Influence of Canopy Cover and Meteorological Factors on the Abundance of Bark and Ambrosia Beetles (Coleoptera: Curculionidae) in Avocado Orchards Affected by Laurel Wilt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Canopy Cover and Sampling of Ambrosia Beetles in Avocado Orchards
2.3. Light Intensity under Canopy Covers
2.4. Meteorological Data
2.5. Statistical Analysis
2.6. Decision Tree (DT) Analysis
3. Results
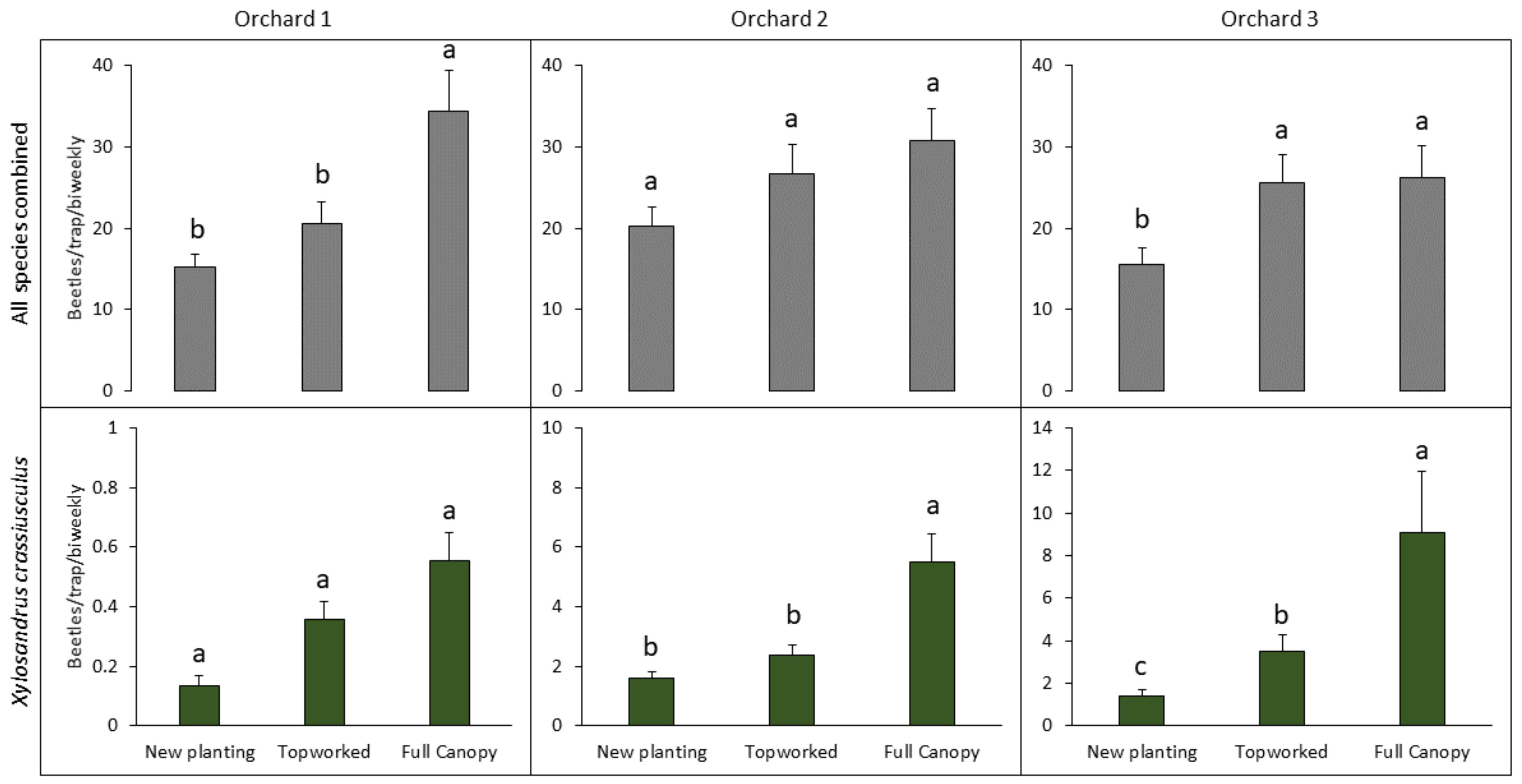
3.1. Effect of Canopy Cover on Ambrosia Beetle Abundance and Composition in LW-Affected Avocado Orchards
3.2. Effect of Light Intensity under Canopy Cover on Abundance of LW Vectors
3.3. Effect of Meteorological Factors on Ambrosia Beetle Flight Activity—Decision Tree Analyses
4. Discussion
4.1. Effect of Canopy Cover on Light Intensity Levels, Ambrosia Beetle Abundance, and Composition in LW-Affected Avocado Orchards
4.2. Effect of Meteorological Factors on Ambrosia Beetle Flight Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT—Countries by Commodity. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity. (accessed on 25 November 2021).
