Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Design, and Treatments
2.2. Soil Sampling and Analyses
2.3. DNA Extraction and Amplicon Sequencing
2.4. Sequence Processing and Data Analysis
3. Results and Discussion
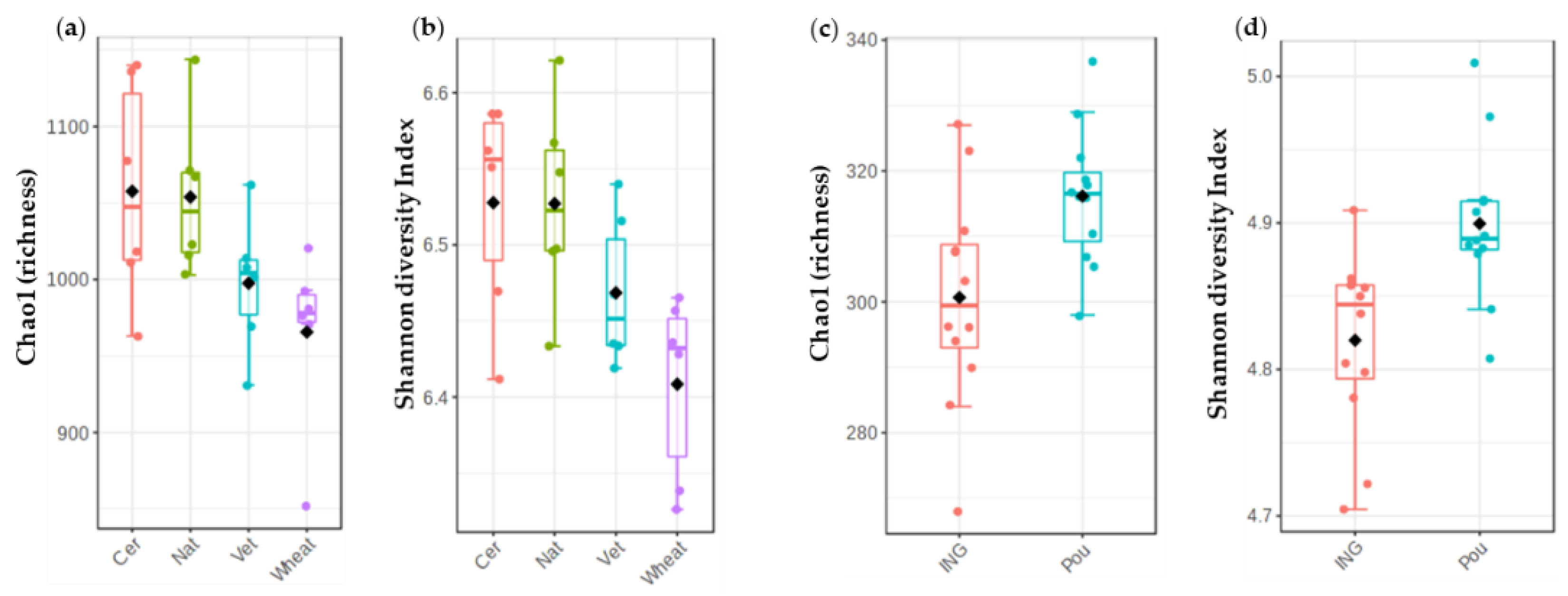
3.1. Alpha Diversity of Microbial Communities
3.1.1. Impact of Cover Crops and Fertilizer Treatments on Bacterial Diversity
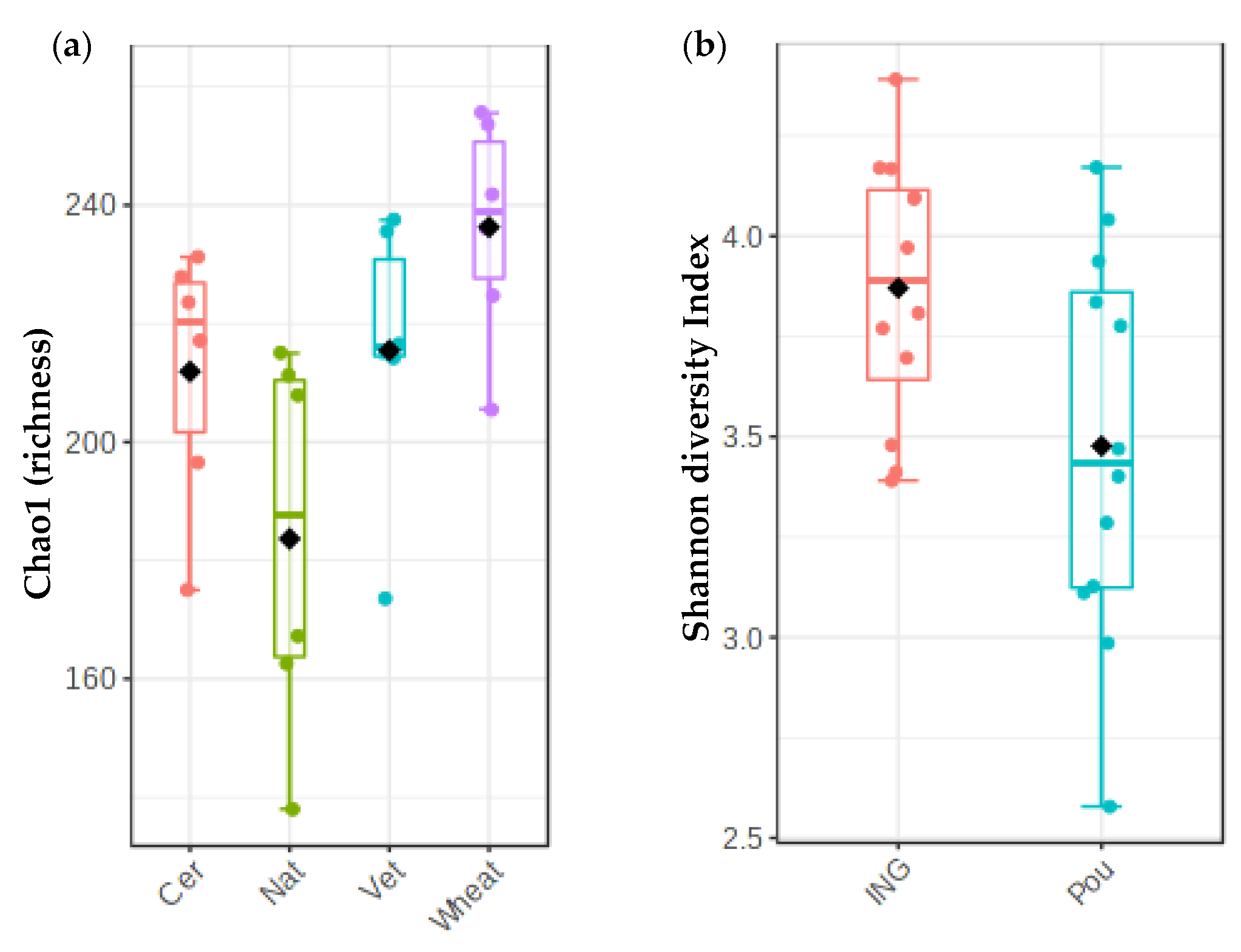
3.1.2. Impact of Cover Crops and Fertilizer Treatments on Fungal Diversity
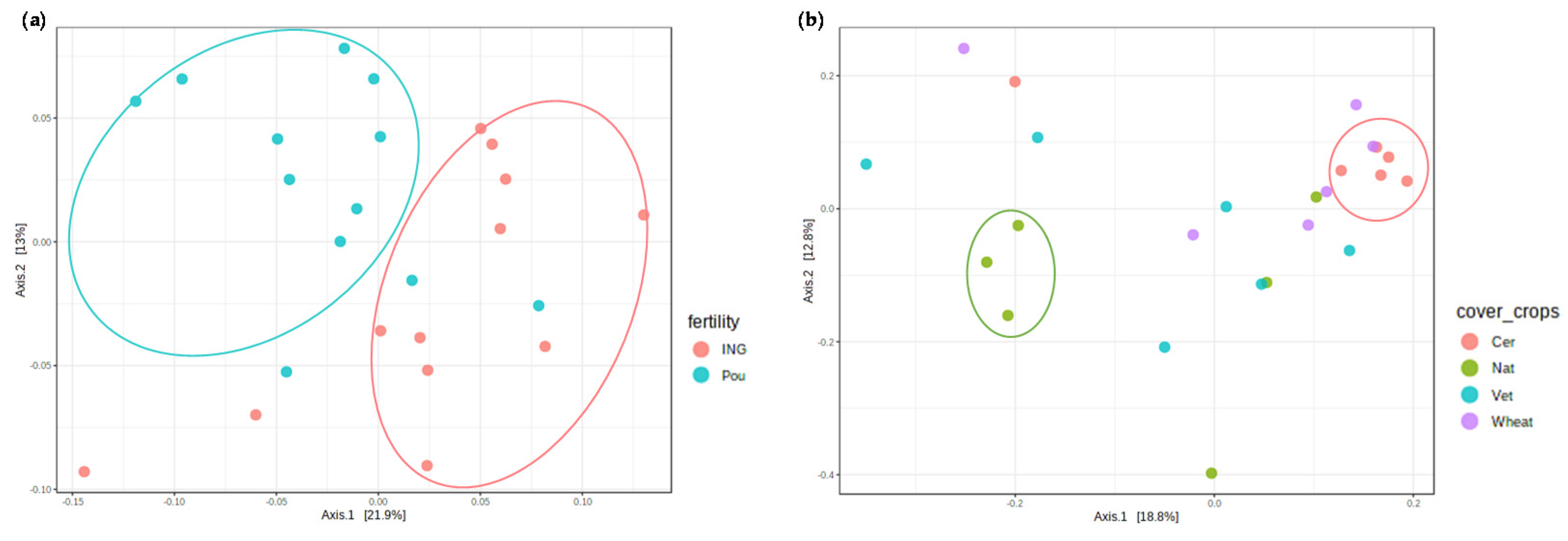
3.2. Beta Diversity of Microbial Communities
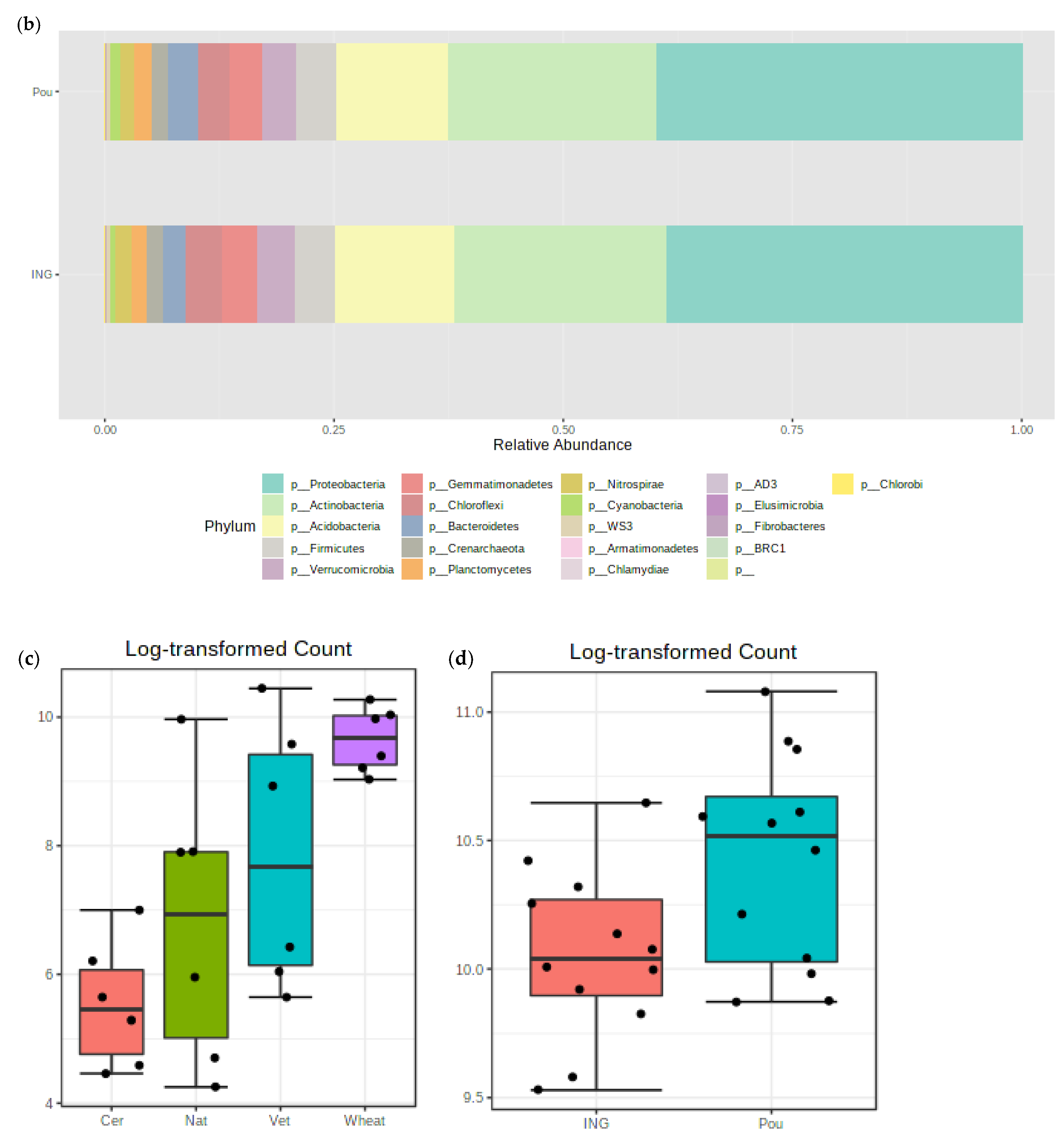
3.3. Impact of Management Practices on the Bacterial Community Abundance
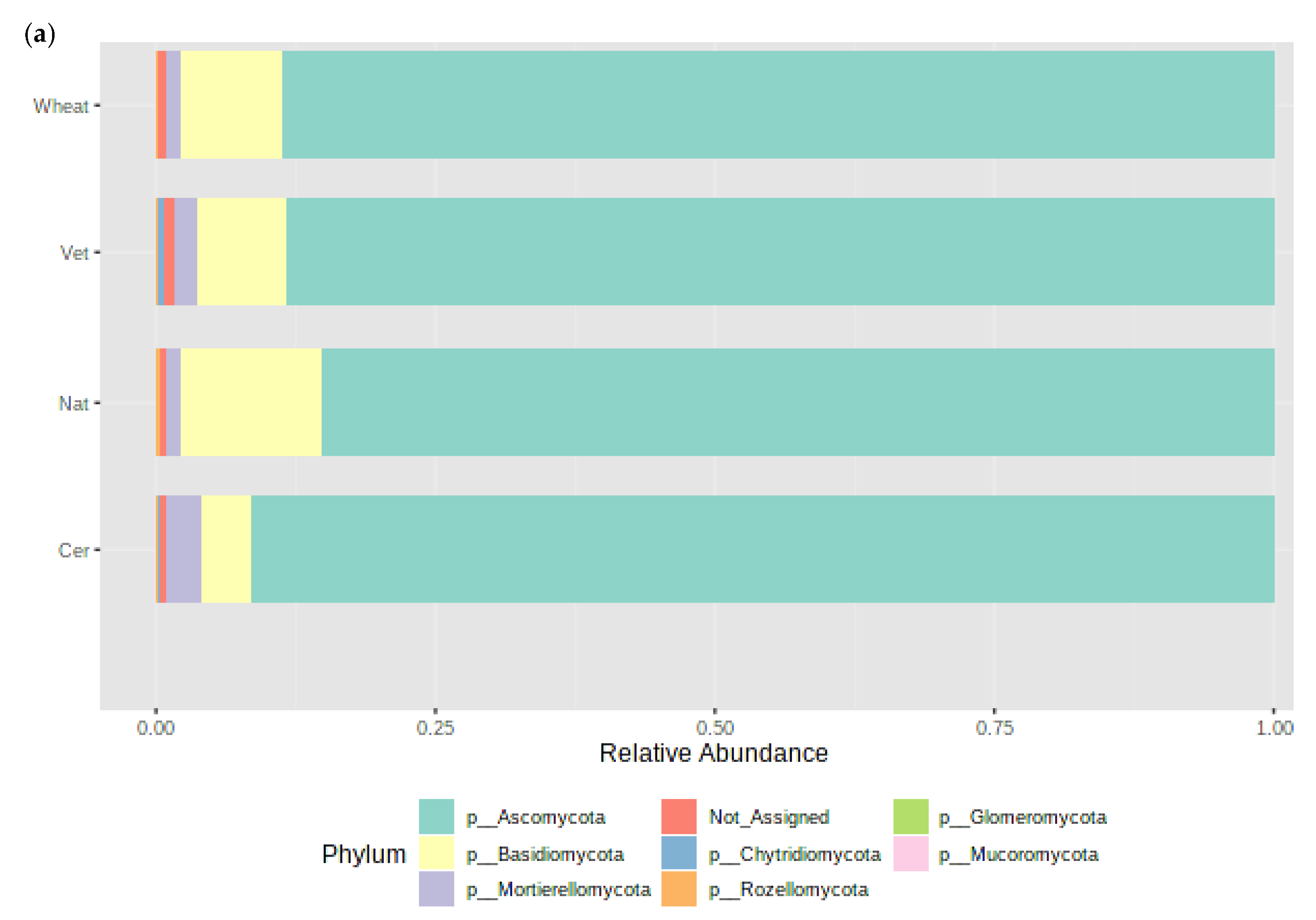
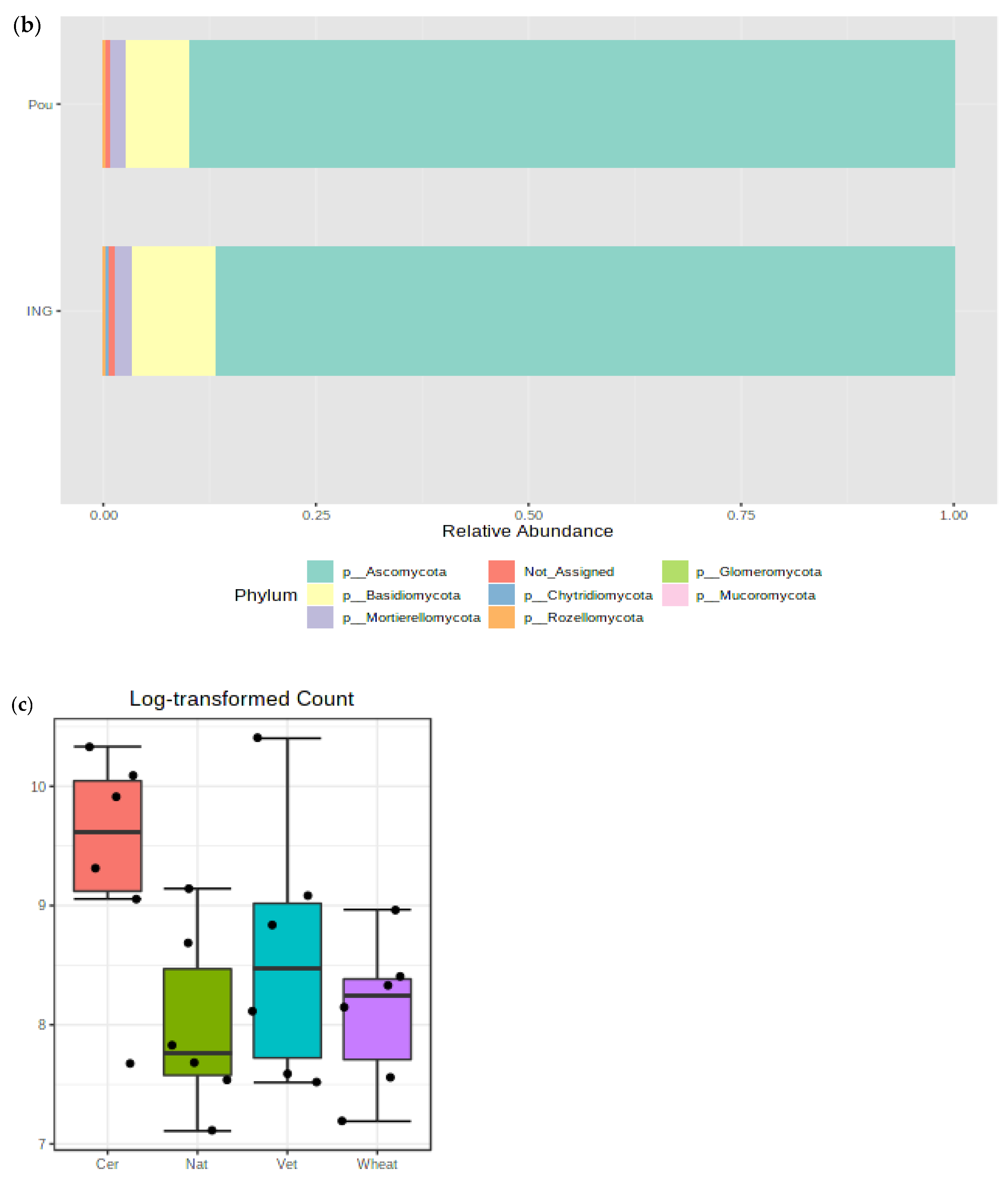
3.4. Impact of Management Practices on the Fungal Community Abundance
3.5. Changes in Soil Physicochemical Properties
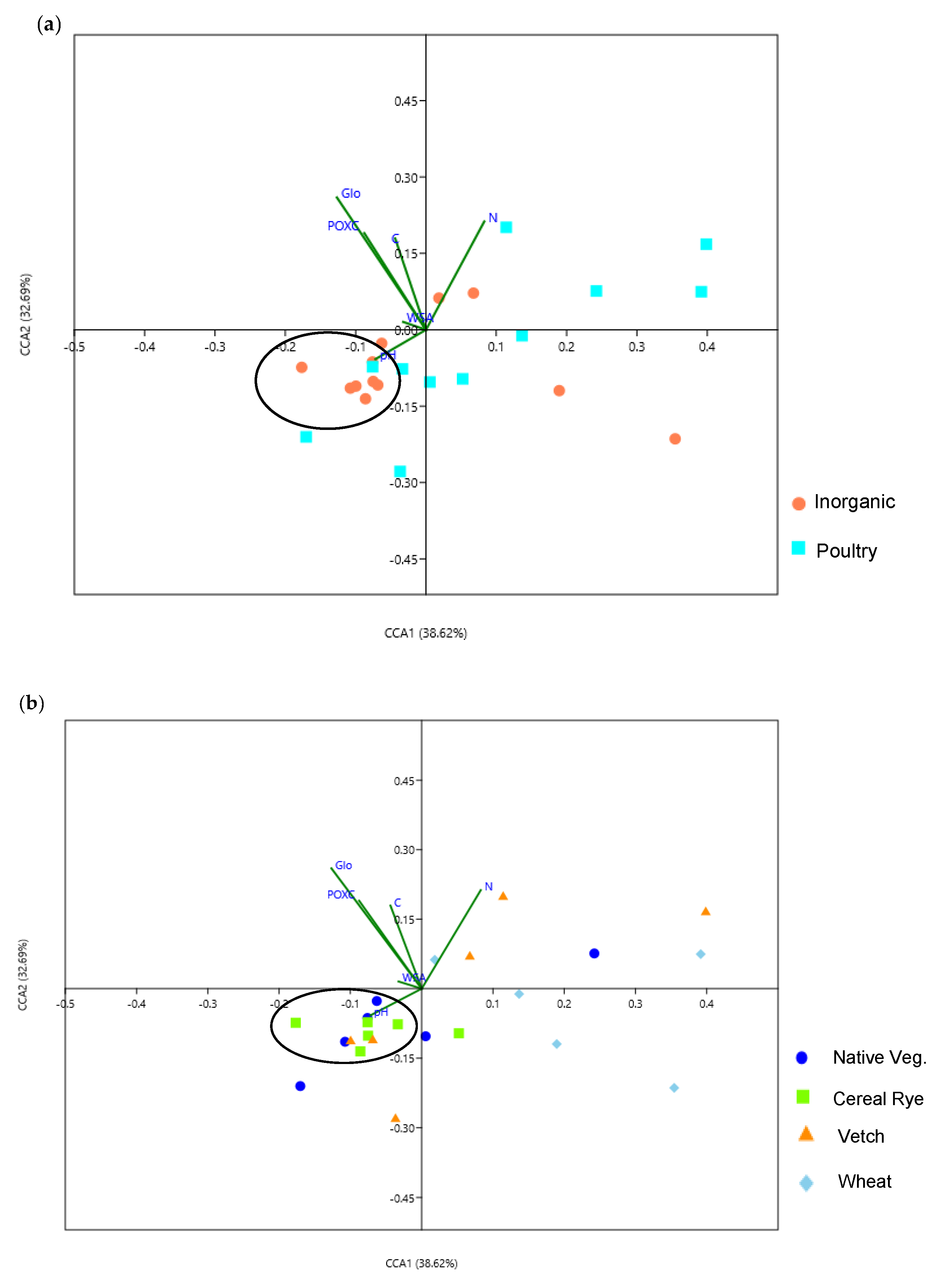
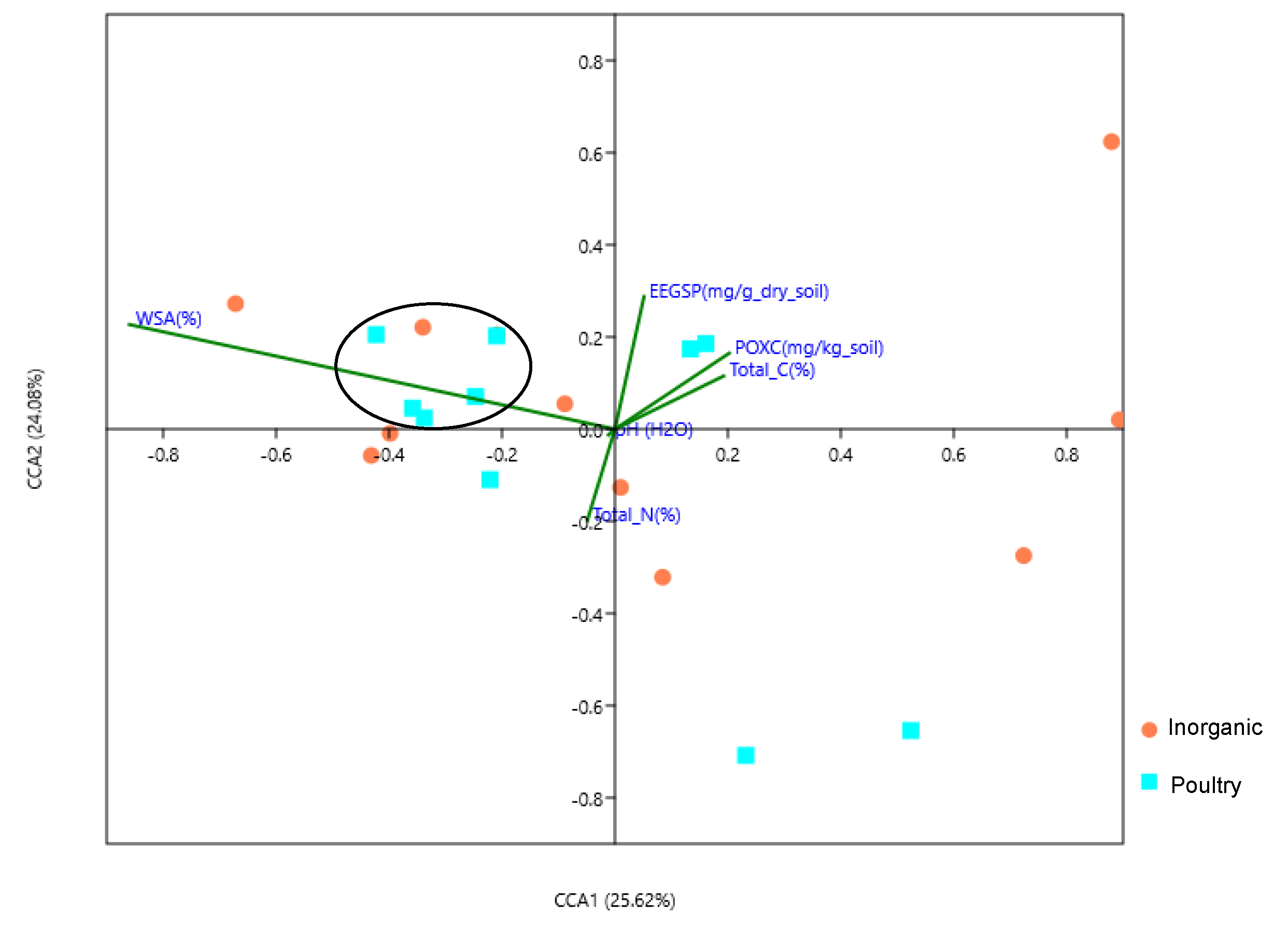
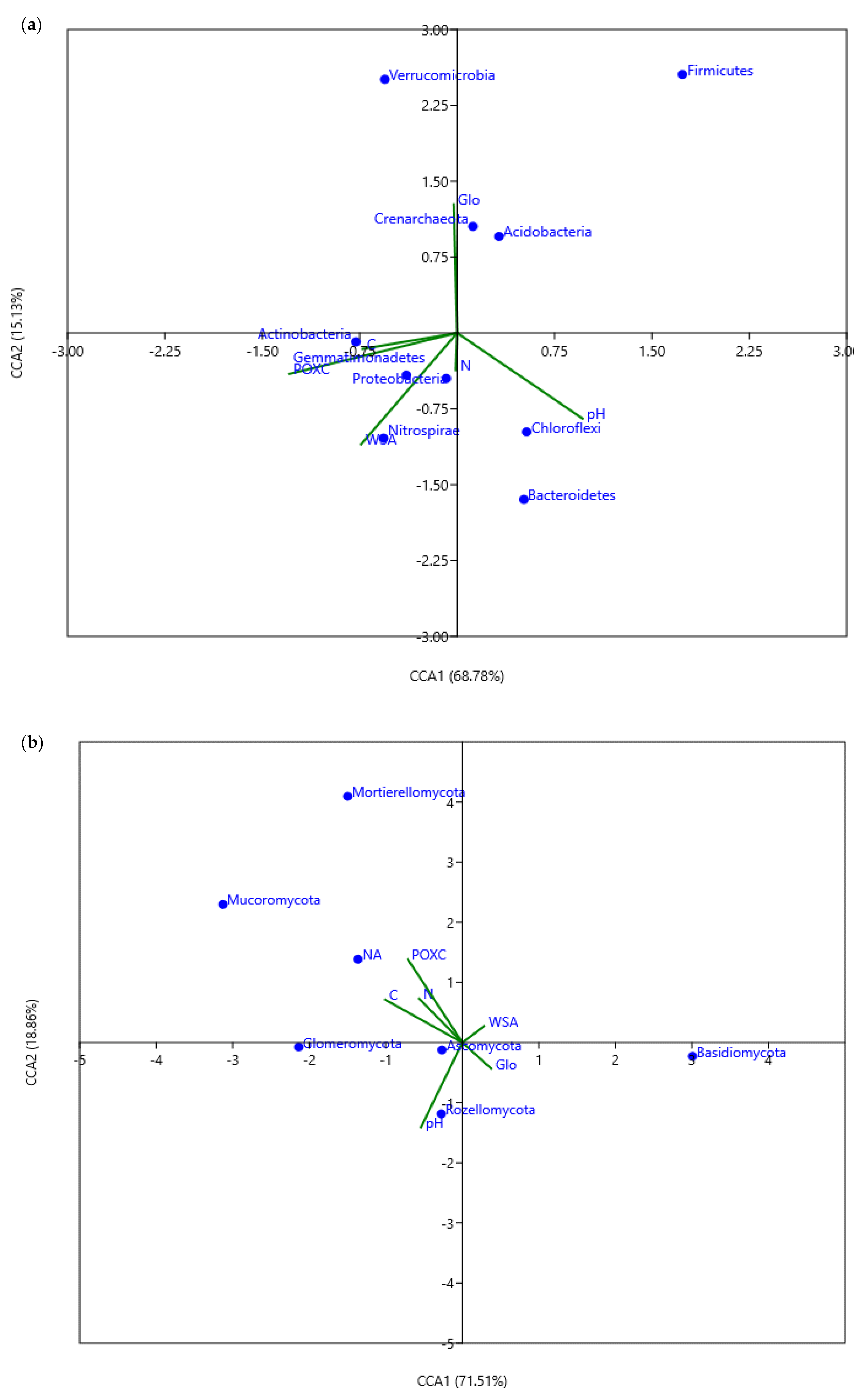
3.6. Association of Soil Physicochemical Properties with the Bacterial and Fungal Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlowski, J. Maximization of Yield and Economic Returns for Non-Irrigated Soybean Production in Mississippi. Available online: https://www.mssoy.org/uploads/files/2016-project-synopses.pdf (accessed on 24 January 2020).
- Pejic, B.; Maksimović, L.; Cimpeanu, S.; Bucur, D.; Milić, S.; Ćupina, B. Response of Soybean to Water Stress at Specific Growth Stages. J. Food Agric. Environ. 2011, 9, 280–284. [Google Scholar]
- Dyer, J.; Mercer, A. Assessment of Spatial Rainfall Variability over the Lower Mississippi River Alluvial Valley. J. Hydrometeorol. 2013, 14, 1826–1843. [Google Scholar] [CrossRef]
- Reba, M.L.; Massey, J.H.; Adviento-Borbe, M.A.; Leslie, D.; Yaeger, M.A.; Anders, M.; Farris, J. Aquifer Depletion in the Lower Mississippi River Basin: Challenges and Solutions. J. Contemp. Water Res. Educ. 2017, 162, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Clark, B. Groundwater Availability of the Mississippi Embayment; US Geological Survey: Reston, VA, USA, 2011. [Google Scholar]
- Hossain, A. Groundwater Depletion in the Mississippi Delta as Observed by the Gravity Recovery and Climate Experiment (GRACE) Satellite System. In Proceedings of the 2014 Mississippi Water Resources Conference, Jackson, MS, USA, 1 April 2014. [Google Scholar]
- Ouyang, Y.; Wan, Y.; Jin, W.; Leininger, T.D.; Feng, G.; Han, Y. Impact of Climate Change on Groundwater Resource in a Region with a Fast Depletion Rate: The Mississippi Embayment. J. Water Clim. Change 2021, 5, 853. [Google Scholar] [CrossRef]
- Lal, R.; Reicosky, D.C.; Hanson, J.D. Evolution of the Plow over 10,000 Years and the Rationale for No-till Farming. Soil Tillage Res. 2007, 93, 1–12. [Google Scholar] [CrossRef]
