Abstract
Efficient approaches aimed at restricting Cuscuta campestris distribution can be based on the control of seed germination. Thus, data on effects of environmental factors, seed age, seed longevity and viability, and hosts on C. campestris seed germination and emergence would provide valuable information in that context. Seeds of 26 populations of C. campestris were collected from different locations in Serbia during the field season August–October between 2005 and 2019. Seeds were collected in three major agronomic regions in Serbia: Banat (13 populations), Srem (11 populations), and Macva (2 populations). The objectives of this study were to investigate the effects of different temperatures and light on seed germination and seedling growth of populations of C. campestris, determine possible correlations between seed age or hosts and total germination and seedling growth, and survey the morphological diversity and genetic variability of seeds of this parasitic plant. Large variability of germination patterns was observed within each agronomic region, and the high variance of seed germination patterns within regions reflects the ability of C. campestris to adapt to local agricultural management practices. For practical purposes, populations that start and complete their emergence earlier are considered harder to control. Thus, farmers should implement effective mechanical and chemical management measures for early-germinating populations.
1. Introduction
The field dodder (Cuscuta campestris Yunck.) is the most prominent among the approximately 200 species in the parasitic plant genus Cuscuta (Convolvulaceae). It is a subcosmopolitan plant regarding its distribution in a variety of habitats [1,2,3]. The presence of parasitic plants contributes to species diversity in natural plant communities by parasitism thwarting host competitiveness and so allowing other present species to thrive [4,5]. Like many other parasitic plants, dodders act as keystone species in their ecosystems [1,6,7]. However, about 15–20 Cuscuta species, most notably C. campestris, are considered agricultural and horticultural pests worldwide [8,9].
The global presence of Cuscuta species depends mostly on the ability of their seeds to disperse, remain viable, and germinate and ultimately on the ability of their seedlings to attach to available hosts. Effective management of weed species, including parasitic plants, in agricultural systems, requires an understanding of such species’ population dynamics [10], and seed fate is a critically important component of such insight. Seed dynamics depend on seed viability and dormancy [11], which affect other processes, such as mortality in the seed bank which occurs through senescence or decay [12], predation [13], and fatal germination [14]. The time of seed germination and the emergence of plants in the field depends on various environmental factors, including light, soil and air temperature, soil moisture, and soil atmosphere [15,16]. Temperature is considered one of the crucial factors for the germination of parasitic plants as well [17]. Additionally, seed dormancy is another significant property of seeds that directly affects their germinating capacity. It is the crucial factor for C. campestris survival and spread in agroecosystems. Many members of the Convolvulaceae family include species with seeds that are capable of undergoing physical dormancy ensured by the water-impermeable mechanical layers in the seed coat [18]. Cuscuta is the only holoparasitic plant capable of this type of dormancy [19,20].
Research on Cuscuta seed morphology, anatomy, embryology, and dispersal is very important, but previous studies had detailed only a few species at a time [21,22,23]. At the same time, some other important studies focused on the selection of hosts by Cuscuta species and their mutual influence. C. campestris was found to be host-nonspecific and able to parasitize almost any dicot plant that comes into contact with it. Dodder habitats vary regarding species diversity and composition, and the host range mainly depends on host availability [2,3,24].
Unlike the important holoparasitic weeds of the genus Orobanche and some hemiparasitic weeds of the genera Striga, Cuscuta species do not require host root exudates to stimulate germination [25]. Seed dormancy is the most important factor for C. campestris survival and spreading in agroecosystems. Predicting the start and duration of seedling emergence can contribute to making better weed control decisions [26] and facilitate the optimal timing of control practices [27].
Patterns of weed emergence are crucial for determining the necessary persistence of herbicides and the effectiveness of nonchemical methods to minimize their deleterious impact on crop yield and quality. Insight into the seed ecology of C. campestris would contribute to better understanding the potential of this parasitic species, as well as its spread and invasiveness. Data on the effects of environmental factors, seed age, seed longevity and viability, and hosts on dodder germination and emergence would provide valuable information in that context. Thus, the objectives of this study were as follows: (i) to investigate the effects of different temperatures and light on seed germination and seedling growth of 26 populations of C. campestris, (ii) to determine possible correlations between seed age or hosts and total germination and seedling growth, (iii) to survey the morphological diversity of C. campestris seeds, and (iv) to examine genetic variability through phylogeny.
2. Materials and Methods
2.1. Seed Collection and Preparation
Seeds of 26 populations of Cuscuta campestris were collected from different locations in Serbia during the field season August–October between 2005 and 2019. Seeds were collected in three major agronomic regions in Serbia: Banat (13 populations), Srem (11 populations), and Macva (2 populations) (Table 1). The seeds were cleaned from vegetative material and seed capsule (pericarp) and stored in paper bags in the laboratory at constant humidity and temperature (50% RH at 20 ± 1 °C).

Table 1.
Primary data on seed materials (voucher specimens).
2.2. Morphological Traits of Seeds
Light microscopy (LM) was used for studying the morphological traits of seeds (length and width). Seed color, based on the seed coat color, was described using the Munsell Color Chart 7.5YR. The light microscope Olympus was used for this purpose, and images were taken by a Moticam 10+ digital camera. The software package Motic Images Plus 3.0 was used for measuring seed morphological characteristics (shape, length, and width (µm)). A batch of 100 seeds was selected to represent each population in measurements of the length and width of seeds, and 1000 seeds were selected from each population for seed weight measurement on the analytic scale.
Scanning electron microscopy (SEM) was used to study the shapes of surface structures of field dodder seeds. They were mounted on stubs with double adhesive tape. The stubs were sputter-coated with gold for 5 min in the apparatus BAL-TEC SCD 005. After coating, the seeds were examined under a scanning electron microscope (Jeol JSM-6390LV).
2.3. Testing Germination and Germination Dynamics In Vitro
The experiment was set up under controlled conditions. Seed germination and seedling growth of C. campestris were examined, focusing on several factors: (1) host (maternal habitats), (2) seed age, (3) temperature, and (4) light. The seeds were scarified with concentrated sulfuric acid (96%) for one hour and then rinsed intensely with sterile distilled water. The seeds were surface sterilized in 5% (w/v) sodium hypochlorite solution (NaOCl) for 3 min and then rinsed three times with sterile distilled water to avoid unwanted inhibition of germination caused by fungal or bacterial toxins. Each population was represented by 400 disinfected seeds (100 seeds per Petri dish), which were placed on filter paper (70 g/m2, 1602 N, Ahlstrom-Munksjö Germany GMBH, Bärenstein, Germany) in Petri dishes. Then, 5 mL of distilled water was added to each dish, and all dishes were kept either in a climate chamber (VELP, Incubator FOC, Serbia) under a range of temperatures (28, 30, and 35 °C) or in a grow box under the following conditions: photoperiod 14 h/10 h, temperature 28/21 ± 1 °C (day/night), humidity 60–70%, and light intensity 300 µE m−2 s−1. All dishes were hermetically sealed with parafilm to avoid evaporation. Germinated seeds were counted daily over one week. Germination percentage was computed and seedling length was measured on the last day. After seven days, germinating seeds were counted at 48 h intervals over the remaining period of 51 days to determine germination dynamics. Germinating seeds developing seedlings of >1 mm length were considered as germinated and removed by sterile tweezers. The germination test was terminated when no more seeds were found germinating for seven consecutive days. After the test, ungerminated seeds were subjected to a standard tetrazolium test [28] to separate dormant from dead seeds and determine seed viability for each population. The experimental design was a randomized complete block with four replications.
