Abstract
Tetracycline (TC) contamination has become hot research topic, but little attention has been paid to its ecotoxicological monitoring. The primary objective of this study is to investigate the impact of TC on human normal liver cells (HL-7702) and find indicators for monitoring their ecotoxicity. The cytotoxicity of TC, at concentrations ranging from 0 to 1000 μg L−1, was assessed on HL-7702 cells. The results showed that TC significantly inhibited the cell viability at a high concentration (1000 μg L−1). The TC at exposure levels ≥ 50–100 μg L−1 significantly increased the levels of extracellular catalase (CAT), malondialdehyde (MDA), alanine transaminase (ALT), and aspartate transaminase (AST), and a significantly positive correlation between the TC concentrations and the values of the above parameters was observed. Swelling of the mitochondrial cristae (MC) and rough endoplasmic reticulum (RER) and the loss of ribosomes in HL-7702 cells, were observed at high TC levels. There was a positive correlation between soil TC concentration and ALT activities. The above results suggest that TC is cytotoxic to HL-7702 cells and that extracellular ALT activities can be used as a sensitive bioindicator for monitoring soil TC contamination. We, therefore, propose that the HL-7702 cell line can be a novel tool for early antibiotics toxicity monitoring.
1. Introduction
Tetracycline (TC) is the most widely used antibiotic in many countries, for human or animal preventive treatment and livestock growth promoters [1,2,3,4]. However, it is normally excreted either unchanged (30–90%) or as other active metabolites after application, due to their incomplete metabolism in humans and livestock [5,6]. The TC disseminates into soils and surface waters [6,7,8], although TC concentrations are below the threshold values (100 μg kg−1) that cause ecotoxic effects in some environments [9], as set by the Steering Committee of the Veterinary International Committee on Harmonization [10]. The chronic exposure to low levels of antibiotics may exert pressure on the development of TC-resistant bacteria and have adverse effects on ecological and human health [8,11,12], which poses a potential threat to human health via the food chain [13,14,15] and is difficult to manage [16]. It is, therefore, imperative to assess the risks of TC on environmental safety and human health, to provide information for developing better mitigation strategies.
In recent years, the adsorption, migration and transformation of antibiotics in soil–water–plant systems have become a research hotspot, and there were many studies reported on the ecotoxicology of TC [8,17,18] and its resistance genes [4,12,19] on the environment. However, still there is a lack of information about an efficient method for diagnosing the toxicity of TC in soil. It has been reported that various indicators were used to identify the toxicants, such as heavy metals or drugs for health risk assessment [20,21,22,23,24]. Many in vitro toxicity indicators can be used as potential indicators to diagnose food, water and air [15,25,26] contamination. As a valuable tool, the determination of cell toxicity in vitro assays can play an important role in the ecological risk assessment of antibiotics [27,28], as these are simple, rapid, sensitive and cost efficient [28,29]. Therefore, the indicators of liver toxicity testing could serve as important biological indicators to assess the ecological safety and health risks of TC.
As a target organ, the liver has an important function, detoxifying toxins taken in from food, as well as exposures to other sources. However, excessive amounts of TC can cause liver injury, which is indicated by the traditional biomarkers, such as alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP); at the same time, the generation of excessive amounts of reactive oxygen species (ROS), following the emergence of some oxidative stress biomarkers, including malondialdehyde (MDA) and hydrogen peroxide (H2O2), which is an underlying cause of liver injury [15], could serve as potential indicators for evaluating the health risks of TC. In addition, the human normal liver cell line HL-7702 is used to establish models of liver injury in vitro and evaluate liver toxicity [15,30]; however, the indicators by which TC affects liver injury and oxidative stress are still unknown. Thus, the objective of this study was to identify sensitive indicators in human liver cells in vitro to diagnose TC contamination in farm soil.