- Carrillo, D.; Duncan, R.E.; Peña, J.E. Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 2012, 95, 573–579. [Google Scholar] [CrossRef]
- Kendra, P.E.; Owens, D.; Montgomery, W.S.; Narvaez, T.I.; Bauchan, G.R.; Schnell, E.Q.; Tabanca, N.; Carrillo, D. α-Copaene is an attractant, synergistic with quercivorol, for improved detection of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2017, 12, e0179416. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Narvaez, T.I.; Carrillo, D. Comparison of trap designs for detection of Euwallacea nr. fornicatus and other Scolytinae (Coleoptera: Curculionidae) that vector fungal pathogens of avocado trees in Florida. J. Econ. Entomol. 2020, 113, 980–987. [Google Scholar] [PubMed]
- Menocal, O.; Kendra, P.E.; Montgomery, W.S.; Crane, J.H.; Carrillo, D. Vertical distribution and daily flight periodicity of ambrosia beetles (Coleoptera: Curculionidae) in Florida avocado orchards affected by laurel wilt. J. Econ. Entomol. 2018, 111, 1190–1196. [Google Scholar] [CrossRef]
- Owens, D.; Kendra, P.E.; Tabanca, N.; Narvaez, T.I.; Montgomery, W.S.; Schnell, E.Q.; Carrillo, D. Quantitative analysis of contents and volatile emissions from α-copaene and quercivorol lures, and longevity for attraction of Euwallacea nr fornicatus in Florida. J. Pest. Sci. 2019, 92, 237–252. [Google Scholar] [CrossRef]
- Owens, D.; Seo, M.; Montgomery, W.S.; Rivera, M.J.; Stelinski, L.L.; Kendra, P.E. Dispersal behavior of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae) in avocado groves and estimation of lure sampling range. Agric. For. Entomol. 2019, 21, 199–208. [Google Scholar] [CrossRef]
- Rivera, M.J.; Martini, X.; Conover, D.; Mafra-Neto, A.; Carrillo, D.; Stelinski, L.L. Evaluation of semiochemical based push-pull strategy for population suppression off ambrosia beetle vectors of laurel wilt disease in avocado. Sci. Rep. 2020, 10, 2670. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, A.F.; Ploetz, R.C.; Peña, P.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant. Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Konkol, J.L.; Narvaez, T.; Duncan, R.E.; Saucedo, R.J.; Campbell, A.; Mantilla, J.; Carrillo, D.; Kendra, P.E. Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida, USA. J. Econ. Entomol. 2017, 110, 347–354. [Google Scholar]
- Saucedo-Carabez, J.R.; Ploetz, R.C.; Konkol, J.L.; Carrillo, D.; Gazis, R. Partnerships between ambrosia beetles and fungi: Lineage-specific promiscuity among vectors of the laurel wilt pathogen, Raffaelea lauricola. Microb. Ecol. 2018, 76, 925–940. [Google Scholar] [CrossRef]
- Basset, Y.; Charles, E.; Hammond, D.S.; Brown, V.K. Short-term effects of canopy openness on insect herbivores in a rain forest in Guyana. J. Appl. Ecol. 2001, 38, 1045–1058. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Moser, A.; Gold, A.; Rotzer, T.; Pauleit, S. Vertical air temperature gradients under the shade of two contrasting urban tree species during different types of summer days. Sci. Total Environ. 2018, 633, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Brar, G.S.; Capinera, J.L.; McLean, S.; Kendra, P.E.; Ploetz, R.C.; Peña, P.E. Effect of trap size, trap height, and age of lure on sampling Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), and its flight periodicity and seasonality. Fla. Entomol. 2012, 95, 1003–1011. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Deyrup, M.A.; Guillén, L.; Epsky, N.D. Xyleborus glabratus, X. affinis, and X. ferrugineus (Coleoptera: Curculionidae: Scolytinae): Electroantennogram responses to host-based attractants and temporal patterns in host-seeking flight. Environ. Entomol. 2012, 41, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Sanchez, J.S.; Deyrup, M.A.; Niogret, J.; Epsky, N.D. Method for collection of live redbay ambrosia beetles, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2012, 95, 513–516. [Google Scholar] [CrossRef]
- Johnson, A.J.; Kendra, P.E.; Skelton, J.; Hulcr, J. Species diversity, phenology, and temporal flight patterns of Hypothenemus pygmy borers (Coleoptera: Curculionidae: Scolytinae) in south Florida. Environ. Entomol. 2016, 45, 627–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudinsky, J.A. Ecology of Scolytidae. Annu. Rev. Entomol. 1962, 7, 327–348. [Google Scholar] [CrossRef]
- Covre, L.de.S.; Melo, A.A.; Flechtmann, C.A.H. Flight activity and spread of Xylosandrus crassiusculus (Motschulsky) (Coleoptera: Curculionidae) in Brazil. Trees For. People. 2021, 4, 100076. [Google Scholar] [CrossRef]
- Martínez, M.; Cognato, A.I.; Guachambala, M.; Boivin, T. Bark and ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) diversity in natural and plantation forests in Ecuador. Environ. Entomol. 2019, 48, 603–613. [Google Scholar] [CrossRef]
- Mezei, P.; Jakuš, R.; Blaženec, M.; Belánová, S.; Šmídt, J. The relationship between potential solar radiation and spruce bark beetle catches in pheromone traps. Ann. For. Res. 2012, 55, 243–252. [Google Scholar]
- Mezei, P.; Potterf, M.; Škvarenina, J.; Rasmussen, J.G.; Jakuš, R. Potential solar radiation as a driver for bark beetle infestation on a landscape scale. Forests 2019, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Aukema, B.H.; Seybold, S.J. The effects of weather on the flight of an invasive bark beetle, Pityophthorus juglandis. Insects 2020, 11, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring North of Mexico, with an illustrated key. Ann. Entomol. Soc. Am. 2006, 99, 1034–1056. [Google Scholar] [CrossRef]
- Atkinson, T.H.; Carrillo, D.; Duncan, R.E.; Peña, J.E. Occurrence of Xyleborus bispinatus (Coleoptera: Curculionidae: Scolytinae) Eichhoff in southern Florida. Zootaxa 2013, 3669, 96–100. [Google Scholar] [CrossRef]
- Gomez, D.F.; Rabaglia, R.J.; Fairbanks, K.E.O.; Hulcr, J. North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys 2018, 768, 19–68. [Google Scholar] [CrossRef] [Green Version]
- De’ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- De’ath, G. Multivariate regression trees: A new technique for modeling species-environment relationships. Ecology 2002, 83, 1105–1117. [Google Scholar]
- Wray, B.A.; Jones, A.T.; Schuhmann, P.W.; Burrus, R.T. Determining the propensity for academic dishonesty using decision tree analysis. Ethics. Behav. 2016, 26, 470–487. [Google Scholar] [CrossRef]
- Shaikhina, T.; Lowe, D.; Daga, S.; Briggs, D.; Higgins, R.; Khovanova, N. Decision tree and random forest models for outcome prediction in antibody incompatible kidney transplantation. Biomed. Signal. Proces. 2019, 52, 456–462. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forest. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Aussenac, G. Interaction between forest stands and microclimatic: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Inskeep, J.R.; Allen, A.P.; Taylor, P.W.; Rempoulakis, P.; Weldon, C.W. Canopy distribution and microclimate preferences of sterile and wild Queensland fruit flies. Sci. Rep. 2021, 11, 13010. [Google Scholar] [CrossRef]