2.4. Ribosomal DNA Gene Sequencing and RAPD Analysis
Vegetative material of C. campestris was bulked from 5 individuals from each population and was used for DNA extraction. Total DNA was extracted with GeneMATRIX Plant & Fungi DNA Purification Kit (EURX, Gdansk, Poland) following the manufacturer’s instructions. The concentration of the obtained DNA was estimated spectrophotometrically by NanoDrop 2000c (Waltham, MA, USA) at A260, and purity was estimated by 260/280 nm absorbance ratio. Sequencing analysis of ribosomal DNA (rDNA) region was used for confirmation of taxonomy and establishment of phylogenetic relationships. PCR amplification of the extracted DNA was performed using primers ITS4 and ITSL [29] for the rDNA region, covering partial fragments of the small subunit ribosomal DNA gene ITS1, 5.8S, and ITS2 regions, and a partial fragment of large subunit ribosomal DNA gene. PCR conditions were as follows: initial denaturation at 94 °C for 5 min; 35 cycles of denaturation at 94 °C for 45 s, primer annealing at 50 °C for 45 s, and extension at 72 °C for 1 min; and final extension at 72 °C for 10 min. Direct sequencing after purification (GeneMATRIX PCR/DNA Clean-Up Purification Kit, EURX) of amplified products was performed by Sanger Sequencing Services of Eurofins Genomics. The amplification products were sequenced in both directions, and assembling (contigs) was constructed from the derived sequences with ChromasPro 2.1.10 (Copyright of Technelysium Pty Ltd., Brisbane, Australia). The MEGA 11.0.10 software was used for alignments, BLAST, and maximum likelihood phylogenetic tree construction [30]. C. campestris sequences available in GenBank (https://www.ncbi.nlm.nih.gov/, accessed on 17 November 2021) were used for BLAST analysis. Sequences were deposited in GenBank.
The extracted DNA was used for randomly amplified polymorphic DNA (RAPD) analysis for population genetic diversity based on polymorphisms’ occurrence in different populations. This was performed, using a single primer (as both forward and reverse) which anneals to different sites in DNA if it varies within populations, through PCR amplification. The PCR reactions were performed under the following conditions: 5 min at 94 °C (initial denaturation); 40 cycles of 1 min at 94 °C, 1 min at 36 °C, and 1 min at 72 °C; and 7 min at 72 °C (final extension). The amplifications were triplicated to prove the presence of fragments and their reproducibility. In these amplifications, four decamer primers were used: OPA03, OPA07 [31], OPB17, and OPAL20 [32]. All above-mentioned amplifications were conducted with KAPA PROBE FAST Master Mix (Merck) in a Techne Thermal Cycler. DNAs and PCR products were visualized on 1% agarose gel with CSL-Runsafe (Cleaver Scientific Ltd., Rugby, UK) under UV light. For the determination of fragment length, DirectLoad Wide Range DNA Marker (Sigma Aldrich, Saint Louis, MI, USA) was applied. Electropherograms from RAPD analysis were analyzed using GelJ v2 software [33], and an unweighted pair group method with arithmetic mean (UPGMA) phylogenetic tree was constructed. Polymorphic information content (PIC) for each primer was calculated according to Roldan-Ruiz [34].
2.5. Statistical Analysis
Data are expressed as means ± standard deviation. Normality and homogeneity of variance of all data were checked using the Kolmogorov–Smirnov and Levene tests. The data were processed in Statistica 8.0 (TIBCO Software) software, using a one-way factorial analysis of variance (ANOVA). Differences in total germination, dormant and dead seeds, and seedling length in each population at different temperatures and under a light regime were evaluated by Fisher’s least significant difference (LSD) test (p < 0.05). Characteristics of seed morphological properties (length and width) for different populations were tested using the t-test (p > 0.05; 0.01 < p < 0.05; p < 0.01).
Cumulative emergence of seedlings in the 26 populations of C. campestris was modeled by the Weibull distribution function [35]:
where Y is the cumulative emergence (%), α is the upper asymptote (theoretical maximum for Y set to 100%), κ is the growth rate, γ is the shape parameter that controls X-ordinate for the point of inflection, and X is either the number of days after planting (DAP) or cumulative temperature accumulation in growing degree days (GDD) with the base temperature (temperature below which germination does not occur) being 10 °C. Weibull parameters (κ and γ) for each model were estimated using the nls2 package [36] of R version 4.0.3 [37] in R Studio [38].
Y = α × exp {−exp [−κ × (log X − log γ)]}
Stepwise regression using backward elimination was employed to determine the best Weibull germination model for C. campestris populations within each of the three major agronomic regions [39]. The procedure starts with fitting a “full” least squares model containing all parameters (e.g., κ and γ fit each C. campestris population within the agronomic region) and then removes parameters that are not helping improve the model (“reduced model”) one at a time until parameter removal does not result in model improvement. The criterion for exclusion of the predictor variable is assessed by F-test (variance ratio) at 0.05 α-level. The procedure aims to prevent model overfitting that occurs when the regression model tends to capture individual observations rather than an overall pattern within the dataset. As a result, the most parsimonious model is used to describe the data, i.e., the model that provides the desired level of explanation with as few parameters as possible [39]. Statistical analyses and graphical representation for the seeding rate study were performed using R [37] and R Studio [38].
3. Results
3.1. Seed Color, Shape, and Size
Morphological characteristics of seeds in different C. campestris populations, determined by LM and SEM, are shown in Table 2 and Figure A1. The color of seeds is important for systematic distinction between and within taxa. The seed color of most test populations was described as brown (4/2, 4/3, 4/4), strong brown (4/6, 5/6), and dark brown (3/2, 3/3) (Figure A1E,F). Four populations (Cus3, 7, 59, and 60) had very dark gray (3/1) seeds, while seeds in the population Cus56 were dark gray (4/1) (Table 2). The seed and cellular shapes can also be of considerable systematic value. Seed shapes varied from globose to oval in all populations (Figure A1A,C). SEM micrographs showed that all 26 populations had irregular, polygonal cell shape in the seed coat epidermal layer (Figure A1B,D).

Table 2.
Morphological traits of C. campestris seeds.
Morphological parameters of seeds (length, width, and weight of 1000 seeds) in the 26 C. campestris populations are shown in Table 2. The acquired parameter data showed that seed width in the test populations ranged from 910.1 ± 57.2 to 1365.1 ± 30.2 µm, while seed length was 1045.6 ± 96.4 to 1503.9 ± 31.7 µm. The weight of 1000 seeds varied from 0.6 to 1.2 g, and the lowest weight was measured in populations Cus3, 5, 8, 11, and 56, while the highest was in populations Cus10 and 62.