2. Materials and Methods
2.1. Cell Culture and Treatments
Human normal liver cell line HL-7702 was purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). Normally, HL-7702 cells were cultured in 25 cm2 flasks (Corning Inc., Corning, NY, USA) that contained Dulbecco’s modified Eagle medium (Gibco, Grand Island, NY, USA) and approximately 15% (v/v) fetal bovine serum (Gibco, Grand Island, NY, USA). Then, the cells were cultured in an incubator (Heraeus, BB15, Hanau, Germany) maintaining 5% CO2, 95% relative humidity and 37 °C air temperature. Phosphate-buffered saline was used to remove the unattached cells, and the cells were subcultured using the 0.25% trypsin–EDTA (Gibco, Grand Island, NY, USA). The culture medium was changed 3–4 times every week. After being 80% confluent, the cells were used for further studies. For cytotoxicity assays, cells at a density of 2 × 105 cells per mL were uniformly seeded in 96-, 24- or 6-well plates (Corning Inc., Corning, NY, USA) in the logarithmic growth phase. After plating for 24 h, HL-7702 cells were exposed to known gradient concentrations of TC (Sigma-Aldrich, St. Louis, MO, USA) (0 (control), 25, 50, 100, 250, 500 and 1000 μg L−1) in the culture medium and incubated for 4 h. Relevant experiments were conducted for markers of cell toxicity determination. The generations of extracellular lactate dehydrogenase (LDH), ALT, AST, ALP, catalase (CAT), H2O2 and MDA were determined by using commercially available assay kits according to the manufacturer protocol (Jiancheng Biochemical Co., Ltd., Nanjing, China). TC reagents were prepared with phosphate-buffered saline solution. All laboratory appliances were sterilized in the experiments.
2.2. Cell Toxicity
2.2.1. Cell Viability
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was performed according to the method of Liu et al. (2019) to evaluate the viability of HL-7702 cells that were exposed to various concentrations of TC.
After HL-7702 cells were exposed to gradient concentrations of TC for 4 h in the 24-well plates, the supernatant of the cell culture medium was collected for the following experiments.
LDH release: LDH activity was determined with a commercially available kit according to the method of Liu et al. (2019). The relative LDH release was calculated by the following equation:
where ‘S’ refers to the reference standard concentration (2 mmol L−1) and ‘N’ refers to the dilute times of samples.
2.2.2. Liver Enzyme Assay
The ALT and AST activities were determined by the method of Liu et al. (2019). ALT and AST values (% of control) were calculated by the ratio of ALT/AST activities of treatment to those of the control group.
The principle of the ALP assay is that the color change is tested when the para-nitrophenol phosphate (colorless) is converted to para-nitrophenol and phosphate (yellow). ALP absorbance at 520 nm was determined with a spectrophotometer (Lambda 35UV-vis, PerkiElmer, Singapore city, Singapore). The ALP value (% of control) was calculated as the ratio of the ALP activity of treatment to that of control.
2.2.3. Oxidative Stress Assays
The CAT assay is based on the hydrolysis of H2O2 by CAT. To calculate the activity of CAT in the supernatant, the OD1 and OD2 values were measured at once and the following 1 min after adding the last reagent, respectively, at a wavelength of 240 nm using a spectrophotometer (Lambda 35UV-vis, PerkiElmer, Singapore city, Singapore). The CAT value was calculated using the following equation:
The H2O2 and MDA contents were determined by the method of Liu et al. (2019), and their contents (% of control) were calculated by the ratio of H2O2/MDA contents in the treatment to those in the control.
2.3. Transmission Electron Microscopy (TEM)
To investigate the morphological changes in HL-7702 cells exposed to TC, the cells were seeded in a 6-well plate following TC treatment (0 (control) and 1000 μg L−1); after 4 h, the samples were harvested, an ultrastructural observation was performed according to the method of Liu et al. (2019). According to the above method, the cells were seeded in a 6-well plate following TC treatment (0 (control) and 1000 μg L−1); after 4 h, the samples were harvested, simultaneously fixed in 2.5% (v/v) glutaraldehyde overnight at 4 °C, and then rinsed 3 times with 0.1 M phosphate-buffered saline (pH = 7.0) and post-fixed for 1 h in 1% (v/v) osmium tetroxide. Sample dehydration was performed by treating with a graded ethanol series (50–100%) and finally with the absolute acetone. Then, the samples were rinsed and impregnated with a 1:3 (v/v) mixture of acetone and Spurr epoxy resin overnight. For ultrastructural observations, the ultrathin sections were prepared and placed on copper grids, and the TEM (JEM-1230, JEOL, Tokyo, Japan) was used to view the cell ultrastructure.
2.4. Soil TC Diagnosis Experiment
Soil antibiotic extraction (water extraction): Two typical soil types, paddy soil (Hangzhou, Zhejiang) and black soil (Harbin, Heilongjiang), were selected to perform the assay. The external tetracycline 0, 0.5 mg and 5 mg were added to 1 kg soil, 5 g of soil samples, were weighed, then distilled water:soil sample = 5:1, extracted overnight, centrifuged at 6000 r/min for 15–20 min. After passing through 0.22 μm filter membrane, the soil TC extraction was obtained and then DMEM culture medium was diluted for cytotoxicity assay.
