Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus coccineus (Ayocote Bean)
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Lectin Extraction
2.3. Hemagglutination Assays
2.4. Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Carbohydrate Content
2.6. Metal Composition Analysis
2.7. The Metal Addition Effect on the Hemagglutination Activity
2.8. Hemagglutination Inhibition by Different Carbohydrates
2.9. Temperature Effect on the Hemagglutination Activity
2.10. pH Effect on the Hemagglutination Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Protein Content
3.2. Lectins Separation
3.3. Hemagglutination Activity
3.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.5. Carbohydrate and Mineral Content
3.6. Effect of the Metal Reincorporation on the Hemagglutination Activity
3.7. Hemagglutination Inhibition by Different Carbohydrates
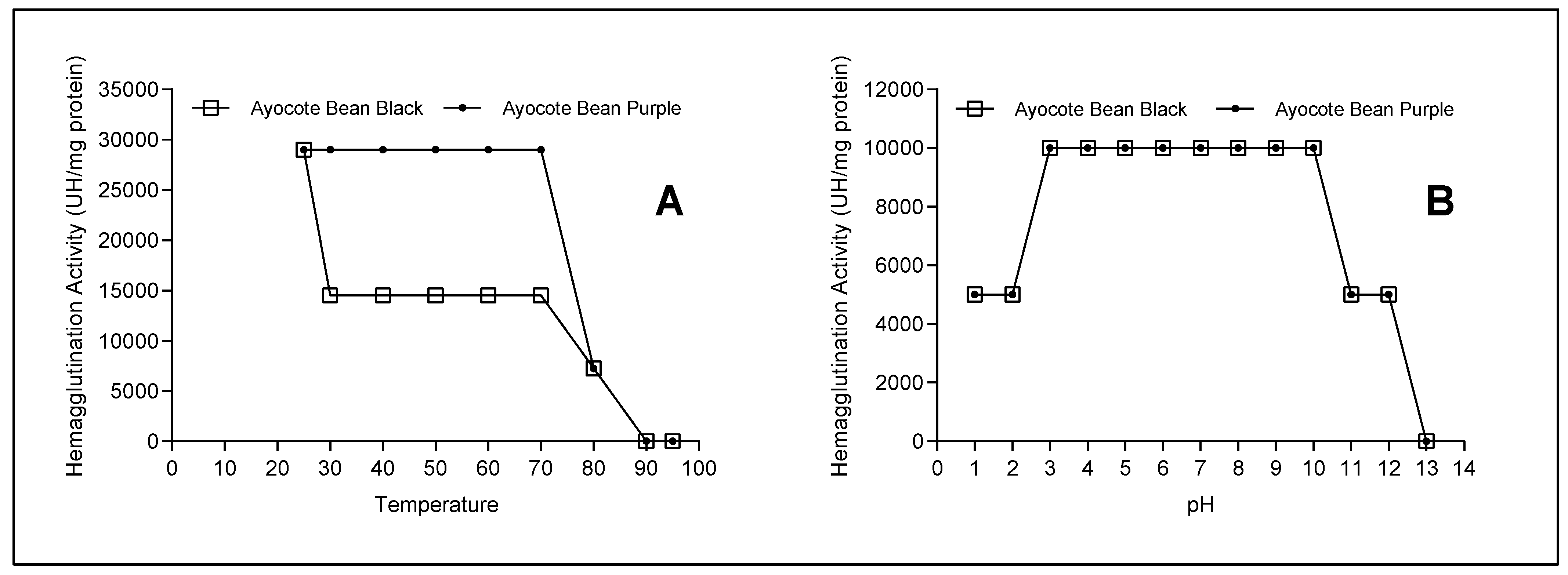
3.8. Temperature and pH Effects on the Hemagglutination Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsaneva, M.; Van Damme, E.J. 130 years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- e Lacerda, R.R.; do Nascimento, E.S.; de Lacerda, J.T.J.G.; da Silva Pinto, L.; Rizzi, C.; Bezerra, M.M.; Ribeiro, P.I.; Pereira, F.S.M.; Texeira, P.V.P.; Cristino, F.G.; et al. Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int. J. Biol. Macromol. 2017, 95, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.W.; Grusak, M.A.; Pinto, E.; Gomes, A.; Ferreira, H.; Balázs, B.; Williams, M. The Biology of Legumes and Their Agronomic, Economic, and Social Impact. In The Plant Family Fabaceae; Springer: Singapore, 2020; pp. 3–25. [Google Scholar]
- Zhang, J.; Shi, J.; Ilic, S.; Jun Xue, S.; Kakuda, Y. Biological properties and characterization of lectin from red kidney bean (Phaseolus vulgaris). Food Rev. Int. 2008, 1, 12–27. [Google Scholar] [CrossRef]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2013, 10, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.K.; Shrivastava, N.; Sharma, B.; Bhagyawant, S.S. Current scenario of legume lectins and their practical applications. J. Crop Sci. Biotechnol. 2018, 28, 217–227. [Google Scholar] [CrossRef]
- Dongre, P.; Shaikh, N.; Gangwal, P.; Pawar, K. Isolation, Partial Purification and Application of lectin isolated from Phaseolus vulgaris (Red Kidney Bean). Int. J. Res. Anal. Rev. 2019, 6, 685–692. [Google Scholar]