- Hansen, N.C.; Allen, B.L.; Baumhardt, R.L.; Lyon, D.J. Research Achievements and Adoption of No-till, Dryland Cropping in the Semi-Arid U.S. Great Plains. Field Crops Res. 2012, 132, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Baligar, V.C.; Bailey, B.A. Role of Cover Crops in Improving Soil and Row Crop Productivity. Commun. Soil Sci. Plant Anal. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Acharya, B.S.; Dodla, S.; Gaston, L.A.; Darapuneni, M.; Wang, J.J.; Sepat, S.; Bohara, H. Winter Cover Crops Effect on Soil Moisture and Soybean Growth and Yield under Different Tillage Systems. Soil Tillage Res. 2019, 195, 104430. [Google Scholar] [CrossRef]
- Jacobs, A.A.; Evans, R.S.; Allison, J.K.; Garner, E.R.; Kingery, W.L.; McCulley, R.L. Cover Crops and No-Tillage Reduce Crop Production Costs and Soil Loss, Compensating for Lack of Short-Term Soil Quality Improvement in a Maize and Soybean Production System. Soil Tillage Res. 2022, 218, 105310. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Linquist, B.A.; Lundy, M.E.; Liang, X.; van Groenigen, K.J.; Lee, J.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. When Does No-till Yield More? A Global Meta-Analysis. Field Crops Res. 2015, 183, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Toliver, D.K.; Larson, J.A.; Roberts, R.K.; English, B.C.; De La Torre Ugarte, D.G.; West, T.O. Effects of No-Till on Yields as Influenced by Crop and Environmental Factors. Agron. J. 2012, 104, 530–541. [Google Scholar] [CrossRef] [Green Version]
- DeFelice, M.S.; Carter, P.R.; Mitchell, S.B. Influence of Tillage on Corn and Soybean Yield in the United States and Canada. Crop Manag. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover Crops and Ecosystem Services: Insights from Studies in Temperate Soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.P.; Shrestha, A.; Mathesius, K.; Scow, K.M.; Southard, R.J.; Haney, R.L.; Schmidt, R.; Munk, D.S.; Horwath, W.R. Cover Cropping and No-Tillage Improve Soil Health in an Arid Irrigated Cropping System in California’s San Joaquin Valley, USA. Soil Tillage Res. 2017, 165, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Bowman, M. Large-Scale Farmer-Led Experiment Demonstrates Positive Impact of Cover Crops on Multiple Soil Health Indicators. Nat. Food. 2021, 2, 97–103. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The Role of Conservation Agriculture in Sustainable Agriculture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.G.; Buehring, N.W.; Prevost, J.D.; Kingery, W.L. Soil Bacterial Community Diversity and Composition as Affected by Tillage Intensity Treatments in Corn-Soybean Production Systems. Microbiol. Res. Int. 2021, 12, 12. [Google Scholar] [CrossRef]
- Ghimire, R.; Ghimire, B.; Mesbah, A.O.; Idowu, O.J.; O’Neill, M.K.; Angadi, S.V.; Shukla, M.K. Current Status, Opportunities, and Challenges of Cover Cropping for Sustainable Dryland Farming in the Southern Great Plains. J. Crop. Improv. 2018, 32, 579–598. [Google Scholar] [CrossRef]
- Kuo, S.; Sainju, U.M.; Jellum, E.J. Winter Cover Crop Effects on Soil Organic Carbon and Carbohydrate in Soil. Soil Sci. Soc. Am. J. 1997, 61, 145–152. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Yu, Q.; Zhang, Y.; Wang, R.; Li, J.; Wang, X. No Tillage Increases Soil Organic Carbon Storage and Decreases Carbon Dioxide Emission in the Crop Residue-Returned Farming System. J. Environ. Manag. 2020, 261, 110261. [Google Scholar] [CrossRef]