The soil TC extraction was used for the cytotoxicity assay as performed above. Human cells HL-7702 were inoculated in 24-well cell culture plates at a density of 2 × 105 cells/mL during the logarithmic growth phase. 24 h after incubation, HL-7702 cells were added to 1000 μL of soil tetracycline extract diluted 100-fold in serum-free DMEM culture medium, and the plates were incubated in a constant temperature incubator at 37 °C and 5% CO2. Three biological replicates were set for each treatment, and the cell cultures were taken for experiments after 4 h of treatment, centrifuged at 1000 r/min for 5 min, and then the ALT activity was detected by the kit.
2.5. Statistical Analysis
All results are presented as the mean ± standard error (SE) using the SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Significant differences between control and exposed treated cells (n = 3) were analyzed by using Student’s t-test and one-way analysis of variance after the data were confirmed to be normal distribution. Kruskal–Wallis tests followed by Mann–Whitney U tests were operated for non-normal distributed data. Statistically significant differences between control cells and those exposed to TC were determined using analysis of variance (ANOVA) with Duncan’s post hoc test.
3. Results
3.1. Effects of TC on Cell Viability (MTT) and Stability (LDH Release) in HL-7702 Cells
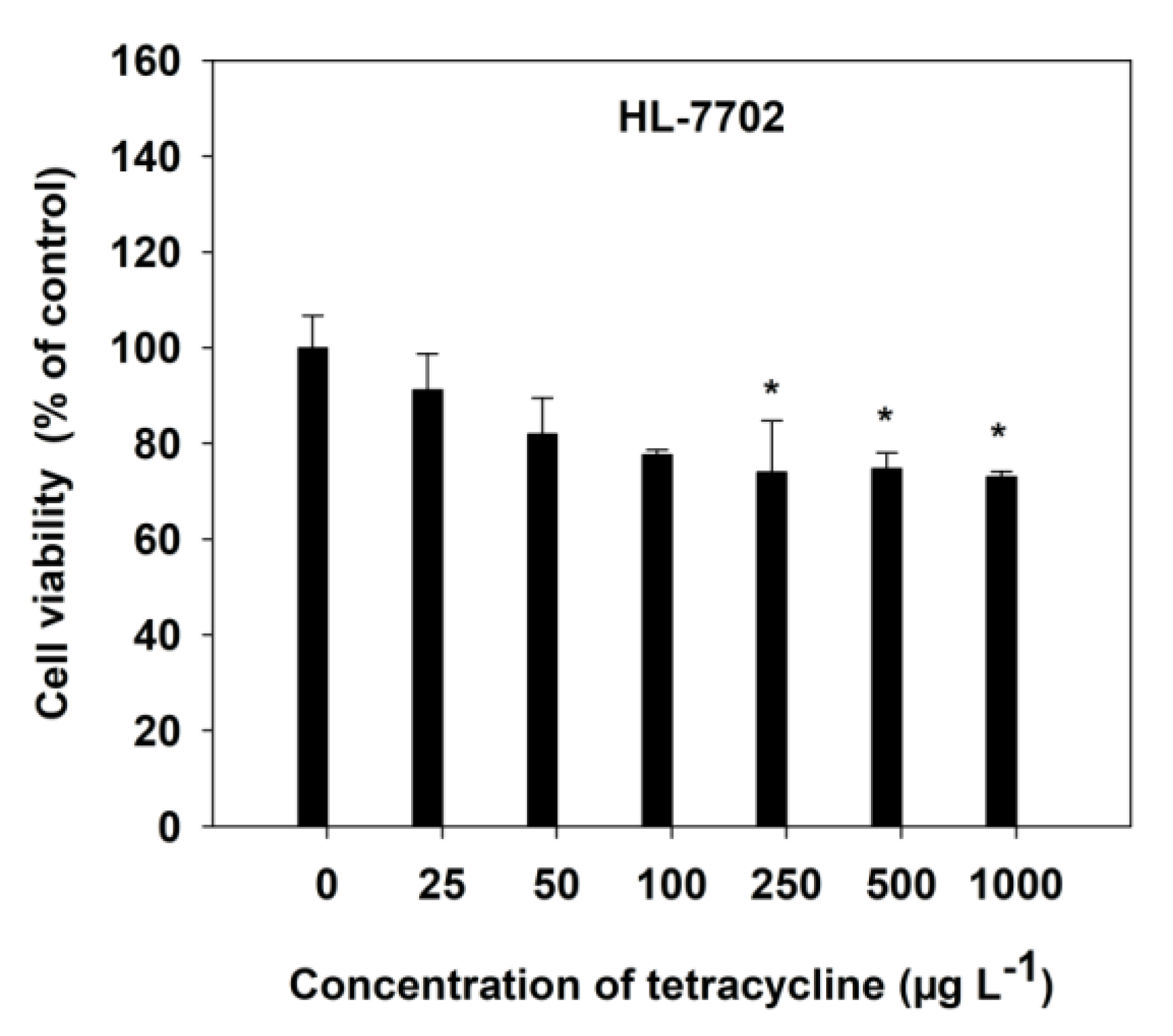
The viability of HL-7702 cells, with increasing TC concentrations, was measured by MTT and LDH release assays, after a 4-h incubation. The dose-dependent toxicity of TC was observed in HL-7702 cells (Figure 1). Generally, a decrease in cell viability was observed, at all varying concentrations of TC, as compared with the respective control. However, a significant (p < 0.05) decrease was observed at 250 μg L−1 of TC (Figure 1). At high concentrations (1000 μg L−1) of TC, the cell viability was found to decrease by 26.94%, compared with the control cells (Figure 1).
