | 1012 c ± 13.3 | 109 b ± 2.2 |
| G 92 ×60% ETc | 19.3 a ± 0.21 | 0.42 a ± 0.022 | 69.8 b ± 1.11 | 405 a ± 8.2 | 1414 a ± 21.0 | 1325 a ± 17.2 | 158 a ± 3.0 |
| Source of Variation | Plant Height (cm) | No. of Fruiting Branches | No. of Total Bolls | No. of Open Bolls | Open Bolls (%) | Average Boll Weight (g) | Seed Cotton Yield | Lint Cotton Yield | Lint (%) | Seed Index |
|---|---|---|---|---|---|---|---|---|---|---|
| (Kantar fad−1) | ||||||||||
| Cultivar (Cv) | * | * | * | * | * | * | * | * | ns | * |
| Giza 86 | 128 b ± 10.2 | 12.7 b ± 1.01 | 36.0 b ± 2.86 | 18.9 b ± 1.699 | 51.6 b ± 4.1 | 2.36 b ± 0.15 | 8.7 b ± 0.70 | 3.51 b ± 0.20 | 41.7 a ± 3.22 | 9.1 b ± 0.70 |
| Giza 92 | 130 a ± 11.1 | 13.2 a ± 1.10 | 37.3 a ± 3.13 | 20.3 a ± 1.72 | 53.2 a ± 4.6 | 2.47 a ± 0.19 | 9.0 a ± 0.71 | 3.66 a ± 0.20 | 41.9 a ± 3.30 | 9.3 a ± 0.75 |
| WRs | * | * | * | ** | * | * | * | * | ns | * |
| 100% ETc | 137 a ± 11.6 | 15.7 a ± 1.3 | 47.8 a ± 3.58 | 27.0 a ± 2.36 | 56.4 b ± 4.6 | 2.95 a ± 0.23 | 11.7 a ± 0.87 | 4.71 a ± 0.26 | 41.7 a ± 3.10 | 11.5 a ± 0.85 |
| 80% ETc | 133 a ± 10.8 | 13.2 b ± 1.0 | 39.4 b ± 3.23 | 22.7 b ± 1.97 | 60.7 a ± 5.3 | 2.38 b ± 0.19 | 9.6 b ± 0.76 | 3.87 b ± 0.21 | 41.5 a ± 3.17 | 9.4 b ± 0.79 |
| 60% ETc | 118 b ± 9.6 | 10.1 c ± 0.9 | 22.9 c ± 2.17 | 9.2 c ± 0.78 | 40.1 c ± 3.3 | 1.93 c ± 0.11 | 5.3 c ± 0.50 | 2.19 c ± 0.15 | 42.3 a ± 3.51 | 6.8 c ± 0.54 |
| Cv × WRs | * | * | * | * | * | * | * | * | * | * |
| G 86 × 100% ETc | 137 a ± 11.1 | 15.4 a ± 1.21 | 46.7 b ± 3.12 | 25.9 b ± 2.31 | 55.5 c ± 4.2 | 2.86 b ± 0.21 | 11.4 b ± 0.91 | 4.59 b ± 0.25 | 42.0 a ± 3.12 | 11.3 a ± 0.80 |
| G 86 × 80% ETc | 132 b ± 10.3 | 12.7 c ± 1.01 | 38.7 d ± 3.04 | 21.9 d ± 2.02 | 59.5 b ± 5.1 | 2.33 c ± 0.17 | 9.7 c ± 0.71 | 3.82 c ± 0.21 | 40.8 a ± 3.02 | 9.4 b ± 0.75 |
| G 86 × 60% ETc | 115 c ± 9.2 | 10.1 d ± 0.82 | 22.6 e ± 2.42 | 9.0 e ± 0.73 | 39.8 d ± 3.1 | 1.89 d ± 0.08 | 5.1 d ± 0.48 | 2.12 d ± 0.15 | 42.3 a ± 3.52 | 6.7 c ± 0.55 |
| G 92 × 100% ETc | 137 a ± 12.0 | 15.9 a ± 1.31 | 48.9 a ± 4.04 | 28.0 a ± 2.41 | 57.3 c ± 5.0 | 3.01 a ± 0.24 | 12.0 a ± 0.82 | 4.82 a ± 0.27 | 41.3 a ± 3.08 | 11.7 a ± 0.90 |
| G 92 × 80% ETc | 133 b ± 11.3 | 13.7 b ± 1.04 | 40.0 c ± 3.42 | 23.5 c ± 1.92 | 61.8 a ± 5.4 | 2.42 c ± 0.21 | 9.6 c ± 0.80 | 3.91 c ± 0.20 | 42.1 a ± 3.32 | 9.5 b ± 0.83 |
