Phenotypic Divergence among Sweet Pepper Landraces Assessed by Agro-Morphological Characterization as a Biodiversity Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Greenhouse Conditions
2.3. Phenotyping Study
2.4. Agronomic Trait
2.5. Statistical Analysis
3. Results
3.1. Phenotypic Differences in Quantitative Vegetative Traits
3.2. Phenotypic Differences in Qualitative Vegetative Traits
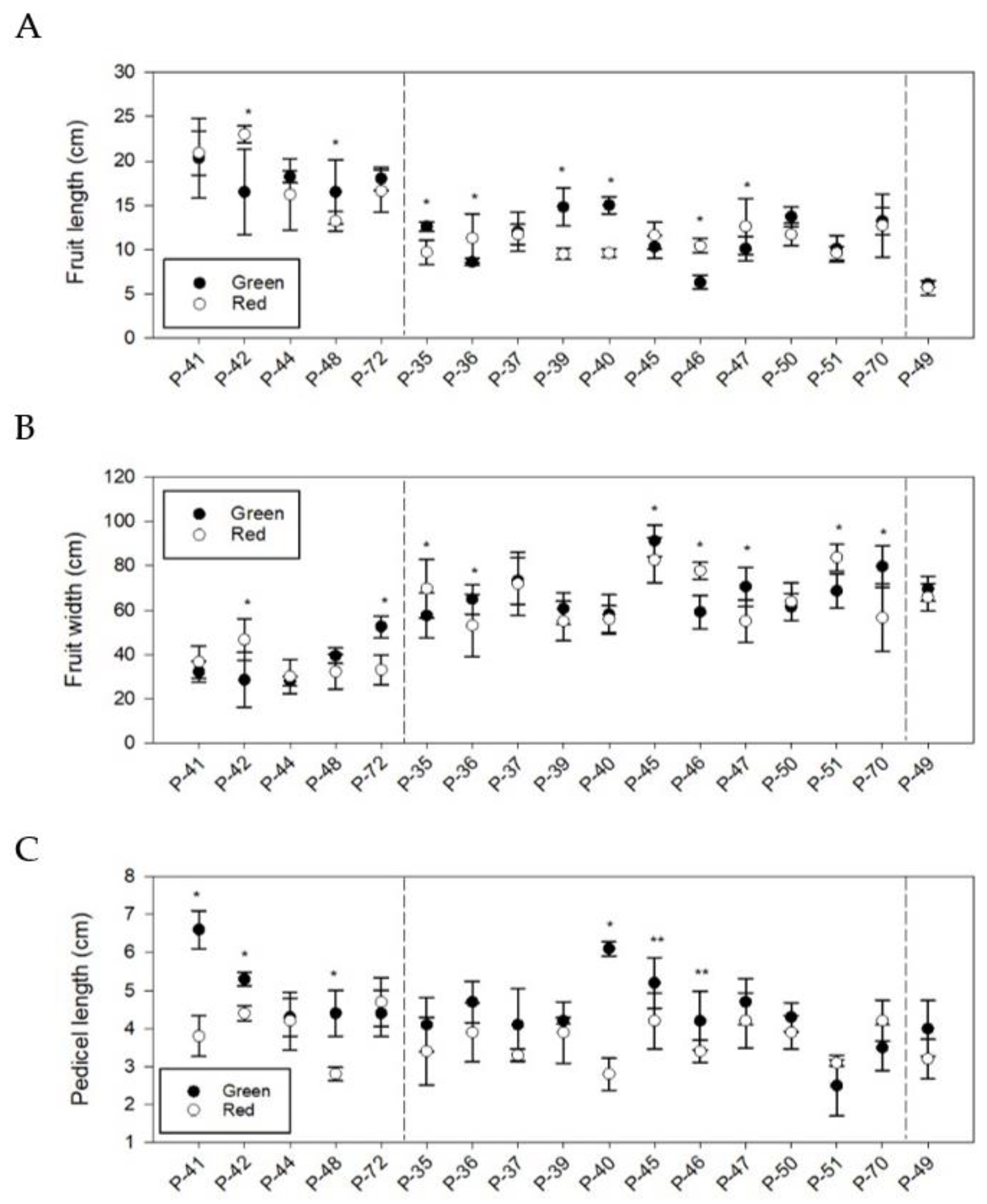
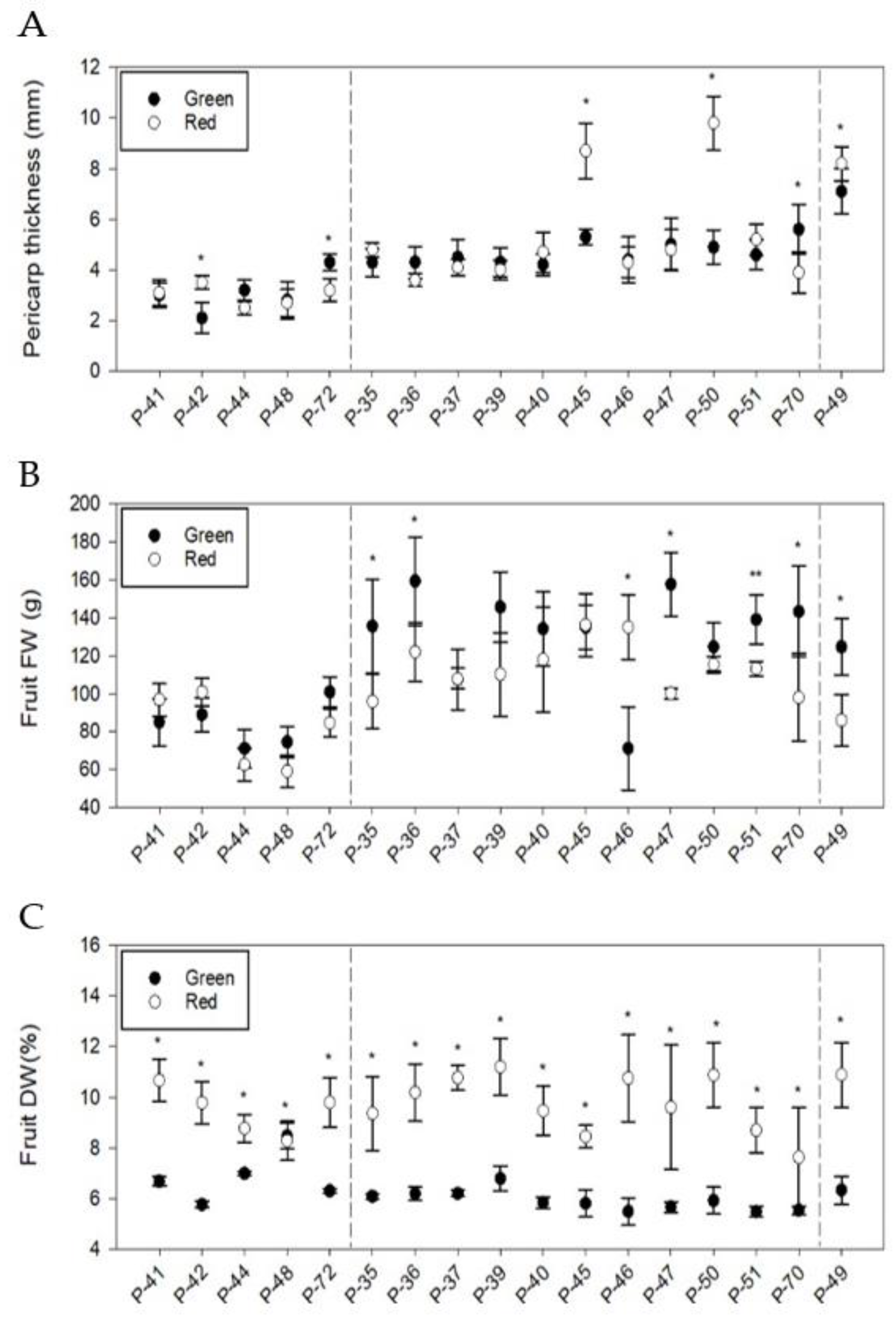
3.3. Phenotypic Differences in Quantitative Fruit Traits
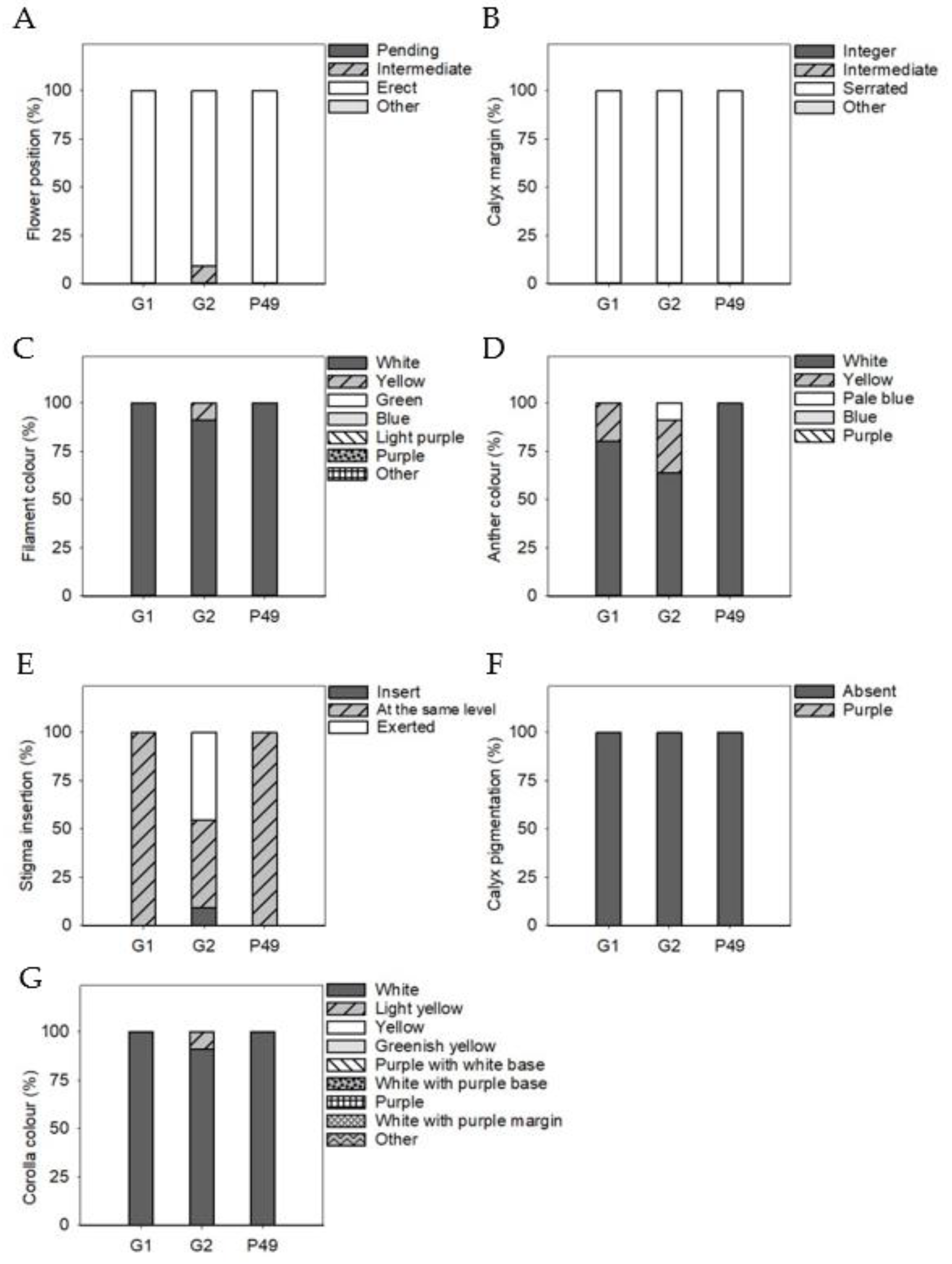
3.4. Phenotypic Differences in Qualitative Fruit Traits
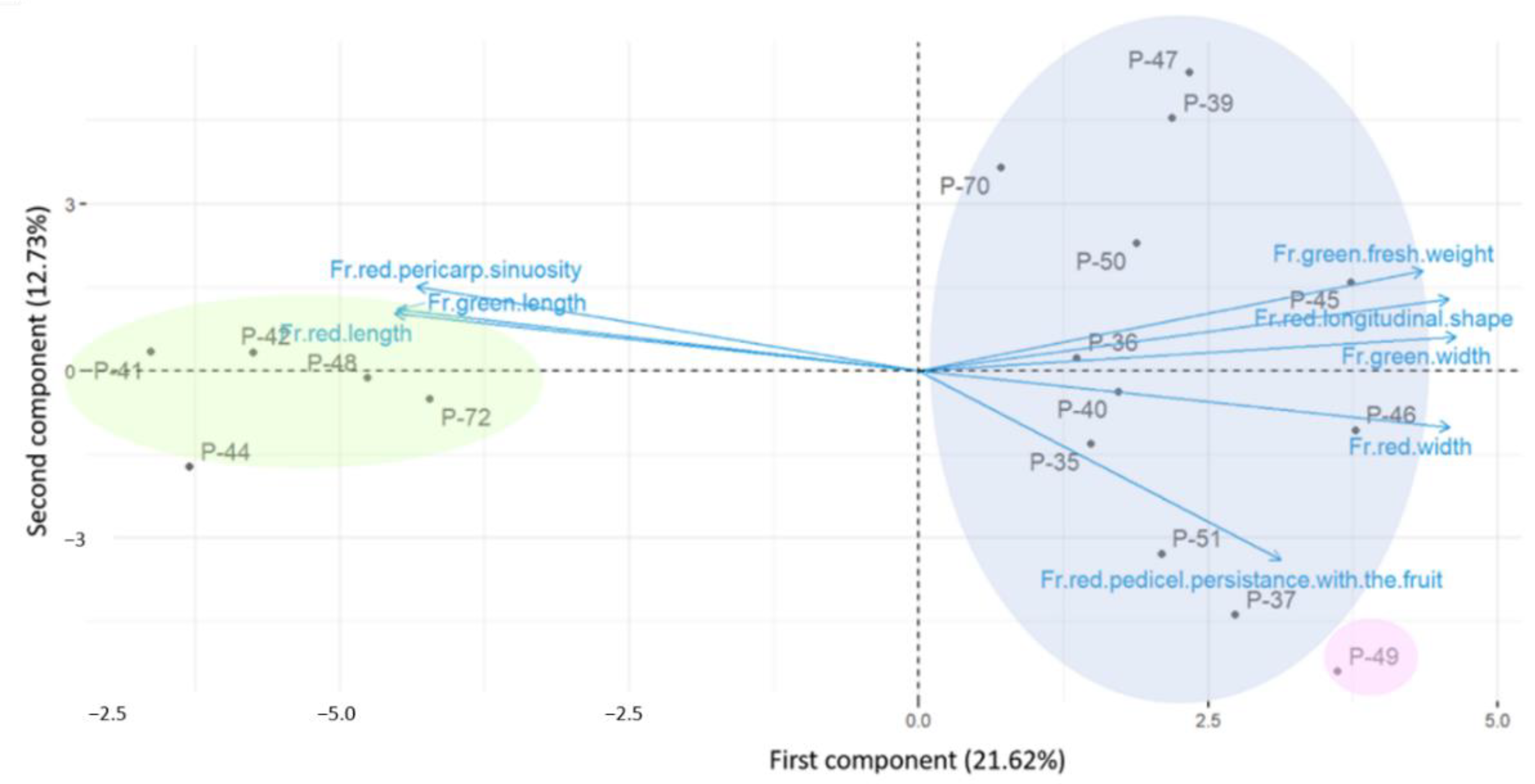
3.5. PCA Analysis
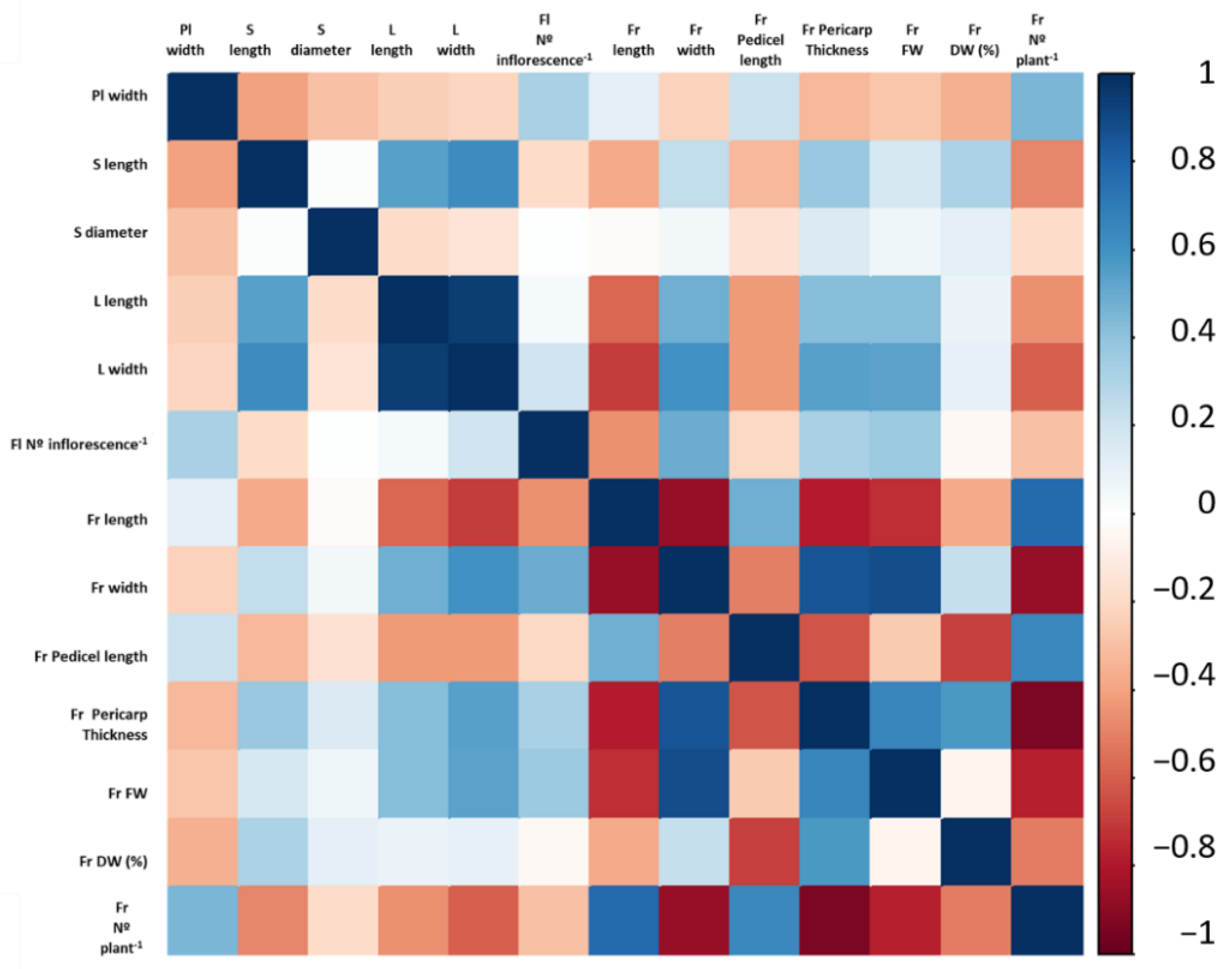
3.6. Correlation among the Selected Quantitative Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Castillo, R. Agricultura tradicional campesina: Características ecológicas. Rev. Tecnol. Marcha 2008, 21, 3–13. [Google Scholar]
- Ebert, A.W. Potential of Underutilized Traditional Vegetables and Legume Crops to Contribute to Food and Nutritional Security, Income and More Sustainable Production Systems. Sustainability 2014, 6, 319–335. [Google Scholar] [CrossRef] [Green Version]
- Kansanga, M.; Andersen, P.; Kpienbaareh, D.; Mason-Renton, S.; Atuoye, K.; Sano, Y.; Antabe, R.; Luginaah, I. Traditional agriculture in transition: Examining the impacts of agricultural modernization on smallholder farming in Ghana under the new Green Revolution. Int. J. Sustain. Dev. World Ecol. 2019, 26, 11–24. [Google Scholar] [CrossRef]