- Gautam, A.K.; Sharma, D.; Sharma, J.; Saini, K.C. Legume lectins: Potential use as a diagnostics and therapeutics against the cancer. Int. J. Biol. Macromol. 2020, 142, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.J.A.; Braga, A.L.; Ribeiro, F.J.; Teixeira, C.S.; da Hora, G.C.; Morais-Braga, M.F.B. A review on the antimicrobial properties of lectins. Int. J. Biol. Macromol. 2022, 195, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Muzquiz, M.; Burbano, C.; Ayet, G.; Pedrosa, M.M.; Cuadrado, C. The investigation of antinutritional factors in Phaseolus vulgaris. Environmental and varietal differences. Biotechnol. Agron. Soc. Environ. 1999, 4, 210–216. [Google Scholar]
- Shi, J.; Xue, S.J.; Kakuda, Y.; Ilic, S.; Kim, D. Isolation and characterization of lectins from kidney beans (Phaseolus vulgaris). Process Biochem. 2007, 10, 1436–1442. [Google Scholar] [CrossRef]
- Bernardino-Nicanor, A.; Acosta-García, G.; Güemes-Vera, N.; Monañéz-Soto, J.L.; Vivar-Vera, M.A.; González-Cruz, L. Fourier transform infrared and Raman spectroscopic study of the effect of the thermal treatment and extraction methods on the characteristics of ayocote bean starches. J. Food Sci. Technol. 2017, 54, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Teniente-Martínez, G.; Bernardino-Nicanor, A.; Cariño-Cortes, R.; Valadez-Vega, M.C.; Montañéz-Soto, J.L.; Acosta-García, G.; González-Cruz, L. Cytotoxic and genotoxic activity of protein isolate of ayocote beans and anticancer activity of their protein fractions. J. Food Meas. Charact. 2019, 13, 1040–1048. [Google Scholar] [CrossRef]
- Alvarado-López, A.N.; Gómez-Oliván, L.M.; Heredia, J.B.; Baeza-Jiménez, R.; García-Galindo, H.S.; Lopez-Martinez, L.X. Nutritional and bioactive characteristics of Ayocote bean (Phaseolus coccienus L.): An underutilized legume harvested in Mexico. CyTA-J. Food 2019, 17, 199–206. [Google Scholar] [CrossRef]
- Lagarda-Diaz, I.; Guzman-Partida, A.M.; Vazquez-Moreno, L. Legume lectins: Proteins with diverse applications. Int. J. Mol. Sci. 2017, 18, 1242. [Google Scholar] [CrossRef] [PubMed]
- Nowakova, N.; Kocourek, J. Studies on phytohemagglutinins: XX. Isolation and characterization of hemagglutinins from scarlet runner seeds (Phaseolus coccineus L.). Biochim. Biophys. Acta BBA-Protein Struct. 1974, 359, 320–333. [Google Scholar] [CrossRef]
- Ochoa, J.L.; Kristiansen, T. Stroma: As an affinity adsorbent for non-inhibitable lectins. FEBS Lett. 1978, 90, 145–148. [Google Scholar] [CrossRef]
- Ochoa, J.L.; Kristiansen, T. Purification and partial characterization of an agglutinin from Phaseolus coccineus var. alubia. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 1982, 705, 396–404. [Google Scholar] [CrossRef]
- Feria, M.; Pérez-Santiago, A.; Cuevas, D.; Martínez, M.; Córdoba, F. Purification and partial characterization of a new anti-A1 lectin of Phaseolus coccineus collected in Oaxaca, Mexico. Prep. Biochem. Biotechnol. 1996, 26, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Calderón, R.A.; Cordoba, F. Immunosuppressive activity of Phaseolus coccineus and Phaseolus vulgaris extracts in mice. Eur. J. Immunol. 1976, 6, 522–525. [Google Scholar] [CrossRef]