Statistical data analysis (t-test) revealed significant differences in the length and width of seeds between most populations (p < 0.01). Differences in seed width were not significant between populations (Cus11:Cus5 p = 0.999853; Cus16:Cus7 p = 0.753841; Cus60:Cus55 p = 1.000000), nor were differences in seed length (Cus1:Cus2 p = 1.000000; Cus10:Cus1 p = 0.264947; Cus10:Cus2 p = 0.828081; Cus61:Cus8 p = 1.000000; Cus15:Cus9 p = 0.933531; Cus60:Cus58 p = 1.000000).
3.2. Seed Age and Hosts
Seed maturity (in years) and hosts of field dodder were additional factors that were examined in this study. The age of test population seeds ranged from 1 (Cus60 and 61) to 15 (Cus58 and 59) years, but most ranged from 10 to 12 years, while two populations (Cus62 and 56) were 3 years old and populations Cus53, 54, 55, and 57 had seeds that were 14 years old (Table 1). In Serbia, C. campestris is primarily hosted by alfalfa and red clover crops, then sugar beet, and finally vegetable crops. It is also important to note that field dodder reaches cultivated crops mostly via its primary hosts (weeds) growing on field edges, and Polygonum aviculare is one of the main hosts of this parasitic flowering plant. Consequently, seeds of different C. campestris populations were sampled for the study from the following hosts: (i) weeds: P. aviculare (13), Ambrosia artemisiifolia (2), and Solanum nigrum (1); (ii) grown crops: Beta vulgaris L. ssp. vulgaris var. altissima Doll (7), Medicago sativa (3), Solanum tuberosum (1), and Foeniculum vulgare (1) (Table 1). The hosts of C. campestris were not found to have any particular effect on seed germination of this parasitic flowering plant under any temperature treatment.
3.3. Germination Tests under Different Temperature and Light Treatments
The temperature had a significant effect on seed germination of different C. campestris populations. The highest germination percentage in all populations, ranging from 64% (Cus62) to 9.3% (Cus59), occurred at 28 °C (Table 3). A correlation was found between germination and seed age as the populations with a seed maturity range of 1 to 3 years germinated significantly better than populations in the 10–15 year maturity range. The data, therefore, indicate a declining trend of germination as seed age advances, which makes it a crucial factor for the germination process (Table 3). The same trend was noted to occur at 30 and 35 °C. It was also confirmed that germination decreases with growing temperature as the highest germination at 35 °C was 20% (Cus62), which was a 3 times lower percentage than that of the same population germinated at 28 °C. The percentage of dead seeds at all temperatures was in reverse correlation with germination percentage.

Table 3.
Total percentage of germinated, dormant, and dead seeds of 26 test populations of C. campestris at 28, 30, and 35 °C temperatures.
Table 3 presents only the significant differences within each population at variable temperatures. The highest coefficient of variation for seed germination was found at 35 °C (4.78%), then 28 °C (3.58%), while the lowest (3.14%) was noted at 30 °C. Corresponding coefficients of variation for dormant and dead seeds were similar and ranged from 1.99 (dormant seeds at 30 °C) to 2.46 (dead seeds at 35 °C) (Table 3).
Light is known to be of crucial influence on the seed germination of most weed species, including field dodder. The data received in this study confirmed such influence. Especially noteworthy is the difference between field dodder germination observed at the temperature of 28 °C in the dark, when the peak was reached, and germination under light treatment at the same temperature. By including light as a factor, the germination percentage increased in all 26 populations inside a range of 1.5–19% (Table 4). The highest germination increase was noted in populations Cus62 (19%) and Cus58 (17%).

Table 4.
Total percentage of germinated, dormant, and dead seeds in 26 populations of C. campestris under light treatment (day/night 28 °C/21 °C) and in the dark (28 °C).
Unlike germination, the parameter of shoot length was found to be less susceptible. Thus, certain temperature or light treatments were not found to significantly stimulate early seedling growth in any of the 26 test populations of C. campestris. This parameter was similar for all populations under light treatment and at all temperatures (28, 30, and 35 °C) since the average values of shoot length under these treatments were 7.7, 6.9, 7.7, and 6.3 cm, respectively (Table 5).

Table 5.
Shoot length of 26 populations of C. campestris at 28, 30, and 35 °C temperatures (dark) and under light treatment (28 °C/21 °C).
3.4. Cumulative Germination
3.4.1. Temperature
The germination pattern of C. campestris seeds was characterized by rapid emergence and a somewhat extended period of completion. Data show that 50% of C. campestris seeds germinated within 5 days, while an additional 15 days were needed to reach 90% germination. Higher average temperatures accelerated C. campestris germination (Table A1, Figure 1a). C. campestris germination was delayed at an average rate of 1.4 days per unit of average temperature (10 days delay for 7 °C increase in temperature). Prediction of cumulative germination using temperature accumulation (GDD) resulted in similar germination patterns across three temperatures (Figure 1b). On average, C. campestris populations needed 40.2 GDD to finish germination. These results also suggest that the temperature-adjusted germination model is preferable as data can be combined across temperatures for more accurate statistical analysis.
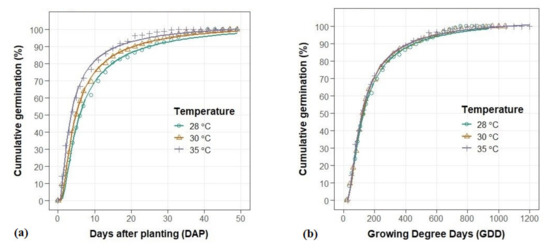
Figure 1.
Effects of temperature on cumulative germination (%) of 26 C. campestris populations using (a) days after planting (DAP) and (b) growing degree days (GDD) as explanatory variables.
3.4.2. Light
Both temperature and light were found to exert a significant impact on C. campestris germination (Table A2, Figure 2 and Figure 3). As field dodder is a small-seeded weed, its germination patterns in the field may vary significantly within a population. Slight variation in soil type, tillage, residue removal, crop competition, and environmental conditions may change soil temperature and illumination in different parts of the field. By burying dodder seeds below the soil surface, where the soil temperature is lower and there is no light, and by planting the crop early in the season and allowing a good stand (ground cover) to form, very beneficial conditions would be created for decreasing the ability of C. campestris to compete for resources. Despite the lower average germination temperature, exposure to light caused C. campestris seeds to germinate 7 days earlier. C. campestris populations in Macva completed germination 3–6 days earlier than in other regions, regardless of light exposure.
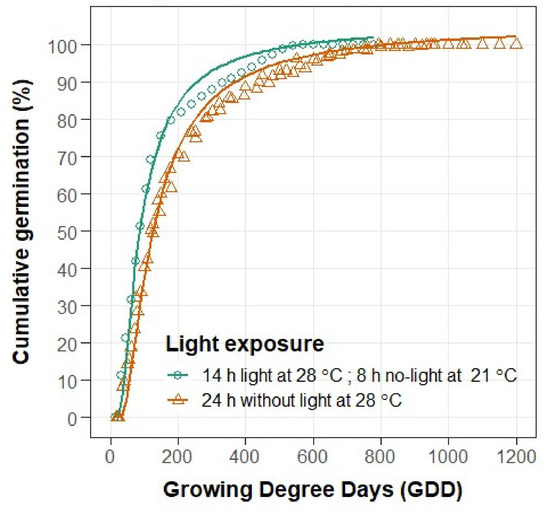
Figure 2.