- White, P.M.; Rice, C.W. Tillage Effects on Microbial and Carbon Dynamics during Plant Residue Decomposition. Soil Sci. Soc. Am. J. 2009, 73, 138–145. [Google Scholar] [CrossRef]
- Finney, D.M.; Buyer, J.S.; Kaye, J.P. Living Cover Crops Have Immediate Impacts on Soil Microbial Community Structure and Function. J. Soils Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Kingery, W.L.; Wood, C.W.; Williams, J.C. Tillage and Amendment Effects on Soil Carbon and Nitrogen Mineralization and Phosphorus Release. Soil Tillage Res. 1996, 37, 239–250. [Google Scholar] [CrossRef]
- Adeli, A.; Brooks, J.P.; Read, J.J.; Shankle, M.W.; Feng, G.; Jenkins, J.N. Poultry Litter and Cover Crop Integration into No-till Cotton on Upland Soil. Agronomy 2019, 111, 2097–2107. [Google Scholar] [CrossRef]
- Pokhrel, S.; Kingery, W.L.; Cox, M.S.; Shankle, M.W.; Shanmugam, S.G. Impact of Cover Crops and Poultry Litter on Selected Soil Properties and Yield in Dryland Soybean Production. Agronomy 2021, 11, 119. [Google Scholar] [CrossRef]
- Adeli, A.; Sistani, K.R.; Rowe, D.E.; Tewolde, H. Effects of Broiler Litter on Soybean Production and Soil Nitrogen and Phosphorus Concentrations. Agronomy 2005, 97, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for Evaluating Soil Quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of Enzymes in Maintaining Soil Health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–42. ISBN 978-3-642-14225-3. [Google Scholar]
- Collins, H.P.; Rasmussen, P.E.; Douglas, C.L., Jr. Crop Rotation and Residue Management Effects on Soil Carbon and Microbial Dynamics. Soil Sci. Soc. Am. J. 1992, 56, 783–788. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do Cover Crops Benefit Soil Microbiome? A Meta-Analysis of Current Research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Ashworth, A.J.; DeBruyn, J.M.; Allen, F.L.; Radosevich, M.; Owens, P.R. Microbial Community Structure Is Affected by Cropping Sequences and Poultry Litter under Long-Term No-Tillage. Soil Biol. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Ashworth, A.; Allen, F.; DeBruyn, J.; Owens, P.; Sams, C. Crop Rotations and Poultry Litter Affect Dynamic Soil Chemical Properties and Soil Biota Long Term. J. Environ. Qual. 2018, 47, 1327–1338. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.P.; Tewolde, H.; Adeli, A.; Shankle, M.W.; Way, T.R.; Smith, R.K.; Pepper, I.L. Effects of Subsurface Banding and Broadcast of Poultry Litter and Cover Crop on Soil Microbial Populations. J. Environ. Qual. 2018, 47, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil Microbiome: A Key Player for Conservation of Soil Health under Changing Climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified Method for Soil Particle-Size Determination to Accompany Soil-Quality Analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 961–1010. ISBN 978-0-89118-866-7. [Google Scholar]