- Pérez-Campos, E.; Lascurain, R.; Sierra, C.; Espinosa, B.; Debray, H.; Bouquelet, S.; Zenteno, E. Erythroagglutinin from Phaseolus coccineus Var. Alubia: Chemical characterization, sugar specificity, and effect on blood coagulation factors. J. Agric. Food Chem. 1997, 45, 3747–3752. [Google Scholar] [CrossRef]
- Morgan, M.R.; Manen, J.F. Lectin variability in Phaseolus coccineus. Phytochemistry 1985, 24, 1981–1985. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Ji, N.; Zhou, J.N.; Bian, H.J.; Li, C.Y.; Chen, F.; Bao, J.K. A novel sialic acid-specific lectin from Phaseolus coccineus seeds with potent antineoplastic and antifungal activities. Phytomedicine 2009, 16, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.L.; Ng, T.B. A dimeric Phaseolus coccineus lectin with anti-oxidative, anti-proliferative and cytokine-inducing activities. Int. J. Biol. Macromol. 2015, 81, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Mejía, E.G.; Hanskins, C.N.; Paredes-López, O.; Shannon, L.M. The lectins and the lectin CRMs of tepary beans (Phaseolus acutifolius) and tepary-common beans (Phaseolus vulgaris) hybrids. J. Food Biochem. 1989, 14, 117–126. [Google Scholar] [CrossRef]
- Sharma, A.; Bun-Ng, T.; Ho-Wong, J.; Lin, P. Purification and characterization of a lectin from Phaseolus vulgaris cv. (Anasazi Beans). J. Biomed. Biotechnol. 2009, 2009, 929568. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Vega, C.; Guzmán-Partida, A.M.; Soto-Cordova, F.J.; Álvarez-Manilla, G.; Morales-González, J.A.; Madrigal-Santillán, E.; Villagómez-Ibarra, J.R.; Zúñiga-Pérez, C.; Gutiérrez-Slinas, J.; Becerril-Flores, M.A. Purification, biochemical characterization, and bioactive properties of a lectin purified from the seeds of white tepary bean (Phaseolus acutifolius Variety Latifolius). Molecules 2011, 16, 2561–2582. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Fei-Fang, E.; Lin, P.; Ho-Wong, J.; Wah-Tsao, S.; Bun-Ng, T. A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J. Agric. Food Chem. 2010, 58, 2221–2229. [Google Scholar] [CrossRef]
- Aremu, M.O.; Olaofe, O.; Basu, S.K.; Abdulazeez, G.; Acharya, S.N. Processed cranberry bean (Phaseolus coccineus L.) seed flour for the African diet. Can. J. Plant Sci. 2010, 90, 719–728. [Google Scholar] [CrossRef]
- Avasilcai, L.; Teliban, G.; Morariu, D.I.; Stoleru, V.; Bibire, N.; Vieriu, M.; Panainte, A.D.; Munteanu, N. Parameters of chemical composition of Phaseolus coccineus L. pods grown in protected areas. Methods 2017, 68, 2955–2958. [Google Scholar] [CrossRef]