Light effects on cumulative seed germination in 26 C. campestris populations depending on growing degree days (GDD).
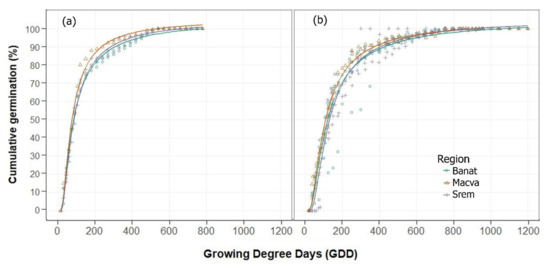
Figure 3.
Cumulative germination (%) of 26 populations of Cuscuta campestris over growing degree days (GDD) by regions (Banat, Srem, Macva, and Belgrade) depending on (a) light and (b) no light.
3.4.3. Locations
The 90% cumulative germination of 26 C. campestris populations was achieved between the 15th and 32nd days (Table A3), suggesting a large genetic variability of C. campestris populations in Serbia. Large variability of germination patterns was also observed within each agronomic region (Figure 4). For example, the difference between the earliest and latest germinating population was 12, 15, and 3 days for Banat, Srem, and Macva, respectively. In the region of Srem, only 30 km separated the earliest (Krcedin) and latest (Pecinci) germinating C. campestris populations. Such high variability of germination patterns within regions reflects the ability of C. campestris to adapt to local agricultural management practices. For practical purposes, populations that start and complete their emergence earlier are considered harder to control. Thus, farmers should implement direct control measures for early-germinating populations.
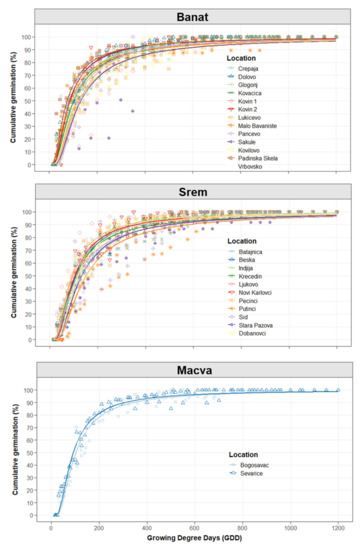
Figure 4.
Cumulative germination (%) by locations grouped by regions during growing degree days (GDD).
3.5. Taxonomic Identity and Genetic Variability
The sequences of internal transcribed spacers (ITS) 1 and 2 and 5.8S ribosomal RNA gene of 24 out of 26 populations of C. campestris were deposited in GenBank at NCBI under accession numbers OL444840 to OL444863. A BLAST search against all accessions in the nucleotide database of GenBank confirmed the taxonomic status of all 24 populations as Cuscuta campestris Yunck. with a percent identity of more than 95%. The acquired sequences were used for the construction of a phylogenetic tree (maximum likelihood method) (Figure 5). Although several clusters could be distinguished, the overall picture revealed substantial genetic variability with no clear relation to geographic distance.
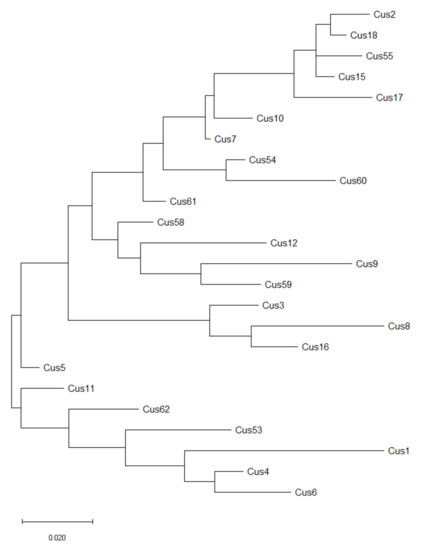
Figure 5.
Maximum likelihood phylogenetic tree of Cuscuta campestris populations based on ITS and 5.8S ribosomal gene DNA sequences. Abbreviations correspond to those represented in Table 1. Sequences are available in GenBank under accession numbers OL444840 to OL444863. Scale bar is genetic distance.
Random amplified polymorphism analysis results are summarized in Table A4. The total number of bands varied between 4 and 5, and the number of polymorphic bands varied between 1 and 4. Two populations, namely Cus55 (Figure A2) and Cus61, did not give amplified products with any of the primers and were omitted from further analysis. The mean value of polymorphic information content (PIC) was 0.38, which is not among the highest acquired by RAPD markers but is still sufficient to assess genetic diversity [40].
According to RAPD analysis, a UPGMA tree was constructed (Figure 6). According to it, two distinct clusters (I and II) could be clearly identified. However, at least four populations (namely Cus8, Cus16, Cus59, and Cus62) are quite distinct from the rest. Similar to the ITS-sequence-generated phylogenic tree, no clear geographic correlation could be identified, but it should be also noted that the clusters between the two trees do not match.
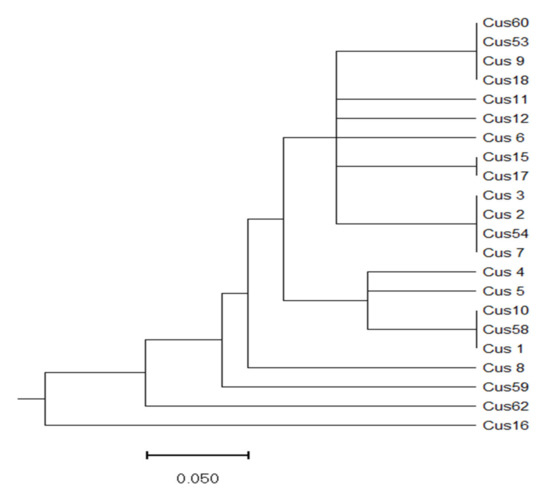
Figure 6.
UPGMA tree of Cuscuta campestris populations based on RAPD analysis with four decamer primers. Scale bar is genetic distance.
4. Discussion
Seed characteristics, such as size, color, shape, and surface structure, are useful parameters for species identification and taxonomy [22,41]. A comparative study of C. campestris populations from our investigation and data reported by Abdel Khalik [22] revealed similar seed sizes. Abdel Khalik [22] and Hamed [42] described the shape and coat color of C. campestris seed, specifying the color as brown, which is consistent with our observation that most test populations were brown, strong brown, and dark brown. We also noticed that all 26 populations had irregular, polygonal cell shape in the epidermal layer of seed coat and that the seed shape varied from globose to oval in all populations, which is consistent with the earlier reports from Abdel Khalik [22] and Hamed [42].
Seed size of some plants is strongly related to their dispersal potential, competitive ability, seed bank behavior, distribution [41], and abundance [43], but Olszewski et al. [23] found no indication of such a relationship in Cuscuta species. In many other plants, seed size is positively correlated with higher seedling survivorship rates when seedlings are facing hazards (e.g., deep shade, drought, physical damage) because larger seeds have more food reserves, while smaller seeds are viewed as having greater dispersal capacity because they are usually produced in larger numbers and have longer seed bank persistence [44,45]. For Cuscuta, the ontogenetic bottleneck of its seedling stage is even more critical because, apart from facing the same abiotic and biotic survival challenges as green plants [46,47], its seedlings also need to find a host plant in the close vicinity and identify and counter the defense of compatible hosts in a narrow time frame [9]. Our results do not confirm a relationship between seed size and germination, early growth, or the dynamic of germination of different populations of C. campestris.