- Thomas, G.W. Soil PH and Soil Acidity. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Madison, WI, USA, 1996; pp. 475–490. ISBN 978-0-89118-866-7. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Madison, WI, USA, 1986; pp. 425–442. ISBN 978-0-89118-864-3. [Google Scholar]
- Reyna, D.L.; Wall, L.G. Revision of Two Colorimetric Methods to Quantify Glomalin-Related Compounds in Soils Subjected to Different Managements. Biol. Fertil. Soils 2014, 50, 395–400. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate Oxidizable Carbon Reflects a Processed Soil Fraction That Is Sensitive to Management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.; Lauber, C.; Walters, W.; Berg-Lyons, D.; Lozupone, C.; Turnbaugh, P.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Orgiazzi, A.; Lumini, E.; Nilsson, R.H.; Girlanda, M.; Vizzini, A.; Bonfante, P.; Bianciotto, V. Unravelling Soil Fungal Communities from Different Mediterranean Land-Use Backgrounds. PLoS ONE 2012, 7, e34847. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From Raw Reads to Community Analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A Web-Based Tool for Comprehensive Statistical, Visual and Meta-Analysis of Microbiome Data. Nucleic. Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for Comprehensive Statistical, Functional, and Meta-Analysis of Microbiome Data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.-Y.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and Resource Factors Influencing High Microbial Diversity in Soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Xia, J.; Yu, X.; Ma, K.; Wan, S. Soil Microbial Community Changes and Their Linkages with Ecosystem Carbon Exchange under Asymmetrically Diurnal Warming. Soil Biol. Biochem. 2011, 43, 2053–2059. [Google Scholar] [CrossRef]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global Biogeography and Quantitative Seasonal Dynamics of Gemmatimonadetes in Soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef] [Green Version]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.; Fierer, N. Temporal Variability in Soil Microbial Communities across Land-Use Types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef]
- Buckley, D.H.; Schmidt, T.M. Diversity and Dynamics of Microbial Communities in Soils from Agro-Ecosystems. Environ. Microbiol. 2003, 5, 441–452. [Google Scholar] [CrossRef]
- Cloutier, M.; Murrell, E.; Barbercheck, M.; Kaye, J.; Finney, D.; García González, I.; Bruns, M. Fungal Community Shifts in Soils with Varied Cover Crop Treatments and Edaphic Properties. Sci. Rep. 2020, 10, 6198. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Powell, J.R.; Hamonts, K.; Reith, F.; Mele, P.; Brown, M.V.; Dennis, P.G.; Ferrari, B.C.; Fitzgerald, A.; Young, A.; et al. Circular Linkages between Soil Biodiversity, Fertility and Plant Productivity Are Limited to Topsoil at the Continental Scale. New Phytol. 2017, 215, 1186–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celestina, C.; Wood, J.L.; Manson, J.B.; Wang, X.; Sale, P.W.G.; Tang, C.; Franks, A.E. Microbial Communities in Top- and Subsoil of Repacked Soil Columns Respond Differently to Amendments but Their Diversity Is Negatively Correlated with Plant Productivity. Sci. Rep. 2019, 9, 8890. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, V.; Smalla, K.; Nesme, J.; Garaycochea, S.; Fresia, P.; Sørensen, S.J.; Babin, D.; Leoni, C. Reduced Tillage, Cover Crops and Organic Amendments Affect Soil Microbiota and Improve Soil Health in Uruguayan Vegetable Farming Systems. FEMS Microbiol. Ecol. 2021, 97, fiab023. [Google Scholar] [CrossRef]