- Daivis, K.F.; Chhatre, A.; Rao, N.; Singh, D.; Ghosh-Jerath, S.; Mridul, A.; DeFries, R. Más allá de la Revolución Verde: Equilibrando múltiples objetivos para la producción sostenible de cereales. Actas Acad. Nac. Cienc. 2019, 116, 25034–25041. [Google Scholar]
- Brush, S.B. Reconsidering the green revolution: Diversity and stability in cradle areas of crop domestication. Hum. Ecol. 1992, 20, 145–167. [Google Scholar] [CrossRef]
- Casañas, F. Varietats tradicionals, obtenció de cultivars amb característiques organoléptiquessuperiors i agricultura en espais periurbans Catalans. Quad. Agrar. 2006, 30, 117–127. [Google Scholar]
- Cebolla-Cornejo, J.; Soler, S.; Nuez, F. Genetic erosion of traditional varieties of vegetable crops in Europe: Tomato cultivation in Valencia (Spain) as a case Study. Int. J. Plant Prod. 2012, 1, 113–128. [Google Scholar] [CrossRef]
- Soler, S.; Prohens, J.; López, C.; Aramburu, J.; Galipienso, L.; Nuez, F. Viruses infecting tomato in Valencia, Spain: Occurence, distribution and effect of seed rigin. J. Phytopathol. 2010, 158, 797–805. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Díez, M.J.; De la Rosa, L.; Martín, I.; Guasch, L.; Cartea, M.E.; Mallor, C.; Casals, J.; Simó, J.; Rivera, A.; Anastasio, G. Plant Genebanks: Present Situation and Proposals for Their Improvement. the Case of the Spanish Network. Front. Plant Sci. 2018, 9, 1794. [Google Scholar] [CrossRef] [Green Version]
- Bozokalfa, M.K.; Eşiyok, D. Evaluation of Morphological and Agronomical Characterization of Turkish Pepper Accessions. Int. J. Veg. Sci. 2011, 17, 115–135. [Google Scholar] [CrossRef]