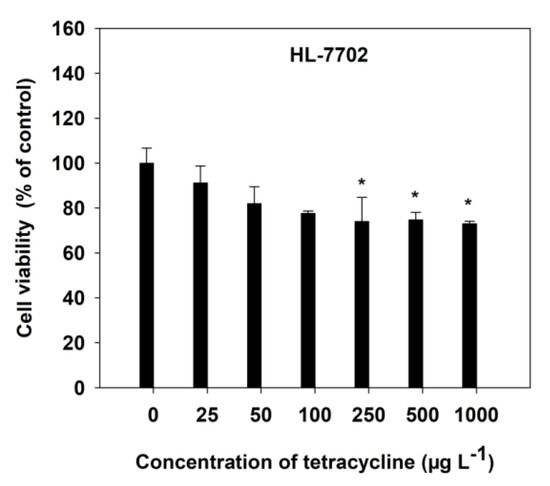
Figure 1.
Effect of TC on cell viability in HL-7702 cells after 4-h incubation. Data are given as mean ± S.E. (n = 6); “*” indicate significant (p < 0.05) differences between controls (0 μg L−1) and treatments.
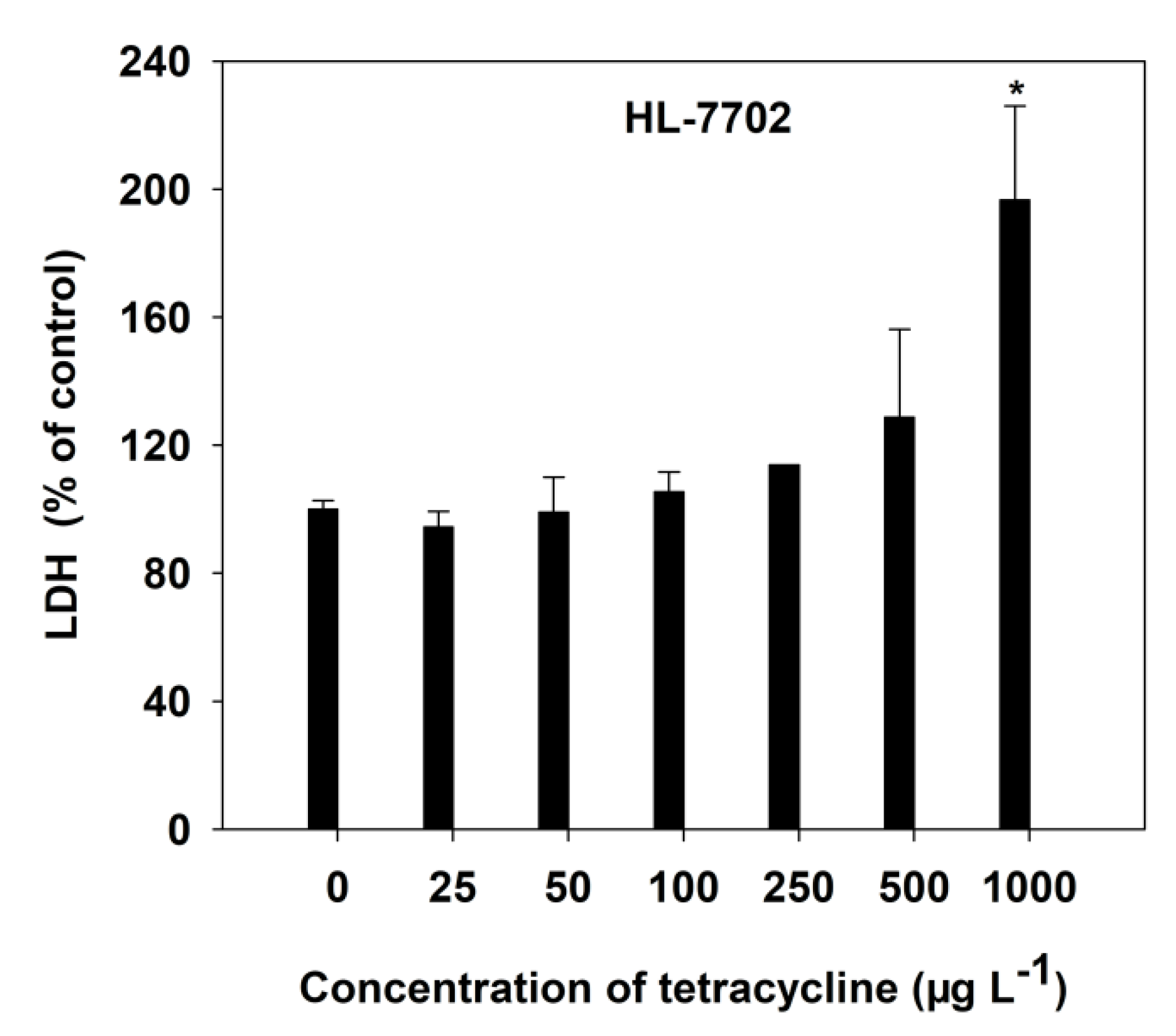
The HL-7702 cell membrane stability, with increasing concentrations of TC, was determined by LDH release into the culture medium (Figure 2). At low TC levels, there was no significant (p > 0.05) LDH release of HL-7702 cells to the culture medium observed. However, TC at the 1000 μg L−1 level significantly (p < 0.05) increased LDH leakage by 96.58%, compared with the control cells (Figure 2). It was found that TC could induce injury to liver cell membranes.
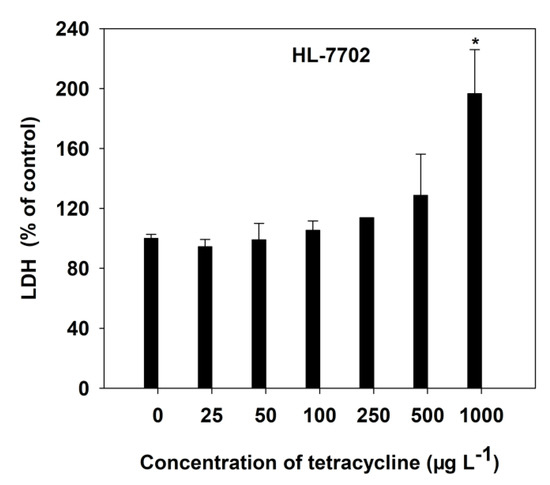
Figure 2.
Effect of TC on LDH release in HL-7702 cells after 4-h incubation. Data are given as mean ± S.E. (n = 3); “*” indicate significant (p < 0.05) differences between controls (0 μg L−1) and treatments.
The regression relationship among cell viability, LDH activity and the concentration of TC is shown in Table 1. Although no significant regression coefficient was found between the values of cell viability and the concentrations of TC at 0–1000 μg L−1, high significant positive linear correlations (p < 0.01) were observed between the LDH activities and the TC concentrations.

Table 1.
Regression equations between cell viability; activities of lactate dehydrogenase (LDH), alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and catalase (CAT); contents of hydrogen peroxide (H2O2) and malondialdehyde (MDA); the concentrations of added tetracycline (TC) in the normal human liver cell line HL-7702.