- Longley, R.; Noel, Z.A.; Benucci, G.M.N.; Chilvers, M.I.; Trail, F.; Bonito, G. Crop Management Impacts the Soybean (Glycine Max) Microbiome. Front. Microbiol. 2020, 11, 1116. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]
- Lori, M.; Symnaczik, S.; Mäder, P.; Deyn, G.D.; Gattinger, A. Organic Farming Enhances Soil Microbial Abundance and Activity—A Meta-Analysis and Meta-Regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [PubMed]
- Wolters, B.; Jacquiod, S.; Sørensen, S.J.; Widyasari-Mehta, A.; Bech, T.B.; Kreuzig, R.; Smalla, K. Bulk Soil and Maize Rhizosphere Resistance Genes, Mobile Genetic Elements and Microbial Communities Are Differently Impacted by Organic and Inorganic Fertilization. FEMS Microbiol. Ecol. 2018, 94, fiy027. [Google Scholar] [CrossRef] [PubMed]
- Elshahed, M.S.; Youssef, N.H.; Spain, A.M.; Sheik, C.; Najar, F.Z.; Sukharnikov, L.O.; Roe, B.A.; Davis, J.P.; Schloss, P.D.; Bailey, V.L.; et al. Novelty and Uniqueness Patterns of Rare Members of the Soil Biosphere. Appl. Environ. Microbiol. 2008, 74, 5422–5428. [Google Scholar] [CrossRef] [Green Version]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, Composition, Diversity and Novelty of Soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, S.-H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and Composition of Soil Acidobacteria and Proteobacteria Communities as a Bacterial Indicator of Past Land-Use Change from Forest to Farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.-W. Effects of Actinobacteria on Plant Disease Suppression and Growth Promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- Franco-Correa, M.; Chavarro-Anzola, V. Actinobacteria as Plant Growth-Promoting Rhizobacteria; IntechOpen: London, UK, 2016; ISBN 978-953-51-2248-7. [Google Scholar]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas Mediated Nutritional and Growth Promotional Activities for Sustainable Food Security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef] [PubMed]
- Combes-Meynet, E.; Pothier, J.F.; Moënne-Loccoz, Y.; Prigent-Combaret, C. The Pseudomonas Secondary Metabolite 2,4-Diacetylphloroglucinol Is a Signal Inducing Rhizoplane Expression of Azospirillum Genes Involved in Plant-Growth Promotion. Mol. Plant Microb. Interact. 2011, 24, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Tang, G.; Niu, J.; Yang, J.; Zhou, Z.; Gao, Y.; Chen, X.; Tian, Y.; Li, Y.; Li, J.; et al. Preparation of a Porphyrin Metal–Organic Framework with Desirable Photodynamic Antimicrobial Activity for Sustainable Plant Disease Management. J. Agric. Food Chem. 2021, 69, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Gkarmiri, K.; Mahmood, S.; Ekblad, A.; Alström, S.; Högberg, N.; Finlay, R. Identifying the Active Microbiome Associated with Roots and Rhizosphere Soil of Oilseed Rape. Appl. Environ. Microbiol. 