- Muñoz-Ramírez, L.S.; Peña-Yam, L.P.; Álvarez-Gil, M.A.; Iglesias-Andreu, L.G.; Avilés-Viñas, S.A.; Canto-Flick, A.; Guzmán-Antonio, A.; Santana-Buzzy, N. Selection of habanero pepper f1 hybrids (Capsicum chinense jacq.) at the yucatan peninsula, mexico with a high potential for different markets. Agriculture 2020, 10, 478. [Google Scholar] [CrossRef]
- Shivakumar, M.S.; Saji, K.V. Association and path coefficient analysis among yield attributes and berry yield in black pepper (Piper nigrum L.). J. Spices Aromat. Crop. 2019, 28, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Prayoga, G.I.; Ropalia; Aini, S.N.; Mustikarini, E.D.; Rosalin, Y. Diversity of black pepper plant (Piper nigrum) in Bangka Island (Indonesia) based on agro-morphological characters. Biodivers. J. Biol. Divers. 2020, 21, 652–660. [Google Scholar] [CrossRef]
- Nsabiyera, V.; Logose, M.; Ochwo-Ssemakula, M.; Sseruwagi, P.; Gibson, P.; Ojiewo, C.O. Morphological characterization of local and exotic hot pepper (Capsicum annuum L.) collections in Uganda. Bioremediat. Biodivers. Bioavailab. 2013, 7, 22–32. [Google Scholar]
- Uddin, M.; Rahman, M.; Hossain, M.; Mian, M. Genetic diversity in eggplant genotypes for heat tolerance. SAARC J. Agric. 2015, 12, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Inicio|Organización de las Naciones Unidas para la Alimentación y la Agricultura. Available online: http://www.fao.org/home/es/ (accessed on 15 April 2021).