In addition to seed size as an important trait affecting plant population dynamics, the evolutionary significance of interspecific seed weight variation has also been documented abundantly in the literature [43,48]. Over the past 30 years, many studies have shown that seed mass is widely variable depending on populations and growing conditions within a population, within a plant species, and even among seeds within a fruit [49,50]. It was also expected that seed traits, along with host preferences, would be affected by genetic variability. As clearly indicated, the studied populations possess substantial genetic diversity, which cannot be properly assessed by a single approach. Further, no clear link between geographic distribution, host preference, and genetic distance could be established, which is in agreement with recent results for Cuscuta campestris [51]. The proposed RAPD markers are generally used to distinguish between Cuscuta species [32]. Moreover, in C. epithymum accessions, they were proved more powerful than ISSR markers [52]. In the present study, they allowed for certain differentiation between different C. campestris populations. Overall, it seems that genetic variability among C. campestris populations in Serbia is quite dispersed and is decisive neither for host preference nor seed traits. It is now well known that individual plants can and often do produce seeds of sizes that are more variable than the sizes within or among populations [48,49]. Besides seed size, a correlation was not detected between seed mass and germination, early growth, or dynamic of germination of different C. campestris populations.
In the past, Cuscuta species were believed to be host-specific and able to infest only one host species, so the names of some dodders (C. epilinum Weihe., C. epithymum (L.) Nath., C. polygonorum Engelm., C. cephalanti Engelm., etc.) refer to species or genera that were initially associated with them [21]. However, when it was confirmed that dodders can parasitize several species and that most of them have a considerable host range, the concept changed, and recent studies have introduced the distinct categories of host-generalist and host-specific dodders [2,3,8,24,53]. Barath and Csiky [24] found in studies conducted over 6 years that C. epithymum and C. campestris were the most widely represented dodder species in Hungary and that the range of their hosts was very wide (341 and 224 host species, respectively). According to Costea and Tardif [9], C. gronovii is the most frequent dodder species in Canada, and C. campestris and C. umbrosa are next most frequent. The existing data for Serbia [54,55] show that C. campestris is the most widely represented species and most important agricultural pest. Of the 26 test populations, 13 were collected from the weed species Polygonum aviculare, while sugar beet was the most frequent species among cultivated crops. Barath and Csiky [24] reported that the most frequent host plant of C. campestris in Hungary was P. aviculare, accounting for 68% of infestation frequency. In our research, the host plant of C. campestris from which seeds were collected had no significant impact on the germination and early growth in any temperature and light treatment. Total germination showed no significant variation in populations of the same age collected from different hosts. Only the population Cus62, aged 3 years, was found to germinate more than any other population, including those that were only 1 year old, and the host was the medicinal plant Foeniculum vulgare L. rich in essential oils. Sarić-Krsmanović et al. [56] noted a negative impact of C. campestris on essential oil yield from fennel seed, as well as oils isolated from peppermint and chamomile leaves and stems [57]. The study did not include determination of the content of C. campestris essential oil, which could have clarified the matter of exchange of secondary metabolites between the parasite and the host as it could indirectly affect the fruiting and viability of this parasitic flowering plant.
Seed response to light is important for preventing germination in places and at times unfavorable for seedling establishment and survival, and de-etiolated dodder seedlings must especially detect and respond to light quality, intensity, and direction to survive. Light provides de-etiolated dodder seedlings with the necessary environmental information that initiates changes in their two modes of vital activity, i.e., from the higher plant mode of growth to the parasitic mode of growth [58,59,60]. In the field, Cuscuta seedlings may grow directly toward a light source and the green canopy of potential nearby hosts [58,59]. In addition, Haidar [59] suggested that stem curling and formation of prehaustoria are influenced by phytochromes (pigments absorbing red and far-red light) as well as cryptochromes (pigments absorbing blue and ultraviolet light). In our research, the highest germination in all populations was noted in the light treatment. The percentage of germinated seeds was the highest (83.50%—Cus62) under light treatment, while the average seedling length was 7.7 cm, which is similar to the statistics in various temperature treatments without illumination (6.3, 6.9, and 7.7 cm on average). This indicates that light had a greater impact on the process of germination than on the subsequent seedling growth (Table 4 and Table 5). Benvenuti et al. [17] examined the influence of temperature on seed germination of C. campestris, and the percentage of seeds that germinated at 20–30 °C, after being scarified with sulfuric acid, ranged from 60% to 80%, while the germination percentage of unscarified seeds was significantly lower (20%). A similar observation was made by Sarić-Krsmanović [55] in tests in which dormancy was broken by soaking seeds in concentrated sulfuric acid, since germination percentage was 59%, 84%, and 97% at temperature of 20, 25, and 30 °C, respectively.
The seeds of Cuscuta species may survive at least 10 years in the field, while dry storage may extend that period, depending on the species, to 50 years or beyond [8,21]. Gaertner [21] reported that C. campestris seed was able to survive in dry storage for 10–20 years, C. gronovii seed up to 30 years, and C. pentagona seed up to 51 years. Gaertner [21] also noted that fresh seeds (immediately after fruiting) of C. campestris were not dormant and were able to germinate while still encapsulated, but several days later 77–95% of the seeds became hard, and their seed coat turned dry, hard, and water-impermeable, consequently becoming dormant. In our research, it was confirmed that a significant number of seeds retained their viability. Moreover, the total germination of C. campestris seeds ranged from 10% to 31% for seeds aged 10 to 14 years.
Germination is a critical stage in the life cycle of weeds and often controls population dynamics, with major practical implications [61]. The results of our studies showed that germination of C. campestris seeds depends on seed characteristics, light, and temperature. However, no clear relation between genetic diversity and seed germination could be established, suggesting that seed germination is mostly dependent on the quality of seeds produced, rather than genetic background. The given data revealed that photoperiod temperatures of 28 °C/21 °C and the temperature of 28 °C are the optimal for germination of C. campestris seeds. Following the obtained results, it is possible to predict the moment of germination of C. campestris in different regions based on climatic parameters.
Author Contributions
Conceptualization, M.S.-K. and L.Z.; methodology, all authors; software, L.Z., D.T. and M.R.; formal analysis, M.S.-K. and J.G.U.; investigation, M.S.-K., L.Z., J.G.U. and D.T.; collecting seed samples S.V., D.B., L.R., M.S.-K. and J.G.U.; data curation, all authors; writing—original draft preparation, M.S.-K., L.Z., M.R. and J.G.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract Nos. 451-03-9/2022-14/200214 and 451-03-9/2022-14/200116) and the Bulgarian National Science Fund (Grant number KП-06-H31/10).