2017, 83, e01938-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Zhong, R.; Jiang, J.; He, L.; Huang, Z.; Shi, G.; Wu, H.; Liu, J.; Xiong, F.; Han, Z.; et al. Cassava/Peanut Intercropping Improves Soil Quality via Rhizospheric Microbes Increased Available Nitrogen Contents. BMC Biotechnol. 2020, 20, 13. [Google Scholar] [CrossRef] [Green Version]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing Aridity Reduces Soil Microbial Diversity and Abundance in Global Drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- Swann, E. Basidiomycota—New World Encyclopedia. Available online: https://www.newworldencyclopedia.org/entry/Basidiomycota (accessed on 20 January 2022).
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Korthals, G.; de Boer, W. The Hidden Potential of Saprotrophic Fungi in Arable Soil: Patterns of Short-Term Stimulation by Organic Amendments. Appl. Soil Ecol. 2020, 147, 103434. [Google Scholar] [CrossRef]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of Indoleacetic Acid, Gibberellic Acid and ACC-Deaminase by Mortierella Strains Promote Winter Wheat Seedlings Growth under Different Conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.M.; Ludwig, A.; Furch, A.C.U.; Mithöfer, A.; Scholz, S.; Reichelt, M.; Oelmüller, R. The Beneficial Root-Colonizing Fungus Mortierella Hyalina Promotes the Aerial Growth of Arabidopsis and Activates Calcium-Dependent Responses That Restrict Alternaria Brassicae-Induced Disease Development in Roots. Mol. Plant Microbe Interact. 2019, 32, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Bonito, G.; Hsu, C.-M.; Hameed, K.; Vilgalys, R.; Liao, H.-L. Mortierella Elongata Increases Plant Biomass among Non-Leguminous Crop Species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA; Hoboken, NJ, USA, 2008; ISBN 978-0-470-27646-4. [Google Scholar]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome Composition and Diversity under the Long-Term Application of Spent Mushroom Substrate and Chicken Manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Uzun, I. Use of Spent Mushroom Compost in Sustainable Fruit Production. J. Fruit Ornam. Plant Res. 2004, 12, 157–165. [Google Scholar]
- Jonathan, S.G.; Lawal, M.M.; Oyetunji, O.J. Effect of Spent Mushroom Compost of Pleurotus Pulmonarius on Growth Performance of Four Nigerian Vegetables. Mycobiology 2011, 39, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadiri, M.; Mustapha, Y. The Use of Spent Mushroom Substrate of L. Subnudus Berk as a Soil Condition for Vegetables. Bayero J. Pure Appl. Sci. 2011, 3, 63212. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Y.; Lin, X.; Zhang, J.; Zeng, J. Dissipation of Polycyclic Aromatic Hydrocarbons (PAHs) in Soil Microcosms Amended with Mushroom Cultivation Substrate. Soil Biol. Biochem. 2012, 47, 191–197. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, L.; Guo, L.; Man, J.; Shang, B.; Pu, R.; Ou, X.; Dai, C.; Liu, P.; Yang, Y.; et al. K Fertilizers Reduce the Accumulation of Cd in Panax Notoginseng (Burk.) F.H. by Improving the Quality of the Microbial Community. Front. Plant Sci. 2020, 11, 888. [Google Scholar] [CrossRef]