- Malik, A.H.; Holm, L.; Johansson, E. Soil and starter fertilizer and its effect on yield and protein composition of malting barley. J. Soil Sci. Plant Nut. 2012, 12, 835–849. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Smith, G.E.; Qiming, S. In vitro binding of bile acids by kidney bean (Phaseolus vulgaris), black gram (Vigna mungo), bengal gram (Cicer arietinum) and moth bean (Phaseolus aconitifolins). Food Chem. 2005, 90, 241–246. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Byanju, B.; Mahfuzur, R.M.; Hojilla-Evangelista, M.P.; Lamsal, B.P. Effect of high-power sonication pretreatment on extraction and some physicochemical properties of proteins from chickpea, kidney bean, and soybean. Int. J. Biol. Macromol. 2020, 145, 712–721. [Google Scholar] [CrossRef]
- Arteaga, T.I.; Castro, G.J.L.; Mendiola, O.E.; García, G.T.; Ángeles, Z.M.V.; García-Santoyo, V.; Torres, C.J.A.; Aguirre, C.; Phinney, B.; Blanco-Labra, A. Characterization of two non-fetuin binding lectins from tepary bean (Phaseolus acutifolius) seeds with differential cytotoxicity on colon cancer cells. J. Glycobiol. 2016, 5, 1–7. [Google Scholar]
- Jiang, B.; Wang, X.; Wang, L.; Lv, X.; Li, D.; Liu, C.; Feng, Z. Two-step isolation, purification, and characterization of lectin from zihua snap bean (Phaseolus vulgaris) seeds. Polymers 2019, 11, 785. [Google Scholar] [CrossRef]
- Khan, F.; Khan, R.H.; Sherwani, A.; Mohmood, S.; Azfer, M.A. Lectins as markers for blood grouping. Med. Sci. Monitor. 2002, 8, RA293–RA300. [Google Scholar]
- Quiróz-Sodi, M.; Mendoza-Díaz, S.; Hernández-Sandoval, L.; Carrillo-Ángeles, I. Characterization of the secondary metabolites in the seeds of nine native bean varieties (Phaseolus vulgaris and Phaseolus coccineus) from Querétaro, Mexico. Bot. Sci. 2018, 96, 650–661. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; Martínez, L.M.; Rito-Palomares, M. Técnicas cromatográficas y su aplicación a estudios de cambios conformacionales, estabilidad y replegamiento de proteínas. Rev. Mex. Ing. Quim. 2012, 11, 415–429. [Google Scholar]
- Casas, R.Z.Y.; Reyes Montaño, E.A.; Vega, C.N.A. Lectinas con dominio de leguminosa: Características estructurales y utilidad como agentes insectistáticos e insecticidas. Chil. J. Agric. Anim. Sci. 2016, 32, 157–169. [Google Scholar] [CrossRef][Green Version]