3.2. Effects of TC on Liver Function (AST, ALP, ALP)
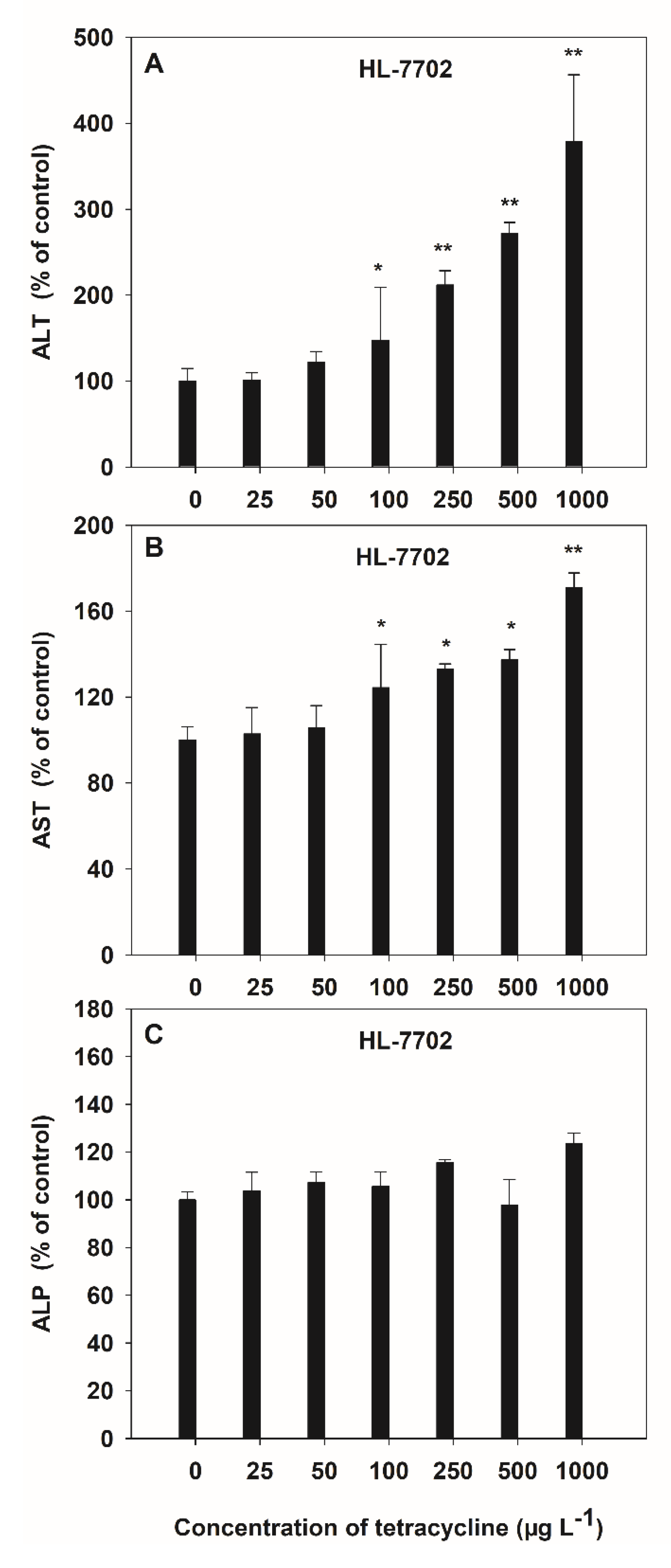
Alanine transaminase, AST and ALP are three liver enzymes damaged by antibiotics and can represent liver function in humans. The toxicity of HL-7702 cells exposed to TC was observed by the activities of ALT and AST, which increased in a dose-dependent manner, and the average increases were 1.05-fold and 0.29-fold over the control values, respectively (Figure 3A,B). However, no significant variation was observed in ALP activity induced by TC (Figure 3C). The activities of ALT and AST were varied at the low dose (100 μg L−1) of TC, while the AST activities were similar for 500 μg L−1 and 1000 μg L−1 of TC. The maximum increase in the activities of ALT (3.78 folds) and AST (1.71 folds) due to TC was observed at 1000 μg L−1 as compared to control.
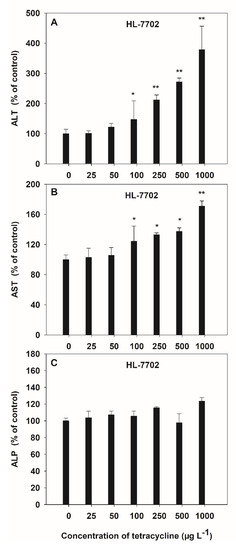
Figure 3.
Effects of TC on the activities of ALT (A), AST (B) and ALP (C) in HL-7702 cells after 4-h incubation. Results are expressed as mean ± S.E. (n = 3); “*” and “**” indicate significant (p < 0.05) and highly significant (p < 0.01) differences between controls and treatments, respectively.
Highly significant positive linear correlations (p < 0.01) between the activities of liver function enzymes (ALT and AST) and the TC concentrations, at 0–1000 μg L−1, was observed, and the correlation coefficients descended as ALT > AST > ALP (Table 1).