Data Availability Statement
Data associated with this study are deposited and published in GenBank at NCBI (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 17 November 2021) under accession numbers from OL444840 to OL444863.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
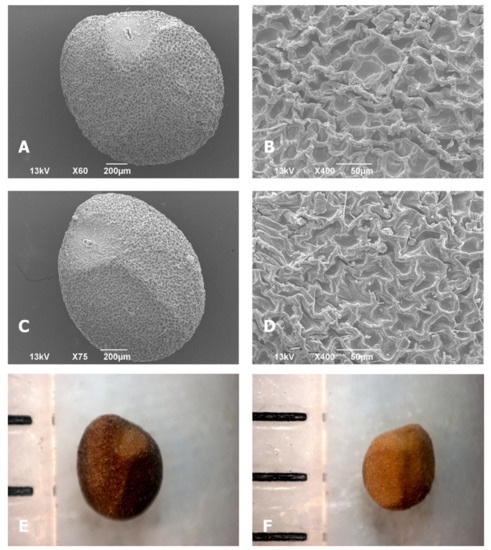
Figure A1.
Cuscuta campestris seed: (A,C) SEM photographs of seed shape; (B,D) cell shape in the epidermal layer of seed coat; (E,F) LM photographs of seed color.
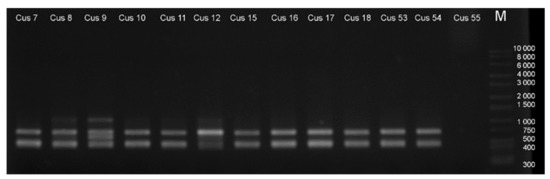
Figure A2.
A representative electropherogram of RAPD amplification products (with primer OPB-17) of C. campestris populations.

Table A1.
Effects of temperature on cumulative germination of 10%, 50%, and 90% of seeds in 26 populations of Cuscuta campestris over growing degree days (GDD) and days after planting (DAP).
Table A1.
Effects of temperature on cumulative germination of 10%, 50%, and 90% of seeds in 26 populations of Cuscuta campestris over growing degree days (GDD) and days after planting (DAP).
| Explanatory Variable | Temp. (°C) | Parameter Estimates (±SE) | GDD | DAP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | e | SE | 10% | 50% | 90% | 10% | 50% | 90% | ||
| global.gd | - | 1.26 | 0.01 | 3.41 | 0.02 | 2 | 5 | 20 | 2 | 5 | 20 |
| global.gdd10 | - | 1.42 | 0.01 | 82.76 | 0.45 | 46 | 107 | 402 | 2 | 5 | 19 |
| GD | 28 | 1.27 | 0.02 | 4.32 | 0.06 | 2 | 6 | 26 | 2 | 6 | 26 |
| GD | 30 | 1.29 | 0.02 | 3.60 | 0.05 | 2 | 5 | 21 | 2 | 5 | 21 |
| GD | 35 | 1.23 | 0.02 | 2.58 | 0.04 | 1 | 3 | 16 | 1 | 3 | 16 |
| GDD | 28 | 1.42 | 0.02 | 95.92 | 1.03 | 53 | 124 | 468 | 3 | 6 | 22 |
| GDD | 30 | 1.47 | 0.02 | 91.59 | 1.01 | 52 | 117 | 423 | 2 | 6 | 20 |
| GDD | 35 | 1.47 | 0.02 | 89.65 | 1.08 | 51 | 115 | 414 | 2 | 5 | 20 |
b—the slope of the line at an inflection point; e—average GDD and DAP resulting in 50% germination between the upper and lower limits; SE—standard error.

Table A2.
Effects of light on cumulative germination of 10%, 50%, and 90% of seeds in 26 populations of Cuscuta campestris over growing degree days (GDD) and days after planting (DAP) and by regions (Banat, Srem, and Macva).
Table A2.
Effects of light on cumulative germination of 10%, 50%, and 90% of seeds in 26 populations of Cuscuta campestris over growing degree days (GDD) and days after planting (DAP) and by regions (Banat, Srem, and Macva).
| Model | Light | Parameter Estimates (±SE) | Germination in GDD | Germination in DAP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | e | SE | 10% | 50% | 90% | 10% | 50% | 90% | ||
| light | yes | 1.46 | 0.01 | 64.18 | 0.58 | 36 | 83 | 301 | 2 | 4 | 14 |
| no light | no | 1.46 | 0.01 | 92.62 | 0.51 | 52 | 119 | 434 | 2 | 6 | 21 |
b—the slope of the line at an inflection point; e—average GDD and DAP resulting in 50% seed germination between the upper and lower limits; SE—standard error.

Table A3.
Region by location models (no light).
Table A3.
Region by location models (no light).
| Region | Location | Parameter Estimates (±SE) | Germination in GDD | Germination in DAP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | e | SE | 10% | 50% | 90% | 10% | 50% | 90% | ||
| Banat | Sakule | 1.51 | 0.07 | 68.81 | 2.27 | 40 | 88 | 305 | 2 | 4 | 15 |
| Lukicevo | 1.23 | 0.05 | 72.90 | 2.63 | 37 | 98 | 453 | 2 | 5 | 22 | |
| Kovačica | 1.42 | 0.06 | 82.09 | 2.58 | 46 | 106 | 402 | 2 | 5 | 19 | |
| Crepaja | 1.40 | 0.06 | 80.52 | 2.57 | 44 | 105 | 400 | 2 | 5 | 19 | |
| Dolovo | 1.48 | 0.07 | 72.69 | 2.36 | 41 | 93 | 334 | 2 | 4 | 16 | |
| Glogonj | 1.72 | 0.08 | 95.23 | 2.52 | 59 | 118 | 351 | 3 | 6 | 17 | |
| Malo Bavaniste | 1.72 | 0.07 | 119.29 | 2.91 | 73 | 148 | 442 | 3 | 7 | 21 | |
| Kovin 1 | 1.54 | 0.07 | 72.68 | 2.31 | 42 | 92 | 313 | 2 | 4 | 15 | |
| Kovin 2 | 1.40 | 0.05 | 114.45 | 3.20 | 63 | 149 | 569 | 3 | 7 | 27 | |
| Pančevo | 1.30 | 0.05 | 98.81 | 3.04 | 52 | 131 | 556 | 2 | 6 | 26 | |
| Padinska skela | 1.41 | 0.06 | 111.26 | 3.52 | 62 | 144 | 549 | 3 | 7 | 26 | |
| Vrbovski | 1.64 | 0.08 | 89.62 | 2.82 | 54 | 112 | 354 | 3 | 5 | 17 | |
| Kovilovo | 1.59 | 0.08 | 106.61 | 3.19 | 63 | 134 | 438 | 3 | 6 | 21 | |
| Srem | Krcedin | 1.44 | 0.06 | 75.57 | 2.39 | 42 | 97 | 360 | 2 | 5 | 17 |
| Batajnica | 1.39 | 0.05 | 103.52 | 2.94 | 57 | 135 | 521 | 3 | 6 | 25 | |
| Beska | 1.47 | 0.06 | 87.20 | 2.57 | 49 | 112 | 405 | 2 | 5 | 19 | |
| Stara Pazova | 1.67 | 0.07 | 98.90 | 2.57 | 60 | 123 | 382 | 3 | 6 | 18 | |
| Novi Karlovci | 1.44 | 0.06 | 75.99 | 2.40 | 43 | 98 | 364 | 2 | 5 | 17 | |
| Pecinci | 1.45 | 0.05 | 142.21 | 3.58 | 80 | 183 | 675 | 4 | 9 | 32 | |
| Ljukovo | 1.57 | 0.07 | 95.25 | 2.60 | 56 | 120 | 399 | 3 | 6 | 19 | |
| Sid | 1.40 | 0.05 | 114.86 | 3.14 | 63 | 149 | 576 | 3 | 7 | 27 | |
| Putinci | 1.32 | 0.05 | 113.92 | 3.23 | 61 | 150 | 627 | 3 | 7 | 30 | |
| Indjija | 1.30 | 0.05 | 88.00 | 2.77 | 46 | 117 | 500 | 2 | 6 | 24 | |
| Dobanovci | 1.91 | 0.10 | 105.11 | 2.84 | 68 | 127 | 341 | 3 | 6 | 16 | |
| Macva | Bogosavac | 1.55 | 0.03 | 92.75 | 1.67 | 54 | 117 | 395 | 3 | 6 | 19 |
| Sevarice | 1.55 | 0.03 | 78.11 | 1.49 | 46 | 99 | 332 | 2 | 5 | 16 | |

Table A4.