- Sane, S.P. The aerodynamics of insect flight. J. Exp. Biol. 2003, 206, 4191–4208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, E.; Srinivasan, M.V.; Zhang, S.; Cowling, A. Visual control of flight speed in honeybees. J. Exp. Biol. 2005, 208, 3895–3905. [Google Scholar] [CrossRef] [Green Version]
- Burrill, R.M.; Dietz, A. The response of honeybees to variations in solar radiation and temperature. Apidologie 1981, 12, 318–329. [Google Scholar] [CrossRef]
- Reber, T.; Vahakainu, A.; Baird, E.; Weckstrom, M.; Warrant, E.; Dacke, M. Effect of light intensity on flight control and temporal properties of photoreceptors in bumblebees. J. Exp. Biol. 2015, 218, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, A.C.S.; Lewis, S.M. The impact of artificial light at night on nocturnal insects: A review and synthesis. Ecol. Evol. 2018, 8, 11337–11358. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Seybold, S.J. Crepuscular flight activity of an invasive insect governed by interacting abiotic factors. PLoS ONE 2014, 9, e105945. [Google Scholar] [CrossRef] [Green Version]
- Bernáth, B.; Gál, J.; Horváth, G. Why is it worth flying at dusk for aquatic insects? Polarotactic water detection is easiest at low solar elevations. J. Exp. Biol. 2004, 207, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Narendra, A.; Reid, S.F.; Hemmi, J.M. The twilight zone: Ambient light levels trigger activity in primitive ants. Proc. R. Soc. B. 2010, 277, 1531–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.M.; Reynolds, D.R.; Smith, A.D.; Chapman, J.W. Orientation cues for high-flying nocturnal insect migrants: Do turbulence-induced temperature and velocity fluctuations indicate the mean wind flow? PLoS ONE 2010, 5, e15758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield III, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southern United States. Plant. Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, J.; Jose, S.; Freeman, J.; Bunyan, M.; Celis, G.; Hagan, D.; Morgan, M.; Pieterson, E.C.; Zak, J. Short-term impacts of laurel wilt on redbay (Persea borbonia [L.] Spreng.) in a mixed evergreen-deciduous forest in Northern Florida. J. For. 2011, 109, 82–88. [Google Scholar]
- Mayfield, A.E., III; Brownie, C. The redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) uses stem silhouette diameter as a visual host-finding cue. Environ. Entomol. 2013, 42, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maner, M.L.; Hanula, J.L.; Horn, S. Population trends of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae): Does utilization of small diameter redbay trees allow populations to persist? Fla. Entomol. 2014, 97, 208–216. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Sheehan, T.N. Trap height considerations for detecting two economically important forest beetle guilds in southeastern US forests. J. Pest. Sci. 2019, 92, 253–265. [Google Scholar] [CrossRef]