3.3. TC-Induced Oxidative Stress in HL-7702 Cells
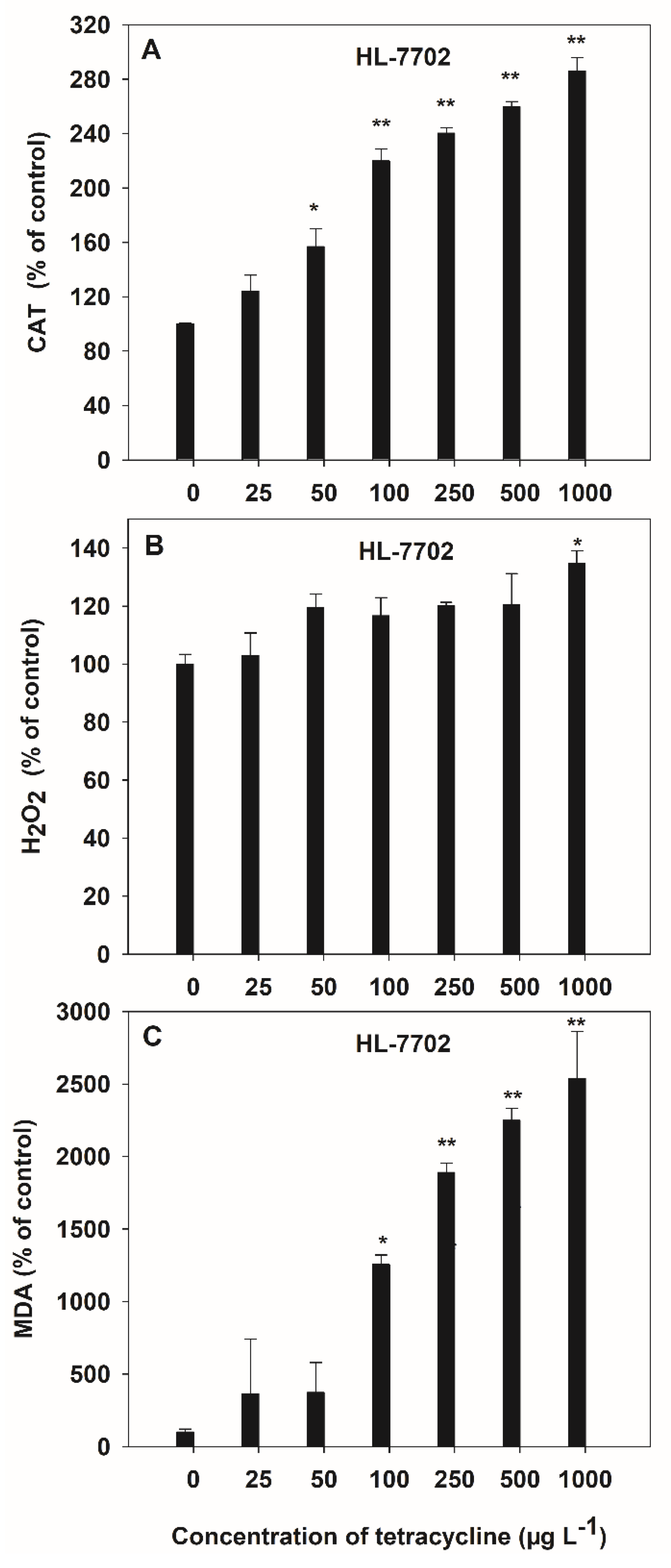
The activities of antioxidant (CAT), oxidant (H2O2 and MDA) enzymes in the culture medium were measured to determine the oxidative stress in HL-7702 cells due to TC contamination (Figure 4). A significant (p < 0.05) increase was observed in CAT activity (2.14-fold) at 50 μg L−1 of TC, as compared to control in HL-7702 cells. H2O2 and MDA contents were significantly (p < 0.05) increased, by 1.19- and 14.45-fold, respectively, at 1000 μg L−1 and 100 μg L−1 of TC, as compared to control in the HL-7702 cell. Extracellular CAT activity, which can maintain the TC tolerance of the cells, significantly increased at 50 μg L−1 of TC (Figure 4A), whereas MDA increased significantly (p < 0.05) at 100 μg L−1 of TC in HL-7702 cells (Figure 4C). The maximum increase by TC was up to 2.86-fold (for CAT) and 25.38-fold (for MDA) over the control at 1000 μg L−1, respectively. However, only high concentrations (1000 μg L−1) of TC induced a significant (p < 0.05) increase in H2O2 content in HL-7702 cells (Figure 4B).
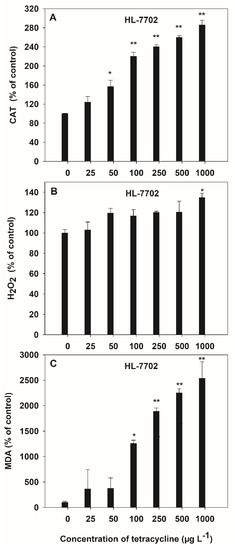
Figure 4.