- Agricultura, Pesca y Alimentación en España 2018. Memoria Annual. Available online: https://www.mapa.gob.es/es/ministerio/servicios/publicaciones/Memoria-MAPA-2018.aspx (accessed on 27 October 2021).
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive characteristics and antioxidant activities of nine peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Martínez, S.; López, M.; González-Raurich, M.; Alvarez, A.B. The effects of ripening stage and processing systems on vitamin C content in sweet peppers (Capsicum annuum L.). Int. J. Food Sci. Nutr. 2005, 56, 45–51. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Martínez-Cuenca, M.-R.; Marsal, J.I.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Calatayud, Á. Bioactive Compounds and Antioxidant Capacity of Valencian Pepper Landraces. Molecules 2021, 26, 1031. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Mateos-Fernández, R.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. Phenotyping Local Eggplant Varieties: Commitment to Biodiversity and Nutritional Quality Preservation. Front. Plant Sci. 2021, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Penella, C.; Nebauer, S.G.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: Physiological responses. J. Plant Physiol. 2014, 171, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Maroto Borrego, J.V. Horticultura Herbácea Especial; Mundi-Prensa: Madrid, Spain, 2002. [Google Scholar]
- IVIA (Instituto Valenciano de Investigaciones Agrarias). Cálculo de Necesidades de Riego. Available online: http://riegos.ivia.es/calculo-de-necesidades-de-riego (accessed on 13 January 2021).