- Bonnardel, F.; Mariethoz, J.; Salentin, S.; Robin, X.; Schroeder, M.; Perez, S.; Lisacek, F.; Imberty, A. UniLectin3D, a database of carbohydrate binding proteins with curated information on 3D structures and interacting ligands. Nucleic Acids Res. 2019, 47, D1236–D1244. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwaraiah, L.Y.; Acharya, A.; Prakash, B. Structural basis of noncanonical polyphenol oxidase activity in DLL-II: A lectin from Dolichos lablab. Biotechnol. Appl. Biochem. 2018, 65, 701–771. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, J.; Dileep, K.V.; Palanimuthu, M.; Geethanandan, K.; Sadasivan, C.; Haridas, M. Metal ions in sugar binding, sugar specificity and structural stability of Spatholobus parviflorus seed lectin. J. Mol. Model. 2013, 19, 3271–3278. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.E.; Awadallah, A.K.; Konozy, E.H. Isolation purification and partial characterization of three lectins from Tamarindus indica seeds with a novel sugar specificity. Int. J. Plant Res. 2016, 6, 13–19. [Google Scholar]
- González-Cuesta, M.; Mellet, C.O.; Fernández, J.M.G. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 39, 5207–5222. [Google Scholar] [CrossRef] [PubMed]
- Bonnardel, F.; Perez, S.; Lisacek, F.; Imberty, A. Structural database for lectins and the UniLectin web platform. In Lectin Purification and Analysis; Humana: New York, NY, USA, 2020; pp. 1–14. [Google Scholar]
- Ayesha, N.; Rao, A.G. Lectins-Robust and Quintessential Proteins of Nature: A Review. Agric. Rev. 2020, 1, 7. [Google Scholar] [CrossRef]
- Jang, H.; Lee, C.; Hwang, Y.; Lee, S.J. Concanavalin A: Coordination diversity to xenobiotic metal ions and biological consequences. Dalton Trans. 2021, 48, 17817–17831. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.T. Carbohydrate-binding properties of lectins: A possible approach to lectin nomenclature and classification. Biosci. Rep. 1984, 8, 621–632. [Google Scholar] [CrossRef]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. 2018, 58, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Boniglia, C.; Fedele, E.; Sanzini, E. Measurement by ELISA of active lectin in dietary supplements containing kidney bean protein. J. Food Sci. 2003, 4, 1283–1286. [Google Scholar] [CrossRef]
- Sun, X.; He, S.; Ye, Y.; Cao, X.; Liu, H.; Wu, Z.; Yue, J.; Jin, R.; Sun, H. Combined effects of pH and thermal treatments on IgE-binding capacity and conformational structures of lectin from black kidney bean (Phaseolus vulgaris L.). Food Chem. 2020, 329, 127183. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.J.; Strzelczyk, A.K.; Feldhof, M.I.; Schmidt, S. Temperature-Switchable Glycopolymers and Their Conformation-Dependent Binding to Receptor Targets. Biomacromolecules 2020, 21, 2913–2921. [Google Scholar] [CrossRef] [PubMed]

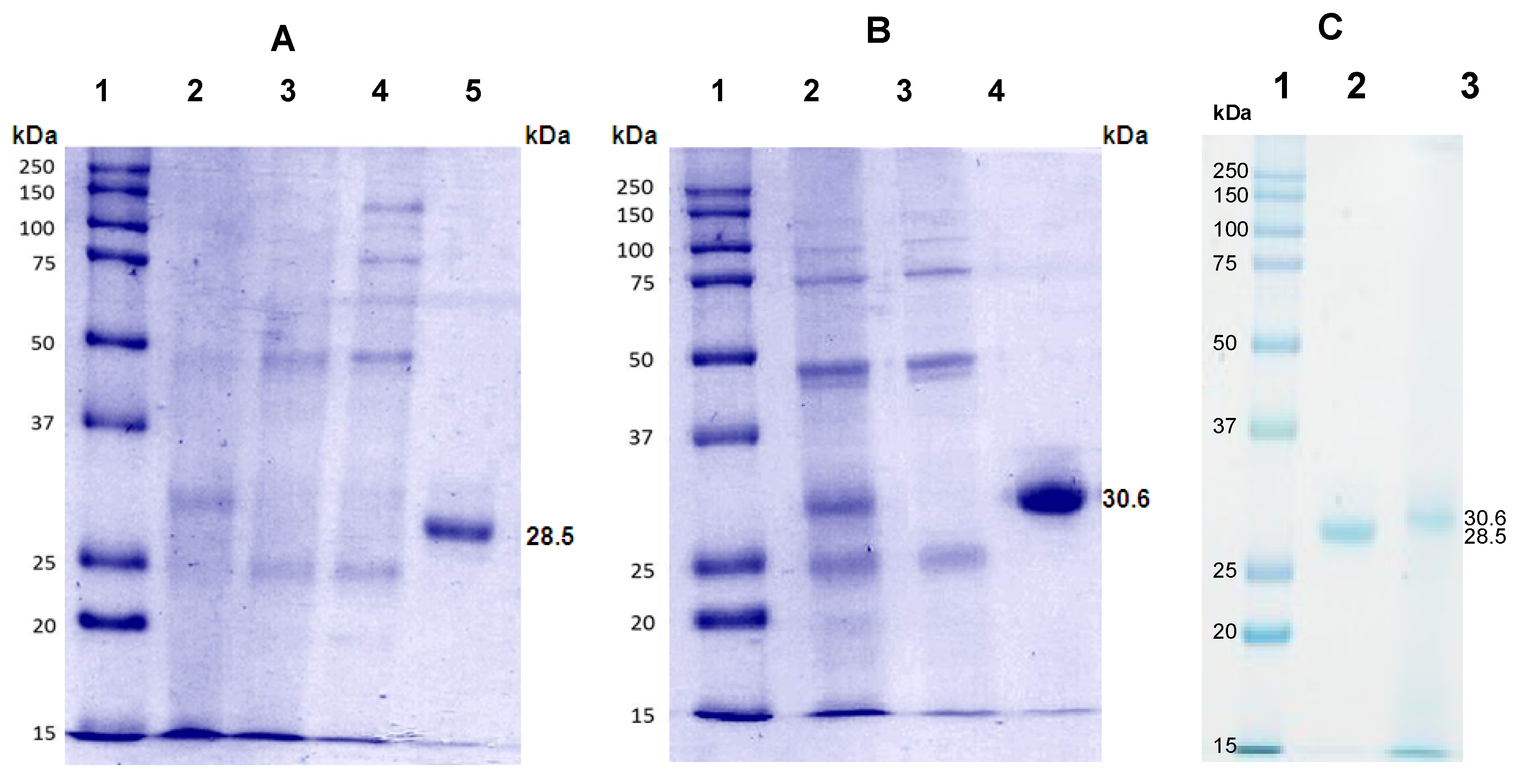
| Purification Step | Volume (mL) | Total Protein (mg) | Hemagglutination Activity | Purification Factor | Lectin (%) |
|---|---|---|---|---|---|