RAPD primers and acquired results from each primer. PIC—polymorphic information content.
Table A4.
RAPD primers and acquired results from each primer. PIC—polymorphic information content.
| Primer | Sequence (5′ -> 3′) | Total No. of Bands | No. of Polymorphic Bands | Size Range of Bands (bp) | PIC |
|---|---|---|---|---|---|
| OPA-03 | AGTCAGCCAC | 4 | 2 | 380–900 | 0.35 |
| OPA-07 | GAAACGGGTG | 5 | 4 | 480–2000 | 0.45 |
| OPB-17 | AGGGAACGAG | 4 | 2 | 500–890 | 0.51 |
| OPAL-20 | AGGAGTCGGA | 5 | 1 | 280–440 | 0.20 |
| Total | 18 | 9 | |||
| Mean | 4.5 | 2.25 | 0.38 |
References
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of infection by parasitic weeds: A review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef]
- García, M.A.; Costea, M.; Kuzmina, M.; Stefanović, S. Phylogeny, character evolution, and biogeography of Cuscuta (Dodders; Convolvulaceae) inferred from coding plastid and nuclear sequences. Am. J. Bot. 2014, 101, 670–690. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule Exchange in Cuscuta–Host Plant Interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz Neto, O.; Leal, I.R.; Santos, J.C.; Lopes, A.V. A holoparasitic plant severely reduces the vegetative and reproductive performance of its host plant in the Caatinga, a Brazilian seasonally dry forest. Acta Bot. Bras. 2017, 31, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Vogel, A.; Schwacke, R.; Denton, A.K.; Usadel, B.; Hollmann, J.; Fischer, K.; Bolger, A.; Schmidt, M.H.-W.; Bolger, M.E.; Gundlach, H.; et al. Footprints of parasitism in the genome of the parasitic flowering plant Cuscuta campestris. Nat. Commun. 2018, 9, 2515. [Google Scholar] [CrossRef]
- Dawson, J.H.; Musselman, L.J.; Wolswinkel, P.; Dörr, I. Biology and control of Cuscuta. Rev. Weed Sci. 1994, 6, 265–317. [Google Scholar]
- Costea, M.; Tardif, F.J. The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. Ex Schult., C. umbrosa Beyr. Ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Can. J. Plant Sci. 2006, 86, 293–316. [Google Scholar] [CrossRef]
- Holst, N.; Rasmussen, I.A.; Bastiaans, L. Field weed population dynamics: A review of model approaches and applications. Weed Res. 2007, 47, 1–14. [Google Scholar] [CrossRef]
- Conn, J.S.; Werdin-Pfisterer, N.R. Variation in seed viability and dormancy of 17 weed species after 24.7 years of burial: The concept of buried seed safe sites. Weed Sci. 2010, 58, 209–215. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Small, J.L.; Torvell, L. Edaphic factors influence the longevity of seeds in the soil. Plant Ecol. 2012, 213, 57–65. [Google Scholar] [CrossRef]
- Westerman, P.R.; Wes, J.S.; Kropff, M.J.; van der Werf, W. Annual losses of weed seeds due to predation in organic cereal fields. J. Appl. Ecol. 2003, 40, 824–836. [Google Scholar] [CrossRef]
- Davis, A.S.; Renner, K.A. Influence of seed depth and pathogens on fatal germination of velvetleaf (Abutilon theophrasti) and giant foxtail (Setaria faberi). Weed Sci. 2007, 55, 30–35. [Google Scholar] [CrossRef]
- Forcella, F.; Benech Arnold, R.L.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crops Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Benvenuti, S.; Dinelli, G.; Bonetti, A.; Catizone, P. Germination ecology, emergence and host detection in Cuscuta campestris. Weed Res. 2005, 45, 270–278. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Baskin, J.M.; Baskin, C.C.; Dixon, K.W. Physical dormancy in the endemic Australian genus stylobasium, a first report for the family Surianaceae (Fabales). Seed Sci. Res. 2006, 16, 229–232. [Google Scholar] [CrossRef]
- Willis, C.G.; Hall, J.C.; de Casas, R.R.; Wang, T.Y.; Donohue, K. Diversification and the evolution of dispersal ability in the tribe Brassicaceae (Brassicaceae). Ann. Bot. 2014, 114, 1675–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaertner, E.E. Studies of seed germination, seed identification, and host relationships in dodders, Cuscuta spp. Mem. Cornell Univ. Agric. Exp. Stn. 1950, 294, 3–56. [Google Scholar]
- Abdel Khalik, K.N. Seed morphology of Cuscuta L. (Convolvulaceae) in Egypt and its systematic significance. Feddes Repert. 2006, 117, 217–224. [Google Scholar] [CrossRef]
- Olszewski, M.; Dilliott, M.; García-Ruiz, I.; Bendarvandi, B.; Costea, M. Cuscuta seeds: Diversity and evolution, value for systematics/identification and exploration of allometric relationships. PLoS ONE 2020, 15, e0234627. [Google Scholar] [CrossRef]
- Baráth, K.; Csiky, J. Host range and host choice of Cuscuta species in Hungary. Acta Bot. Croat. 2012, 71, 215–227. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, Y.; Miryamchik, H.; Rubin, B.; Eizenberg, H. Field dodder (Cuscuta campestris)—A new model describing temperature-dependent seed germination. Weed Sci. 2016, 64, 53–60. [Google Scholar] [CrossRef]
- Grundy, A.C. Predicting weed emergence: A review of approaches and future challenges. Weed Res. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Association for Seed Testing: Zurich, Switzerland, 2008. [Google Scholar]
- Hsiao, C.; Chatterton, N.J.; Asay, K.H.; Jensen, K.B. Phylogenetic relationships of 10 grass species: An assessment of phylogenetic utility of the internal transcribed spacer region in nuclear ribosomal DNA in monocots. Genome 1994, 37, 112–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Zahid, N.Y.; Abbasi, N.A.; Hafiz, I.A.; Ahmad, Z. Genetic diversity of indigenous fennel (Foeniculum vulgare Mill.) germplasm in Pakistan assessed by RAPD Markers. Pak. J. Bot. 2009, 41, 1759–1767. [Google Scholar]
- Elsiddig, M.A.; Mahadi, Y.M.; Mohamed, E.H.; Alamin, S.E.; Alrahim, A.I.A.; Haroun, N.E.; Eltayeb, A.H.; Ibraheem, Y.M. Genetic diversity of dodder (Cuscuta spp.) collected from Khartoum and Gezira States. Int. J. Sci. Res. Publ. 2018, 8, 634–640. [Google Scholar] [CrossRef]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GeIJ—A tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015, 16, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldán-Ruiz, I.; Dendauw, J.; van Bockstaele, E.; Depicker, A.; de Loose, M. AFLP Markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol. Breed. 2000, 6, 125–134. [Google Scholar] [CrossRef]
- Seber, G.A.F.; Wild, C.J. Growth models. In Nonlinear Regression; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1989; pp. 325–365. [Google Scholar]
- Grothendieck, G. Nls2: Non-Linear Regression with Brute Force. R Package Version 0.2. CRAN. 2013. Available online: http://cran.uvigo.es/web/packages/nls2/nls2.pdf (accessed on 14 April 2021).