- International Plant Genetic Resources Institute. Descriptors for Capsicum (Capsicum spp.); International Plant Genetic Resources Institute: Rome, Italy, 1995. [Google Scholar]
- Nankar, A.N.; Kostova, D.; Tringovska, I.; Grozeva, S.; Ganeva, D. Tomato phenotypic diversity determined by combined approaches of conventional and high-throughput tomato analyzer phenotyping. Plants 2020, 9, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudré, C.P.; Gonçalves, L.S.A.; Rodrigues, R.; Amaral Júnior, A.D.; Riva-Souza, E.M.; Bento, C.D.S. Genetic variability in domesticated Capsicum spp. as assessed by morphological and agronomic data in mixed statistical analysis. Genet. Mol. Res. 2010, 9, 283–294. [Google Scholar] [CrossRef]
- Bianchi, P.A.; da Silva, L.R.A.; Alencar, A.A.D.S.; Santos, P.H.A.D.; Pimenta, S.; Sudré, C.P.; Corte, L.E.-D.; Gonçalves, L.S.A.; Rodrigues, R. Biomorphological Characterization of Brazilian Capsicum Chinense Jacq. Germplasm. Agronomy 2020, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Do Rêgo, E.R.; do Rêgo, M.M.; Cruz, C.D.; Finger, F.L.; Casali, V.W.D. Phenotypic diversity, correlation and importance of variables for fruit quality and yield traits in Brazilian peppers (Capsicum baccatum). Genet. Resour. Crop Evol. 2010, 58, 909–918. [Google Scholar] [CrossRef]
- Dos Pessoa, A.M.; Rêgo, E.R.D.; de Carvalho, M.G.; Santos, C.A.P.; do Rêgo, M.M. Genetic diversity among accessions of Capsicum annuum L. Through morphoagronomic characters. Genet. Mol. Res. 2018, 17, gmr16039883. [Google Scholar] [CrossRef] [Green Version]
- Tsonev, S.; Todorova, V.; Groseva, S.; Popova, T.; Todorovska, E.G. Evaluation of diversity in Bulgarian pepper cultivars by agronomical traits and issr markers. Genetika 2017, 49, 647–662. [Google Scholar] [CrossRef]
- Rivera, A.; Mallor, C.; Garcés-Claver, A.; García-Ulloa, A.; Pomar, F.; Silvar, C. Assessing the genetic diversity in onion (Allium cepa L.) landraces from northwest Spain and comparison with the European variability. N. Z. J. Crop Hortic. Sci. 2016, 44, 103–120. [Google Scholar] [CrossRef]
- Cardoso, R.; Ruas, C.F.; Giacomin, R.M.; Ruas, P.M.; Ruas, E.A.; Barbieri, R.L.; Rodrigues, R.; Gonçalves, L.S.A. Genetic variability in Brazilian Capsicum baccatum germplasm collection assessed by morphological fruit traits and AFLP markers. PLoS ONE 2018, 13, e0196468. [Google Scholar] [CrossRef] [Green Version]
- Occhiuto, P.N.; Peralta, I.E.; Asprelli, P.D.; Galmarini, C.R. Characterization of Capsicum germplasm collected in northwestern Argentina based on morphological and quality traits. AgriScientia 2014, 31, 63–73. [Google Scholar] [CrossRef]
- Zewdie, Y.; Tong, N.; Bosland, P. Establishing a core collection of Capsicum using a cluster analysis with enlightened selection of accessions. Genet. Resour. Crop Evol. 2004, 51, 147–151. [Google Scholar] [CrossRef]
- Nankar, A.N.; Todorova, V.; Tringovska, I.; Pasev, G.; Radeva-Ivanova, V.; Ivanova, V.; Kostova, D. A step towards Balkan Capsicum annuum L. core collection: Phenotypic and biochemical characterization of 180 accessions for agronomic, fruit quality, and virus resistance traits. PLoS ONE 2020, 15, e0237741. [Google Scholar] [CrossRef] [PubMed]
- Nankar, A.N.; Tringovska, I.; Grozeva, S.; Todorova, V.; Kostova, D. Application of high-throughput phenotyping tool Tomato Analyzer to characterize Balkan Capsicum fruit diversity. Sci. Hortic. 2019, 260, 108862. [Google Scholar] [CrossRef]
- Yada, B.; Tukamuhabwa, P.; Alajo, A.; Mwanga, R.O. Morphological Characterization of Ugandan Sweetpotato Germplasm. Crop Sci. 2010, 50, 2364–2371. [Google Scholar] [CrossRef]
- Cericola, F.; Portis, E.; Toppino, L.; Barchi, L.; Acciarri, N.; Ciriaci, T.; Sala, T.; Rotino, G.L.; Lanteri, S. The Population Structure and Diversity of Eggplant from Asia and the Mediterranean Basin. PLoS ONE 2013, 8, e73702. [Google Scholar] [CrossRef] [Green Version]
- Tembe, K.O.; Chemining’wa, G.; Ambuko, J.; Owino, W. Evaluation of African tomato landraces (Solanum lycopersicum) based on morphological and horticultural traits. Agric. Nat. Resour. 2018, 52, 536–542. [Google Scholar] [CrossRef]
- Grozeva, S.; Nankar, A.N.; Ganeva, D.; Tringovska, I.; Pasev, G.; Kostova, D. Characterization of tomato accessions for morphological, agronomic, fruit quality, and virus resistance traits. Can. J. Plant Sci. 2021, 101, 476–489. [Google Scholar] [CrossRef]
- Wasonga, D.O.; Ambuko, J.L.; Cheminingwa, G.N.; Odeny, D.A.; Crampton, B.G. Morphological Characterization and Selection of Spider Plant:Accessions from Kenya and South Africa. Asian J. Agric. Sci. 2015, 7, 36–44. [Google Scholar] [CrossRef]
- Baba, V.Y.; Rocha, K.R.; Gomes, G.P.; de Fátima Ruas, C.; Ruas, P.M.; Rodrigues, R.; Gonçalves, L.S.A. Genetic diversity of Capsicum chinense accessions based on fruit morphological characterization and AFLP markers. Genet. Resour. Crop Evol. 2015, 63, 1371–1381. [Google Scholar] [CrossRef]
- Moreira, A.F.P.; Ruas, P.M.; de Fátima Ruas, C.; Baba, V.Y.; Giordani, W.; Arruda, I.M.; Rodrigues, R.; Gonçalves, L.S.A. Genetic diversity, population structure and genetic parameters of fruit traits in Capsicum chinense. Sci. Hortic. 2018, 236, 1–9. [Google Scholar] [CrossRef]
- Lahbib, K. Genetic diversity evaluation of pepper (Capsicum annuum L.) in Tunisia based on morphologic characters. Afr. J. Agric. Res. 2012, 7, 3413–3417. [Google Scholar] [CrossRef]