| G 92 × 60% ETc | 120 c ± 10.0 | 10.1 d ± 0.95 | 23.2 e ± 1.92 | 9.4 e ± 0.83 | 40.4 d ± 3.4 | 1.97 d ± 0.13 | 5.5 d ± 0.51 | 2.26 d ± 0.14 | 42.3 a ± 3.50 | 6.8 c ± 0.52 |
| Source of Variation | Micronaire Value | Maturity Ratio | Fiber Length (mm) | Uniformity Ratio (%) | Elongation (%) | Fiber Strength (g tex−1) | Yellowness Degree | Reflection (%) |
|---|---|---|---|---|---|---|---|---|
| Cultivar (Cv) | ns | ns | * | * | ns | * | * | * |
| Giza 86 | 4.13 a ± 0.070 | 93.3 a ± 0.345 | 32.4 b ± 0.510 | 83.1 b ± 0.31 | 7.55 a ± 0.040 | 38.4 b ± 0.38 | 10.5 a ± 0.23 | 74.2 b ± 1.11 |
| Giza 92 | 4.25 a ± 0.100 | 93.9 a ± 0.432 | 32.8 a ± 0.571 | 85.5 a ± 0.41 | 7.54 a ± 0.051 | 39.1 a ± 0.41 | 9.8 b ± 0.24 | 76.1 a ± 1.45 |
| WRs | * | ns | * | * | * | * | * | * |
| 100% ETc | 4.38 a ± 0.096 | 92.4 a ± 0.358 | 31.0 b ± 0.523 | 86.7 a ± 0.38 | 8.25 a ± 0.056 | 36.7 b ± 0.31 | 12.4 a ± 0.30 | 68.1 b ± 1.08 |
| 80% ETc | 4.14 b ± 0.080 | 94.3 a ± 0.396 | 33.6 a ± 0.552 | 83.9 b ± 0.36 | 7.28 b ± 0.044 | 39.9 a ± 0.45 | 8.8 c ± 0.19 | 79.2 a ± 1.41 |
| 60% ETc | 4.05 b ± 0.079 | 94.2 a ± 0.412 | 33.2 a ± 0.547 | 82.4 b ± 0.36 | 7.12 b ± 0.038 | 39.6 a ± 0.42 | 9.2 b ± 0.22 | 78.3 a ± 1.36 |
| Cv × WRs | * | ns | * | * | * | * | * | * |
| G 86 × 100% ETc | 4.35 a ± 0.081 | 92.4 a ± 0.314 | 30.9 b ± 0.484 | 85.7 b ± 0.34 | 8.18 a ± 0.051 | 36.5 b ± 0.29 | 12.4 a ± 0.29 | 67.9 d ± 0.92 |
| G 86 × 80% ETc | 4.07 b ± 0.065 | 93.8 a ± 0.371 | 33.5 a ± 0.523 | 82.5 cd ± 0.30 | 7.33 b ± 0.036 | 39.5 a ± 0.44 | 9.2 c ± 0.18 | 77.9 bc ± 1.20 |
| G 86 × 60% ETc | 3.97 c ± 0.064 | 93.8 a ± 0.351 | 32.7 a ± 0.523 | 81.1 d ± 0.30 | 7.15 c ± 0.033 | 39.1 a ± 0.40 | 9.9 b ± 0.21 | 76.9 c ± 1.21 |
| G 92 × 100% ETc | 4.41 a ± 0.111 | 92.3 a ± 0.402 | 31.1 b ± 0.562 | 87.7 a ± 0.42 | 8.31 a ± 0.060 | 36.8 b ± 0.32 | 12.4 a ± 0.31 | 68.2 d ± 1.23 |
| G 92 × 80% ETc | 4.21 b ± 0.095 | 94.8 a ± 0.421 | 33.7 a ± 0.580 | 85.2 b ± 0.41 | 7.23 b ± 0.051 | 40.3 a ± 0.46 | 8.4 d ± 0.19 | 80.5 a ± 1.62 |
| G 92 × 60% ETc | 4.13 c ± 0.094 | 94.6 a ± 0.473 | 33.7 a ± 0.571 | 83.6 c ± 0.41 | 7.08 c ± 0.042 | 40.1 a ± 0.44 | 8.5 d ± 0.22 | 79.7 ab ± 1.51 |