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-Project.Org (accessed on 14 April 2021).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.Rstudio.Com (accessed on 14 April 2021).
- Wang, M.; Wright, J.; Brownlee, A.; Buswell, R. A Comparison of approaches to stepwise regression on variables sensitivities in building simulation and analysis. Energy Build. 2016, 127, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Refika Akçali Giachino, R. Investigation of the genetic variation of anise (Pimpinella anisum L.) using RAPD and ISSR markers. Genet. Resour. Crop Evol. 2020, 67, 763–780. [Google Scholar] [CrossRef]
- Kanwal, D.; Abid, R.; Qaiser, M. The seed atlas of Pakistan III. Cuscutaceae. Pak. J. Bot. 2010, 42, 703–709. [Google Scholar]
- Hamed, K.A. Pollen and seed characters of certain Cuscuta species growing in Egypt with a reference to a taxonomic treatment of the genus. Int. J. Agric. Biol. 2005, 7, 325–332. [Google Scholar]
- Guo, Q.; Brown, J.H.; Valone, T.J. Constraints of seed size on plant distribution and abundance. Ecology 2000, 81, 2149–2155. [Google Scholar] [CrossRef]
- Venable, D.L.; Brown, J.S. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 1988, 131, 360–384. [Google Scholar] [CrossRef]
- Bekker, R.M.; Bakker, J.P.; Grandin, U.; Kalamees, R.; Milberg, P.; Poschlod, P.; Thompson, K.; Willems, J.H. Seed size, shape and vertical distribution in the soil: Indicators of seed longevity. Funct. Ecol. 1998, 12, 834–842. [Google Scholar] [CrossRef]
- Isselstein, J.; Tallowin, J.R.B.; Smith, R.E.N. Factors affecting seed germination and seedling establishment of fen-meadow species. Restor. Ecol. 2002, 10, 173–184. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Michaels, H.J.; Benner, B.; Hartgerink, A.P.; Lee, T.D.; Rice, S.; Willson, M.F.; Bertin, R.I. Seed size variation: Magnitude, distribution, and ecological correlates. Evol. Ecol. 1988, 2, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Obeso, J.R. Seed mass variation in the perennial herb Asphodelus albus: Sources of variation and position effect. Oecologia 1993, 93, 571–575. [Google Scholar] [CrossRef]
- Susko, D.J.; Lovett-Doust, L. Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata (Brassicaceae). Am. J. Bot. 2000, 87, 56–66. [Google Scholar] [CrossRef]
- Masanga, J.; Oduor, R.; Alakonya, A.; Ngugi, M.; Ojola, P.; Bellis, E.S.; Runo, S. Comparative phylogeographic analysis of Cuscuta campestris and Cuscuta reflexa in Kenya: Implications for management of highly invasive vines. Plants People Planet 2021, 1–14. [Google Scholar] [CrossRef]
- Kazemitabar, S.K.; Azoush, S.; Alikelayeh, A.S. Comparison of RAPD and ISSR molecular markers to determine of genetic diversity in weed dodder (Cuscuta Epithymum L.). J. Biodivers. Environ. Sci. 2014, 4, 19–26. [Google Scholar]
- Koch, A.M.; Binder, C.; Sanders, I.R. Does blackwell Publishing, Ltd. the generalist parasitic plant Cuscuta campestris selectively forage in heterogeneous plant communities? New Phytol. 2004, 162, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Kojic, M.; Vrbicanin, S. Parasitic weeds—Main characteristics, taxonomy, biodiversity and distribution. Dodders (Cuscuta L.). Acta Herbol. 2000, 9, 21–28. (in Serbian). [Google Scholar]
- Saric-Krsmanovic, M. Biologija Viline Kosice (Cuscuta campestris Yunck.) i Mogućnosti Njenog Suzbijanja. Ph.D. Thesis, University of Belgrade, Faculty of Agriculture, Belgrade, Serbia, 2013. (In Serbian). [Google Scholar]
- Sarić-Krsmanović, M.; Matković, A.; Radivojević, L.; Gajić-Umiljendić, J.; Šantrić, L.; Đurović-Pejčev, R. Alterations in yield of seed essential oil from Foeniculum vulgare upon infection by Cuscuta campestris. In Proceedings of the Book of Abstracts of the VIII Congress on Plant Protection: Integrated Plant Protection for Sustainable Crop Production and Forestry, Zlatibor, Serbia, 25–29 November 2019; Tanovi’c, B., Dolzhenko, V., Nicot, P., Eds.; Plant Protection Society of Serbia: Zlatibor, Serbia, 2019; p. 162. [Google Scholar]
- Sarić-Krsmanović, M.; Dragumilo, A.; Gajić Umiljendić, J.; Radivojević, L.; Šantrić, L.; Đurović-Pejčev, R. Infestation of field dodder (Cuscuta campestris Yunck.) promotes changes in host dry weight and essential oil production in two aromatic plants, peppermint and chamomile. Plants 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Orr, G.L.; Haidar, M.A.; Orr, D.A. Smallseed dodder (Cuscuta planiflora) phototropism toward far-red when in white light. Weed Sci. 1996, 44, 233–240. [Google Scholar] [CrossRef]
- Haidar, M.A. Characterisation of the interaction between cryptochromes and phytochromes in blue light-induced coiling and prehaustoria development of dodder (Cuscuta campestris) seedlings. Ann. Appl. Biol. 2003, 143, 57–62. [Google Scholar] [CrossRef]
- Haidar, M.; Shabala, S. Ion flux kinetics in blue light-grown field dodder (Cuscuta campestris) seedlings: Correlation between ion and blue light. Weed Biol. Manag. 2015, 15, 159–164. [Google Scholar] [CrossRef]
- Keller, M.; Kollmann, J. Effects of seed prove-nance on germination of herbs for agricultural com-pensation sites. Agric. Ecosyst. Environ. 1999, 72, 87–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).