- Finger, F.; Lannes, S.; Schuelter, A.; Doege, J.; Comerlato, A.; Gonçalves, L.; Ferreira, F.; Clovis, L.; Scapim, C. Genetic diversity of Capsicum chinensis (Solanaceae) accessions based on molecular markers and morphological and agronomic traits. Genet. Mol. Res. 2010, 9, 1852–1864. [Google Scholar] [CrossRef]
- López-Sesé, A.I.; Staub, J.; Katzir, N.; Gómez-Guillamón, M.L. Estimation of between and within accession variation in selected Spanish melon germplasm using RAPD and SSR markers to assess strategies for large collection evaluation. Euphytica 2002, 127, 41–51. [Google Scholar] [CrossRef]
- Toklu, F.; Tuba Biçer, B.; Karaköy, T. Agro-morphological characterization of the Turkish lentil landraces. Afr. J. Biotechnol. 2009, 8, 4121–4127. [Google Scholar] [CrossRef]
- Muller, M.H.; Délieux, F.; Fernández-Martínez, J.M.; Garric, B.; Lecomte, V.; Anglade, G.; Leflon, M.; Motard, C.; Segura, R. Occurrence, distribution and distinctive morphological traits of weedy Helianthus annuus L. populations in Spain and France. Genet. Resour. Crop Evol. 2009, 56, 869–877. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic variability and interrelationship among various morphological and quality traits in quinoa (Chenopodium quinoa Willd.). Field Crop. Res. 2007, 101, 104–116. [Google Scholar] [CrossRef]
- Panthee, R.B.; Regmi, H.N.; Subedi, P.P.; Bhattarai, S.; Dhakal, J. Diversity Analysis of Garlic (Allium sativum L.) Germplasms Available in Nepal Based on Morphological Characters. Genet. Resour. Crop Evol. 2006, 53, 205–212. [Google Scholar] [CrossRef]
- Prohens, J.; Blanca, J.M.; Nuez, F. Morphological and molecular variation in a collection of eggplants from a secondary center of diversity: Implications for conservation and breeding. J. Am. Soc. Hortic. Sci. 2005, 130, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Tümbilen, Y.; Frary, A.; Mutlu, S.; Doganlar, S. Genetic diversity in Turkish eggplant (Solanum melongena) varieties as determined by morphological and molecular analyses. Int. Res. J. Biotechnol. 2001, 2, 16–25. [Google Scholar]
- Paran, I.; van der Knaap, E. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. J. Exp. Bot. 2007, 58, 3841–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Domenico, C.I.; Coutinho, J.P.; Godoy, H.T.; de Melo, A.M. Caracterização agronômica e pungência em pimenta de cheiro. Hortic. Bras. 2012, 30, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.Q.; Jasmim, J.M.; Santos, J.O.; Bento, C.S.; Sudré, C.P.; Rodrigues, R. Fenotipagem e seleção de genitores em acessos de pimentas para fins ornamentais. Hortic. Bras. 2015, 33, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Bento, C.S. Descritores qualitativos e multicategóricos na estimativa da variabilidade fenotípica entre acessos de pimentas. Sci. Agrar. 2014, 8, 147–154. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Ceccanti, C.; Padilla, Y.G.; López-Galarza, S.; Calatayud, Á.; Conte, G.; Guidi, L. Effect of Grafting on the Production, Physico-Chemical Characteristics and Nutritional Quality of Fruit from Pepper Landraces. Antioxidants 2020, 9, 501. [Google Scholar] [CrossRef]
- Lannes, S.D.; Finger, F.L.; Schuelter, A.R.; Casali, V.W.D. Growth and quality of Brazilian accessions of Capsicum chinense fruits. Sci. Hortic. 2007, 112, 266–270. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwai, K. Chapter 4 Constituents of Red Pepper Species: Chemistry, Biochemistry, Pharmacology, and food Science of the Pungent Principle of Capsicum Species. Alkaloids Chem. Pharmacol. 1984, 23, 227–299. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Cecon, P.R.; Finger, F.L.; e Silva, F.F.; Puiatti, M. Divergência genética entre genótipos de pimenta com base em caracteres morfo-agrônomicos. Hortic. Bras. 2011, 29, 354–358. [Google Scholar] [CrossRef]
- Melo, L.F.; Gomes, R.L.F.; da Silva, V.B.; Monteiro, E.R.; Lopes, Â.C.A.; Peron, A.P. Potencial ornamental de acessos de pimenta. Ciência Rural 2014, 44, 2010–2015. [Google Scholar] [CrossRef]
- De, A.K. Capsicum. The Genus Capsicum; CRC Press: London, UK, 2003. [Google Scholar]
- Malone, M.; Andrews, J. The distribution of xylem hydraulic resistance in the fruiting truss of tomato. Plant Cell Environ. 2001, 24, 565–570. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Tilbrook, J.; Pardo, C.; Kotula, L.; Sullivan, W.; Steudle, E. Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cv Shiraz and Chardonnay. Aust. J. Grape Wine Res. 2004, 10, 170–181. [Google Scholar] [CrossRef]
- Trifilò, P.; Raimondo, F.; Lo Gullo, M.A.; Nardini, A.; Salleo, S. Hydraulic connections of leaves and fruit to the parent plant in Capsicum frutescens (hot pepper) during fruit ripening. Ann. Bot. 2010, 106, 333. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chaim, A.; Paran, I. Genetic analysis of quantitative traits in pepper (Capsicum annuum). J. Am. Soc. Hortic. Sci. 2000, 125, 66–70. [Google Scholar] [CrossRef]
- Poulos, J.M. Pepper breeding (Capsicum spp.): Achievements, challenges and possibilities. Plant Breed. Abstr. 1994, 64, 143–155. [Google Scholar]
- Fonseca, R.M.; Lopes, R.; Barros, W.S.; Lopes, M.T.G.; Ferreira, F.M. Morphologic characterization and genetic diversity of Capsicum chinense Jacq. accessions along the upper Rio Negro-Amazonas. Crop Breed. Appl. Biotechnol. 2008, 8, 187–194. [Google Scholar] [CrossRef]
- Munchi, A.D.; Behera, T.K. Correlation and path coefficient analysis in chilli. Indian J. Hortic. Res. 2000, 11, 93–97. [Google Scholar]