- Niogret, J.; Epsky, N.D.; Schnell, R.J.; Boza, E.J.; Kendra, P.E.; Heath, R.R. Terpenoid variations within and among half-sibling avocado trees, Persea americana Mill. (Lauraceae). PLoS ONE 2013, 8, e73601. [Google Scholar] [CrossRef] [Green Version]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Pruett, G.E.; Mayfield, A.E., III; MacKenzie, M.; Deyrup, M.A.; Bauchan, G.R.; Ploetz, R.C.; Epsky, N.D. North American Lauraceae: Terpenoid emissions, relative attraction and boring preferences of redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2014, 9, e102086. [Google Scholar] [CrossRef]
- Rodríguez, C.S.; Cognato, A.I.; Righi, C.A. Bark and ambrosia beetle (Curculionidae: Scolytinae) diversity found in agricultural and fragmented forests in Piracicaba-SP, Brazil. Environ. Entomol. 2017, 46, 1254–1263. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Efficacy of α-copaene, cubeb, and eucalyptol lures for detection of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2016, 109, 2428–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, G.S.; Kendra, P.E.; David, A.S.; Lake, E.C.; Sigmon, J.W.; Palacios, J.; Donlan, E.M. Community of bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) infesting Brazilian peppertree treated with herbicide and the volatile tree response. Environ. Entomol. 2021, 50, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Roling, M.P.; Kearby, W.H. Seasonal flight and vertical distribution of Scolytidae attracted to ethanol in an oak-hickory forest in Missouri. Can. Ent. 1975, 107, 1315–1320. [Google Scholar] [CrossRef]
- Weber, B.C.; McPherson, J.E. Seasonal flight patterns of Scolytidae (Coleoptera) in black walnut plantations in North Carolina and Illinois. Coleopt. Bull. 1991, 45, 45–56. [Google Scholar]
- Oliver, J.B.; Mannion, C.M. Ambrosia beetle (Coleoptera: Scolytidae) species attacking chestnut and captured in ethanol-baited traps in middle Tennessee. Environ. Entomol. 2001, 30, 909–918. [Google Scholar] [CrossRef]
- Reding, M.; Oliver, J.; Schultz, P.; Ranger, C. Monitoring flight activity of ambrosia beetles in ornamental nurseries with ethanol-baited traps: Influence of trap height on captures. J. Environ. Hort. 2010, 28, 85–90. [Google Scholar] [CrossRef]
- Reding, M.E.; Schultz, P.B.; Ranger, C.M.; Oliver, J.B. Optimizing ethanol-baited traps for monitoring damaging ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in ornamental nurseries. J. Econ. Entomol. 2011, 104, 2017–2024. [Google Scholar] [CrossRef]
- Viloria, Z.; Villanueva, R.T.; Bessin, R.; O’Neal, P.; Ranger, C.M.; Dunwell, W. Scolytinae in nursery and fruit crops of western Kentucky and seasonal population patterns of four invasive ambrosia beetles. J. Entomol. Sci. 2021, 56, 374–386. [Google Scholar] [CrossRef]
- Wermelinger, B.; Seifert, M. Analysis of temperature dependent development of the spruce bark beetle Ips typographus. (L.) (Col., Scolytidae). J. Appl. Entomol. 1998, 122, 185–191. [Google Scholar] [CrossRef]
- Aukema, B.H.; Clayton, M.K.; Raffa, K.F. Modeling flight activity and population dynamics of the pine engraver, Ips pini, in the Great Lakes region: Effects of weather and predators over short time scales. Popul. Ecol. 2005, 47, 61–69. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Williams, K.K.; Hofstetter, R.W.; McMillin, J.D.; DeGomez, T.E.; Wagner, M.R. Influence of temperature on spring flight initiation for southwestern ponderosa pine bark beetles (Coleoptera: Curculionidae, Scolytinae). Environ. Entomol. 2008, 37, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Sittichaya, W.; Permkam, S.; Cognato, A.I. Species composition and flight pattern of Xyleborini ambrosia beetles (Col.: Curculionidae: Scolytinae) from agricultural areas in southern Thailand. Environ. Entomol. 2012, 41, 776–784. [Google Scholar] [CrossRef]