- Liu, E.; Ghirmai, S.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; Liu, Q. Long-Term Effects of No-Tillage Management Practice on Soil Organic Carbon and Its Fractions in the Northern China. Geoderma 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Cai, L.; Guo, Z.; Zhang, J.; Gai, Z.; Liu, J.; Meng, Q.; Liu, X. No Tillage and Residue Mulching Method on Bacterial Community Diversity Regulation in a Black Soil Region of Northeastern China. PLoS ONE 2021, 16, e0256970. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. A Comprehensive Analysis of the Response of the Fungal Community Structure to Long-Term Continuous Cropping in Three Typical Upland Crops. J. Integr. Agric. 2020, 19, 866–880. [Google Scholar] [CrossRef]
- Pierre, W.H. Nitrogenous Fertilizers and Soil Acidity: I. Effect of Various Nitrogenous Fertilizers on Soil Reaction1. Agronomy 1928, 20, 254–269. [Google Scholar] [CrossRef] [Green Version]
- Malhi, S.S.; Harapiak, J.T.; Nyborg, M.; Gill, K.S. Effects of Long-term Applications of Various Nitrogen Sources on Chemical Soil Properties and Composition of Bromegrass Hay. J. Plant Nutr. 2000, 23, 903–912. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Anderson, T.-H. Interactive Effects of PH and Substrate Quality on the Fungal-to-Bacterial Ratio and QCO2 of Microbial Communities in Forest Soils. Soil Biol. Biochem. 1998, 30, 1269–1274. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.-H. Comparison of Soil Fungal/Bacterial Ratios in a PH Gradient Using Physiological and PLFA-Based Techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and Anthropogenic Controls over Bacterial Communities in Wetland Soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef] [Green Version]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil PH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Kowalchuk, G.A.; Stephen, J.R.; De Boer, W.; Prosser, J.I.; Embley, T.M.; Woldendorp, J.W. Analysis of Ammonia-Oxidizing Bacteria of the Beta Subdivision of the Class Proteobacteria in Coastal Sand Dunes by Denaturing Gradient Gel Electrophoresis and Sequencing of PCR-Amplified 16S Ribosomal DNA Fragments. Appl. Environ. Microbiol. 1997, 63, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Jackson, R.B. The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The Influence of Soil Properties on the Structure of Bacterial and Fungal Communities across Land-Use Types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Wright, S.F.; Franke-Snyder, M.; Morton, J.B.; Upadhyaya, A. Time-Course Study and Partial Characterization of a Protein on Hyphae of Arbuscular Mycorrhizal Fungi during Active Colonization of Roots. Plant Soil 1996, 181, 193–203. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large Contribution of Arbuscular Mycorrhizal Fungi to Soil Carbon Pools in Tropical Forest Soils. Plant Soil 2001, 233, 167–177. [Google Scholar] [CrossRef]