- Do Rêgo, E.R.; do Rêgo, M.M.; de Matos, I.W.F.; Barbosa, L.A. Caracterização morfológica e química de frutos de acessos do gênero Capsicum spp. Hortic. Bras. 2011, 29, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Do Rêgo, E.R.; do Rêgo, M.M.; Finger, F.L.; Cruz, C.D.; Casali, V.W.D. A diallel study of yield components and fruit quality in chilli pepper (Capsicum baccatum). Euphytica 2009, 168, 275–287. [Google Scholar] [CrossRef]
- De Carvalho, É.B.; Vitolo, M.R.; Gama, C.M.; Lopez, F.A.; Taddei, J.A.C.; de Morais, M.B. Fiber intake, constipation, and overweight among adolescents living in Sao Paulo City. Nutrition 2006, 22, 744–749. [Google Scholar] [CrossRef]
- Barroso, P.A.; Rêgo, E.R.; Rêgo, M.M.; Nascimento, K.S.; Nascimento, N.F.F.; Nascimento, M.F.; Soares, W.S.; Ferreira, K.T.C.; Otoni, W.C. Analysis of segregating generation for components of seedling and plant height of pepper (Capsicum annuum L.) for medicinal and ornamental purposes. Acta Hortic. 2012, 953, 269–276. [Google Scholar] [CrossRef]
- Kumar, S.R.; Arumugam, T.; Ulaganathan, V.; Kumar, S.R. Genetic diversity in eggplant germplasm by principal component analysis. J. Breed. Genet. 2016, 48, 162–171. [Google Scholar]
- Ferreira, K.T.C.; Rêgo, E.R.; Rêgo, M.M.; Fortunato, F.L.G.; Nascimento, N.F.F.; De Lima, J.A.M. Combining ability for morpho-agronomic traits in ornamental pepper. Acta Hortic. 2015, 1087, 187–194. [Google Scholar] [CrossRef]
- Do Rêgo, E.R.; Nascimento, M.F.; do Nascimento, N.F.F.; dos Santos, R.M.C.; Fortunato, F.L.G.; do Rêgo, M.M. Comparação de métodos para a produção de frutos autofecundados em pimenteiras ornamentais. Hortic. Bras. 2012, 30, 669–672. [Google Scholar] [CrossRef] [Green Version]




| Abbreviation Code | Germplasm Code | Group | Fruit Description | Origin/Cultivated Zone (3) | Intended Use (Consumption) |
|---|---|---|---|---|---|
| P-35 | BGV005087 (1) | G2 | Rectangular shape, blocky and with four shoulders and locule marked | Fanzara, Castellón, Spain | Roasted, preferably when mature (red) |
| P-36 | BGV005035 (1) | G2 | Irregular, rectangular-conical shape, but an inconsistent pattern, slightly marked shoulders | Chelva, Valencia, Spain | Roasted |
| P-37 | BGV005097 (1) | G2 | Triangular shape and apex truncated | Castillo de Villamalefa, Castellón, Spain | Roasted, preferably when mature (red) |
| P-39 | BGV005115 (1) | G2 | Triangular shape and apex truncated | Alicante, Spain | Roasted, preferably when mature (red) |
| P-40 | BGV005125 (1) | G2 | Rounded- elongated triangular shape and apex truncated | Elda, Alicante, Spain | Roasted, preferably when mature (red) |
| P-41 | BGV014141 (1) | G1 | Elongated (horn type), only slightly marked shoulders | Vinaroz, Castellón, Spain | Fried, preferably when immature (green) |
| P-42 | BGV014145 (1) | G1 | Elongated (horn type), only slightly marked shoulders | Almenara, Castellón, Spain | Fried, preferably when immature (green) |
| P-44 | BGV016188 (1) | G1 | Elongated (horn type), only slightly marked shoulders | Guardamar del Segura, Alicante, Spain | Fried, preferably when immature (green) |
| P-45 | BGV005064 (1) | G2 | Triangular shape and apex truncated | Ademuz, Valencia, Spain | Roasted, preferably when mature (red) |
| P-46 | BGV005085 (1) | G2 | Rectangular shape, blocky and with four shoulders and locule marked | Onda, Castellón, Spain | Roasted |
| P-47 | BGV005040 (1) | G2 | Rounded-elongated triangular shape and apex truncated | Siete Aguas, Valencia, Spain | Roasted |
| P-48 | BGV005034 (1) | G1 | Elongated (horn type), only slightly marked shoulders | Chelva, Valencia, Spain | Fried, preferably when immature (green) |
| P-49 | BGV005046 (1) | - | Ball-like shape with only slightly marked shoulders | Benissa, Alicante, Spain | Roasted, preferably when mature (red) |
| P-50 | BGV005116 (1) | G2 | Triangular shape and apex truncated | Rojales, Alicante, Spain | Roasted |
| P-51 | BGV014553 (1) | G2 | Rectangular shape, blocky and with four shoulders and locule marked | Tales, Castellón, Spain | Roasted |
| P-70 | IVIA 70 (2) | G2 | Rounded- elongated triangular shape and apex truncated | Moncada, Valencia, Spain | Roasted, preferably when immature (green) |
| P-72 | IVIA 72 (2) | G1 | Elongated (horn type), only slightly marked shoulders | Canal de Navarrés, Valencia, Spain | Fried, preferably when immature (green) |
| Group | Landrace | Stem L to 1st Bifurcation (cm) | Stem Diameter (mm) | Plant W (cm) | Leaf L (cm) | Leaf W (cm) | Flowers Per Axile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | P-41 | 22.4 | c | 15.7 | 64.8 | ab | 16.7 | c | 9.2 | 1.00 | |||
| P-42 | 28.9 | b | 15.6 | 61.6 | b | 19.1 | ab | 10.2 | 1.00 | ||||
| P-44 | 33.9 | a | 14.4 | 61.4 | b | 20.1 | a | 10.2 | 1.00 | ||||
| P-48 | 21.9 | c | 15.3 | 68.1 | a | 17.0 | bc | 9.5 | 1.00 | ||||
| P-72 | 32.3 | ab | 14.6 | 68.3 | ab | 18.3 | abc | 9.9 | 1.00 | ||||
| G2 | P-35 | 31.4 | b | 15.6 | bc | 65.6 | abc | 16.7 | f | 10.1 | d | 1.28 | b |
| P-36 | 30.0 | bc | 15.2 | bcd | 66.6 | ab | 19.3 | de | 10.3 | d | 1.00 | c | |
| P-37 | 23.3 | ef | 15.1 | bcd | 69.3 | a | 19.3 | de | 10.6 | cd | 2.00 | a | |
| P-39 | 28.4 | bcd | 16.0 | bc | 50.3 | e | 22.9 | ab | 11.7 | bc | 1.00 | c | |
| P-40 | 43.0 | a | 14.5 | cd | 64.4 | abcd | 23.2 | a | 14.8 | a | 1.00 | c | |
| P-45 | 29.9 | bc | 16.6 | b | 71.0 | a | 21.7 | abc | 12.7 | b | 2.29 | a | |
| P-46 | 22.1 | f | 14.0 | d | 70.4 | a | 20.2 | cd | 11.8 | b | 1.50 | b | |