- Kautz, M.; Schopf, R.; Osher, J. The “Sun-effect”: Microclimatic alterations predispose forest edges to bark beetle infestations. Eur. J. For. Res. 2013, 132, 453–465. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Gregoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Gomez, D.F.; Skelton, J.; María, M.de.; Hulcr, J. Influence of temperature and precipitation anomaly on the seasonal emergence of invasive bark beetles in subtropical South America. Neotrop. Entomol. 2020, 49, 347–352. [Google Scholar] [CrossRef]








| Species | Orchard 1 | Orchard 2 | Orchard 3 |
|---|---|---|---|
| Subfamily Scolytinae | |||
| Tribe Xyleborini | |||
| Ambrosiodmus lecontei Hopkins § | 135 | 91 | 99 |
| Ambrosiodmus minor (Stebbing) | 24 | 11 | 1 |
| Euwallacea perbrevis (Schedl) | 54 | 50 | 34 |
| Premnobius cavipennis Eichhoff | 96 | 23 | 38 |
| Theoborus ricini (Eggers) | 136 | 24 | 10 |
| Xyleborinus andrewesii (Blandford) § | 354 | 131 | 22 |
| Xyleborinus gracilis (Eichhoff) § | 37 | 9 | 7 |
| Xyleborinus saxesenii (Ratzeburg) | 1586 | 6802 | 3925 |
| Xyleborus affinis Eichhoff § | 189 | 317 | 240 |
| Xyleborus bispinatus Eichhoff § | 53 | 135 | 137 |
| Xyleborus ferrugineus (Fabricius) § | 44 | 40 | 46 |
| Xyleborus volvulus (Fabricius) § | 419 | 687 | 558 |
| Xylosandrus crassiusculus (Motschulsky) § | 141 | 1278 | 1894 |
| Tribe Cryphalini | |||
| Hypothenemus spp. | 5452 | 726 | 1941 |
| Subfamily Platypodinae | |||
| Euplatypus parallelus (Fabricius) | 94 | 60 | 34 |
| Total ε | 8814 | 10384 | 8986 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menocal, O.; Kendra, P.E.; Padilla, A.; Chagas, P.C.; Chagas, E.A.; Crane, J.H.; Carrillo, D. Influence of Canopy Cover and Meteorological Factors on the Abundance of Bark and Ambrosia Beetles (Coleoptera: Curculionidae) in Avocado Orchards Affected by Laurel Wilt. Agronomy 2022, 12, 547. https://doi.org/10.3390/agronomy12030547
Menocal O, Kendra PE, Padilla A, Chagas PC, Chagas EA, Crane JH, Carrillo D. Influence of Canopy Cover and Meteorological Factors on the Abundance of Bark and Ambrosia Beetles (Coleoptera: Curculionidae) in Avocado Orchards Affected by Laurel Wilt. Agronomy. 2022; 12(3):547. https://doi.org/10.3390/agronomy12030547
Chicago/Turabian StyleMenocal, Octavio, Paul E. Kendra, Armando Padilla, Pollyana C. Chagas, Edvan A. Chagas, Jonathan H. Crane, and Daniel Carrillo. 2022. "Influence of Canopy Cover and Meteorological Factors on the Abundance of Bark and Ambrosia Beetles (Coleoptera: Curculionidae) in Avocado Orchards Affected by Laurel Wilt" Agronomy 12, no. 3: 547. https://doi.org/10.3390/agronomy12030547
APA StyleMenocal, O., Kendra, P. E., Padilla, A., Chagas, P. C., Chagas, E. A., Crane, J. H., & Carrillo, D. (2022). Influence of Canopy Cover and Meteorological Factors on the Abundance of Bark and Ambrosia Beetles (Coleoptera: Curculionidae) in Avocado Orchards Affected by Laurel Wilt. Agronomy, 12(3), 547. https://doi.org/10.3390/agronomy12030547