- DuPont, S.T.; Culman, S.W.; Ferris, H.; Buckley, D.H.; Glover, J.D. No-Tillage Conversion of Harvested Perennial Grassland to Annual Cropland Reduces Root Biomass, Decreases Active Carbon Stocks, and Impacts Soil Biota. Agric. Ecosyst. Environ. 2010, 137, 25–32. [Google Scholar] [CrossRef]
- Padre, A.; Ladha, J. Assessing the Reliability of Permanganate-Oxidizable Carbon as an Index of Soil Labile Carbon. Soil Sci. Soc. Am. J. 2004, 68, 969–978. [Google Scholar] [CrossRef]
- Mohanty, M.; Sinha, N.; Reddy, K.; Chaudhary, R.; Rao, A.; Dalal, R.; Menzies, N. How Important Is the Quality of Organic Amendments in Relation to Mineral N Availability in Soils? Agric. Res. 2013, 2, 99–110. [Google Scholar] [CrossRef]

| Fertilizer Source | Cover Crops | pH | Total C (%) | Total N (%) | WSA% | EE-GRSP | POXC | Clay | Sand | Silt |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg g−1) | (mg kg−1) | (%) | (%) | (%) | ||||||
| Poultry Litter | Wheat | 5.8 ± 0.1 | 1.6 ± 0.3 | 0.2 ± 0 | 52.0 ± 7.2 | 86.5 ± 11.4 | 509.7 ± 126.7 | 14.2 ± 0.6 | 17.9 ± 1.9 | 67.9 ± 1.4 |
| Vetch | 5.6 ± 0.5 | 1.9 ± 0.3 | 0.2 ± 0 | 56.0 ± 7 | 88.8 ± 15.1 | 579.4 ± 117.2 | 14.9 ± 1.7 | 17.4 ± 1 | 67.7 ± 1.5 | |
| Cereal Rye | 5.7 ± 0.2 | 1.8 ± 0.3 | 0.2 ± 0 | 54.4 ± 3.7 | 87.7 ± 4.7 | 543.6 ± 60.7 | 13.5 ± 1.5 | 17.8 ± 1.4 | 68.6 ± 1.8 | |
| Native veg. | 5.8 ± 0.1 | 1.6 ± 0.2 | 0.2 ± 0 | 60.4 ± 3.8 | 86.1 ± 1.9 | 527.1 ± 6.3 | 14.2 ± 0.6 | 16.9 ± 3.7 | 68.9 ± 4.3 | |
| Inorganic Fertilizer | Wheat | 5.8 ± 0.2 | 1.5 ± 0.2 | 0.1 ± 0 | 47.1 ± 5.4 | 73.5 ± 15.6 | 509.7 ± 72.2 | 13.9 ± 1.0 | 17.6 ± 0.9 | 68.5 ± 0.9 |
| Vetch | 5.4 ± 0.2 | 1.7 ± 0.3 | 0.2 ± 0 | 57.1 ± 7.7 | 80.1 ± 8.3 | 573.2 ± 112.5 | 15.2 ± 1.2 | 17.8 ± 1.6 | 67.0 ± 1.6 | |
| Cereal Rye | 5.8 ± 0.2 | 1.7 ± 0.2 | 0.2 ± 0 | 48.0 ± 12.7 | 78.3 ± 7.9 | 563.5 ± 80.3 | 14.5 ± 0.6 | 15.9 ± 0.5 | 69.5 ± 0.9 | |
| Native veg. | 5.6 ± 0.1 | 1.5 ± 0.4 | 0.2 ± 0 | 50.1 ± 11.2 | 90.6 ± 8.5 | 502.8 ± 103.4 | 13.5 ± 1.5 | 16.5 ± 2.4 | 70.0 ± 3.9 |
| Mantle Test | Bacterial Communities | |||||
|---|---|---|---|---|---|---|
| pH | C | N | WSA | EE-GRSP | POXC | |
| R | 0.654 | −0.120 | −0.158 | −0.01 | 0.362 | −0.067 |
| p-Value | <0.001 | 0.287 | 0.178 | 0.931 | <0.001 | 0.609 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodadinne Narayana, N.; Kingery, W.L.; Shankle, M.W.; Ganapathi Shanmugam, S. Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System. Agronomy 2022, 12, 618. https://doi.org/10.3390/agronomy12030618
Kodadinne Narayana N, Kingery WL, Shankle MW, Ganapathi Shanmugam S. Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System. Agronomy. 2022; 12(3):618. https://doi.org/10.3390/agronomy12030618
Chicago/Turabian StyleKodadinne Narayana, Nisarga, William L. Kingery, Mark W. Shankle, and Shankar Ganapathi Shanmugam. 2022. "Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System" Agronomy 12, no. 3: 618. https://doi.org/10.3390/agronomy12030618
APA StyleKodadinne Narayana, N., Kingery, W. L., Shankle, M. W., & Ganapathi Shanmugam, S. (2022). Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System. Agronomy, 12(3), 618. https://doi.org/10.3390/agronomy12030618