| P-47 | 30.9 | b | 16.2 | bc | 54.3 | de | 20.9 | bcd | 12.7 | b | 1.37 | b | |
| P-50 | 30.1 | bc | 25.4 | a | 55.9 | cde | 17.9 | ef | 10.1 | d | 1.25 | bc | |
| P-51 | 26.6 | cde | 15.2 | bcd | 69.9 | a | 20.8 | bcd | 11.7 | bc | 1.00 | c | |
| P-70 | 24.5 | def | 15.2 | bcd | 57.1 | bcde | 19.3 | de | 10.1 | d | 1.00 | c | |
| - | P-49 | 33.9 | 15.7 | 59.7 | 21.0 | 12.4 | 1.00 | ||||||
| Group | Landrace | Length (cm) | Width (cm) | L/W | Pedicel L (cm) | Wall Thickness (mm) | Locule Number | FW (g) | DW (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | P-41 | 20.3 | a | 3.2 | c | 6.4 | a | 6.6 | a | 3.0 | b | 2.5 | b | 84.9 | bc | 6.7 | bc |
| P-42 | 16.5 | b | 2.9 | c | 6.4 | a | 5.3 | b | 2.1 | c | 2.8 | b | 89.0 | ab | 5.8 | d | |
| P-44 | 18.2 | ab | 2.8 | c | 6.5 | a | 4.3 | c | 3.2 | b | 3.5 | a | 71.2 | c | 7.0 | b | |
| P-48 | 16.5 | b | 3.9 | b | 4.4 | b | 4.4 | c | 2.8 | b | 3.0 | ab | 74.6 | bc | 8.5 | a | |
| P-72 | 18.0 | ab | 5.3 | a | 3.5 | b | 4.4 | c | 4.3 | a | 2.6 | b | 100.9 | ab | 6.3 | c | |
| G2 | P-35 | 12.6 | bc | 5.8 | f | 2.4 | b | 4.1 | cde | 4.3 | d | 3.5 | bc | 135.6 | bc | 6.1 | bc |
| P-36 | 8.6 | e | 6.5 | def | 1.4 | de | 4.7 | bc | 4.3 | d | 3.0 | d | 159.4 | ab | 6.2 | b | |
| P-37 | 12.0 | cd | 7.3 | bc | 1.5 | cd | 4.1 | de | 4.5 | cd | 3.0 | cd | 107.6 | e | 6.2 | b | |
| P-39 | 14.8 | a | 6.1 | f | 2.3 | b | 4.2 | cde | 4.3 | cd | 3.5 | bcd | 145.7 | abc | 6.8 | a | |
| P-40 | 15.0 | a | 5.8 | f | 2.7 | a | 6.1 | a | 4.2 | d | 3.8 | ab | 131.8 | cd | 5.8 | bcd | |
| P-45 | 10.3 | d | 9.1 | a | 1.1 | e | 5.2 | b | 5.3 | ab | 4.0 | a | 157.6 | abc | 5.8 | bcd | |
| P-46 | 6.3 | f | 5.9 | f | 1.1 | e | 4.2 | cd | 4.4 | cd | 3.8 | a | 172.8 | a | 5.5 | d | |
| P-47 | 10.1 | d | 7.1 | cd | 1.5 | cd | 4.7 | bcd | 5.0 | abc | 4.0 | a | 134.3 | bcd | 5.7 | cd | |
| P-50 | 13.7 | ab | 6.1 | ef | 2.3 | b | 4.3 | cd | 5.0 | bcd | 3.0 | d | 135.1 | bc | 5.9 | bcd | |
| P-51 | 10.1 | d | 6.9 | cde | 1.5 | cd | 2.5 | f | 4.6 | cd | 3.5 | b | 139.1 | bc | 5.5 | d | |
| P-70 | 13.2 | bc | 8.0 | b | 1.7 | c | 3.5 | e | 5.6 | a | 3.5 | b | 143.4 | bc | 5.5 | d | |
| - | P-49 | 6.1 | 7.0 | 0.9 | 4.0 | 7.1 | 2.8 | 124.7 | 6.3 | ||||||||
| Group | Landrace | L (cm) | W (cm) | L/W | Pedicel L (cm) | Wall Thickness (mm) | Locule number | FW (g) | DW (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | P-41 | 20.9 | a | 3.7 | b | 5.9 | a | 3.8 | b | 3.1 | a | 2.7 | b | 96.7 | a | 9.8 | c |
| P-42 | 23.0 | ab | 4.7 | a | 5.0 | ab | 4.4 | a | 3.5 | a | 3.4 | a | 100.8 | a | 8.8 | bc | |
| P-44 | 16.2 | a | 3.0 | b | 5.5 | a | 4.2 | ab | 2.5 | b | 2.9 | ab | 62.7 | bc | 10.8 | b | |
| P-48 | 13.2 | b | 3.2 | b | 4.5 | b | 2.8 | c | 2.7 | b | 3.4 | a | 59.1 | c | 13.8 | a | |
| P-72 | 16.6 | ab | 3.0 | b | 5.1 | ab | 4.7 | a | 3.3 | a | 2.6 | b | 84.6 | ab | 9.8 | bc | |
| G2 | P-35 | 9.7 | d | 7.0 | bc | 1.4 | e | 3.4 | bc | 4.8 | cde | 3.6 | a | 95.9 | c | 9.4 | bcde |
| P-36 | 11.3 | abcd | 5.3 | d | 2.0 | bc | 3.9 | ab | 3.6 | g | 3.4 | a | 122.0 | ab | 10.2 | abc | |
| P-37 | 11.7 | ab | 7.6 | bc | 1.5 | de | 3.3 | bcd | 4.1 | efg | 3.3 | a | 107.9 | bc | 10.8 | ab | |
| P-39 | 9.5 | cd | 5.5 | d | 1.8 | cd | 3.9 | ab | 4.0 | defg | 3.8 | a | 110.2 | abc | 11.2 | a | |
| P-40 | 9.6 | d | 5.6 | d | 1.8 | c | 2.8 | d | 4.7 | cde | 3.7 | a | 118.0 | abc | 10.7 | ab | |
| P-45 | 11.6 | ab | 8.2 | a | 1.4 | e | 4.2 | a | 8.7 | b | 3.6 | a | 136.3 | a | 9.6 | abcd | |
| P-46 | 10.4 | bcd | 7.8 | ab | 1.4 | ef | 3.4 | bc | 4.3 | def | 3.7 | a | 135.2 | a | 8.3 | de | |
| P-47 | 12.6 | a | 5.5 | d | 2.3 | a | 4.2 | a | 4.8 | cd | 3.6 | a | 100.1 | bc | 9.5 | abcde | |
| P-50 | 11.7 | abc | 6.4 | cd | 1.8 | c | 3.9 | ab | 9.8 | a | 3.3 | a | 115.4 | abc | 8.4 | cde | |
| P-51 | 9.6 | d | 8.4 | a | 1.1 | f | 3.1 | cd | 5.2 | c | 3.4 | a | 113.3 | abc | 8.7 | cde | |
| P-70 | 12.7 | a | 5.7 | d | 2.3 | ab | 4.2 | a | 3.9 | fg | 3.3 | a | 97.9 | c | 7.6 | e | |
| - | P-49 | 5.7 | 6.7 | 0.9 | 3.2 | 8.2 | 3.3 | 86.1 | 10.9 | ||||||||
| Group | Landrace | Number Plant−1 |
|---|---|---|
| G1 | P-41 | 22.3 |
| P-42 | 24.3 | |
| P-44 | 10.5 | |
| P-48 | 19.8 | |
| P-72 | 11.0 | |
| Mean | 17.6 | |
| G2 | P-35 | 8.50 |
| P-36 | 16.00 | |
| P-37 | 21.75 | |
| P-39 | 11.75 | |
| P-40 | 15.25 | |
| P-45 | 16.00 | |
| P-46 | 11.25 | |
| P-47 | 13.75 | |
| P-50 | 12.25 | |
| P-51 | 17.00 | |
| P-70 | 12.25 | |
| Mean | 14.2 | |
| - | P-49 | 10.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Mateos-Fernández, R.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. Phenotypic Divergence among Sweet Pepper Landraces Assessed by Agro-Morphological Characterization as a Biodiversity Source. Agronomy 2022, 12, 632. https://doi.org/10.3390/agronomy12030632
Martínez-Ispizua E, Calatayud Á, Marsal JI, Mateos-Fernández R, Díez MJ, Soler S, Valcárcel JV, Martínez-Cuenca M-R. Phenotypic Divergence among Sweet Pepper Landraces Assessed by Agro-Morphological Characterization as a Biodiversity Source. Agronomy. 2022; 12(3):632. https://doi.org/10.3390/agronomy12030632
Chicago/Turabian StyleMartínez-Ispizua, Eva, Ángeles Calatayud, José Ignacio Marsal, Rubén Mateos-Fernández, María José Díez, Salvador Soler, José Vicente Valcárcel, and Mary-Rus Martínez-Cuenca. 2022. "Phenotypic Divergence among Sweet Pepper Landraces Assessed by Agro-Morphological Characterization as a Biodiversity Source" Agronomy 12, no. 3: 632. https://doi.org/10.3390/agronomy12030632