| Traits | SCY | EL | MSI | Chls | Phenols | MDA | ProC | SOD | CAT | POD |
|---|---|---|---|---|---|---|---|---|---|---|
| EL | −0.955 ** | |||||||||
| MSI | 0.966 ** | −0.991 ** | ||||||||
| TChC | 0.930 ** | −0.976 ** | 0.980 ** | |||||||
| PhC | −0.872 ** | 0.901 ** | −0.898 ** | −0.895 ** | ||||||
| MDA | −0.875 ** | 0.895 ** | −0.895 ** | −0.892 ** | 0.993 ** | |||||
| ProC | −0.842 ** | 0.870 ** | −0.861 ** | −0.859 ** | 0.982 ** | 0.983 ** | ||||
| SOD | −0.906 ** | 0.897 ** | −0.901 ** | −0.885 ** | 0.964 ** | 0.969 ** | 0.957 ** | |||
| CAT | −0.853 ** | 0.895 ** | −0.894 ** | −0.904 ** | 0.968 ** | 0.969 ** | 0.958 ** | 0.936 ** | ||
| POD | −0.858 ** | 0.905 ** | −0.907 ** | −0.918 ** | 0.960 ** | 0.962 ** | 0.956 ** | 0.930 ** | 0.984 ** | |
| APX | −0.885 ** | 0.903 ** | −0.907 ** | −0.903 ** | 0.973 ** | 0.976 ** | 0.975 ** | 0.960 ** | 0.969 ** | 0.982 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, M.A.M.; El-hady, M.A.A.; Abdelkader, M.A.; Abd-Elkrem, Y.M.; El-Gabry, Y.A.; El-temsah, M.E.; El-Areed, S.R.M.; Rady, M.M.; Alamer, K.H.; Alqubaie, A.I.; et al. Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars under Water Limitations. Agronomy 2022, 12, 803. https://doi.org/10.3390/agronomy12040803
Eid MAM, El-hady MAA, Abdelkader MA, Abd-Elkrem YM, El-Gabry YA, El-temsah ME, El-Areed SRM, Rady MM, Alamer KH, Alqubaie AI, et al. Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars under Water Limitations. Agronomy. 2022; 12(4):803. https://doi.org/10.3390/agronomy12040803
Chicago/Turabian StyleEid, Mohamed A. M., Mohamed A. Abd El-hady, Mohamed A. Abdelkader, Yasser M. Abd-Elkrem, Yasser A. El-Gabry, Mohamed E. El-temsah, Sherif R. M. El-Areed, Mostafa M. Rady, Khalid H. Alamer, Ahmad I. Alqubaie, and et al. 2022. "Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars under Water Limitations" Agronomy 12, no. 4: 803. https://doi.org/10.3390/agronomy12040803
APA StyleEid, M. A. M., El-hady, M. A. A., Abdelkader, M. A., Abd-Elkrem, Y. M., El-Gabry, Y. A., El-temsah, M. E., El-Areed, S. R. M., Rady, M. M., Alamer, K. H., Alqubaie, A. I., & Ali, E. F. (2022). Response in Physiological Traits and Antioxidant Capacity of Two Cotton Cultivars under Water Limitations. Agronomy, 12(4), 803. https://doi.org/10.3390/agronomy12040803