| ABBL | |||||
| Crude extract | 60.5 | 282.53 | 2192.72 | 1.00 | |
| Proteinic extract | 23.5 | 131.59 | 3618.37 | 1.65 | |
| Protein bound to fetuin (Lectin) | 26.8 | 1.12 | 65,437.14 | 29.84 | 0.39 |
| ABPL | |||||
| Crude extract | 60.5 | 284.59 | 1088.43 | 1.00 | |
| Proteinic extract | 23.0 | 152.33 | 3125.76 | 2.87 | |
| Protein bound to fetuin (Lectin) | 28.0 | 1.344 | 53,333.33 | 49.00 | 0.47 |
| Metal Ion (ppm) | Lectin | |||
|---|---|---|---|---|
| Black | Purple | |||
| Native | Demetallized | Native | Demetallized | |
| Ca+2 | 3528.3 | 2629.6 | 2375.4 | 1577.5 |
| Cu+2 | ND | ND | 176.1 | 97.3 |
| Fe+2 | 58.2 | 41.7 | 41.8 | 23.6 |
| Mg+2 | 1965.6 | 1581.3 | 1470.1 | 846.2 |
| Mn+2 | 168.6 | 35.1 | 98.5 | 19.3 |
| Zn+2 | 464.8 | 395.5 | 235.8 | 118.4 |
| Hemagglutination Activity | |
| ABBL | |
| Native | 65,437 |
| Demetallized | 8000 |
| Demetallized with metal ions addition | 64,000 |
| ABPL | |
| Native | 53,333 |
| Demetallized | 25,600 |
| Demetallized with metal ions addition | 51,200 |
| Hemagglutinating Activity | ||
| Monosaccharides | ABBL | ABPL |
| glucose | n.i. | n.i. |
| fructose | n.i. | n.i. |
| galactose | n.i. | n.i. |
| mannose | n.i. | n.i. |
| xylose | n.i. | n.i. |
| arabinose | n.i. | n.i. |
| ramnose | n.i. | n.i. |
| Disaccharides | ||
| maltose | n.i. | n.i. |
| trehalose | n.i. | n.i. |
| Trisaccharides | ||
| raffinose | n.i. | n.i. |
| Glycoproteins | ||
| ovalbumin | n.i. | n.i. |
| fetuin | * 0.0217 mg/mL | * 0.0217 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cruz, L.; Valadez-Vega, C.; Juárez-Goiz, J.M.S.; Flores-Martínez, N.L.; Montañez-Soto, J.L.; Bernardino-Nicanor, A. Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus coccineus (Ayocote Bean). Agronomy 2022, 12, 716. https://doi.org/10.3390/agronomy12030716
González-Cruz L, Valadez-Vega C, Juárez-Goiz JMS, Flores-Martínez NL, Montañez-Soto JL, Bernardino-Nicanor A. Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus coccineus (Ayocote Bean). Agronomy. 2022; 12(3):716. https://doi.org/10.3390/agronomy12030716
Chicago/Turabian StyleGonzález-Cruz, Leopoldo, Carmen Valadez-Vega, José Mayolo Simitrio Juárez-Goiz, Norma Leticia Flores-Martínez, José Luis Montañez-Soto, and Aurea Bernardino-Nicanor. 2022. "Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus coccineus (Ayocote Bean)" Agronomy 12, no. 3: 716. https://doi.org/10.3390/agronomy12030716
APA StyleGonzález-Cruz, L., Valadez-Vega, C., Juárez-Goiz, J. M. S., Flores-Martínez, N. L., Montañez-Soto, J. L., & Bernardino-Nicanor, A. (2022). Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus coccineus (Ayocote Bean). Agronomy, 12(3), 716. https://doi.org/10.3390/agronomy12030716