Effect of TC on the activities of CAT (A), H2O2 (B) and MDA (C) contents in HL-7702 cells after 4-h incubation. Data are given as mean ± S.E. (n = 3); “*” and “**” indicate significant (p < 0.05) and highly significant (p < 0.01) differences between controls and treatments, respectively.
Significant positive linear correlations (p < 0.05) were observed between the activities of the antioxidant (CAT) and oxidant (H2O2 and MDA) enzyme, at the TC exposure of 0–1000 μg L−1 (Table 1). The order of correlation coefficients, according to the regression equations, was MDA > H2O2 > CAT for TC, at concentrations from 0 to 1000 μg L−1.
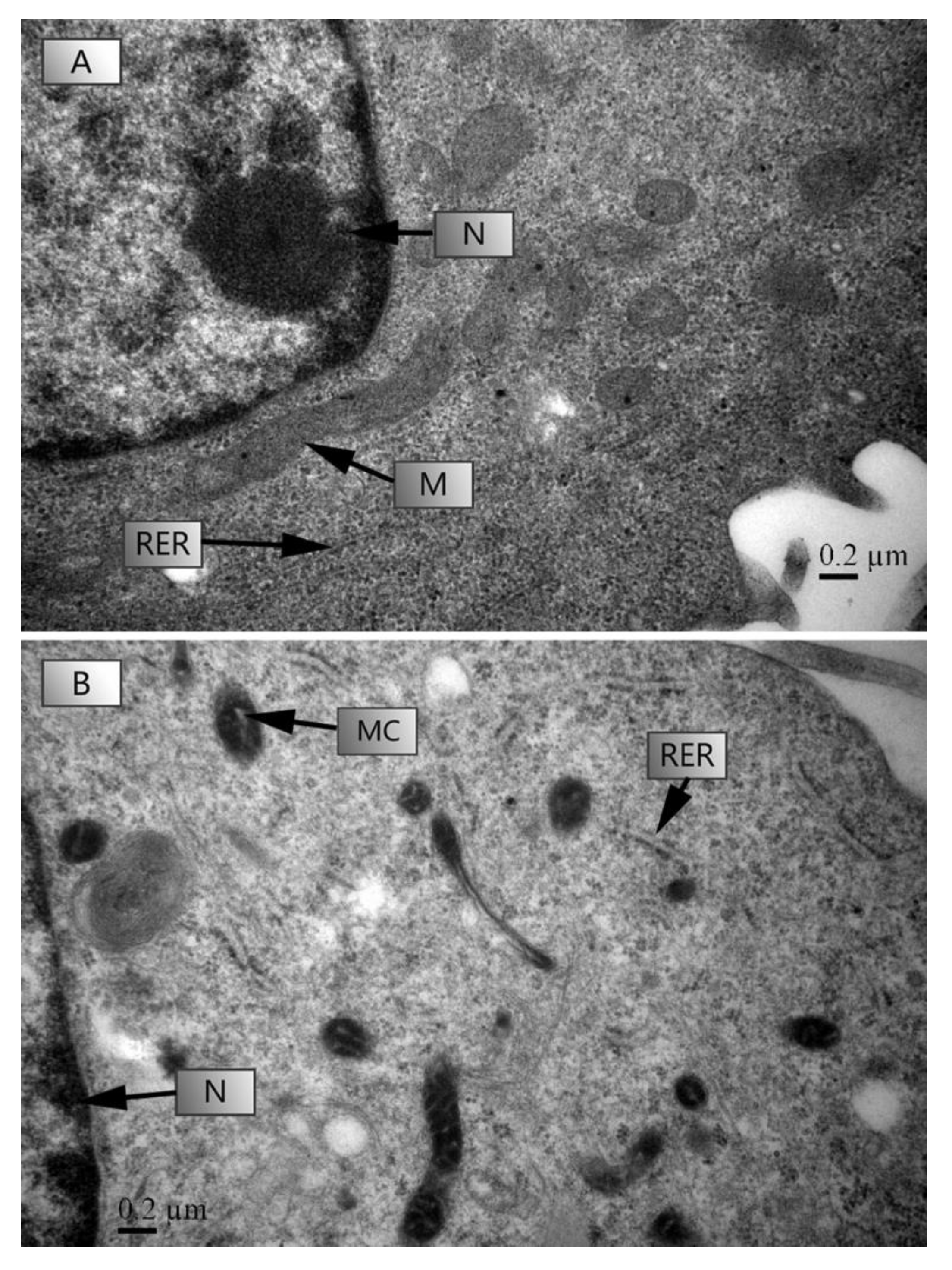
The TEM microscopic photographs of HL-7702 cells under a control (0 μg L−1) and 1000 μg L−1 stress are shown in Figure 5. After 4 h of culture, TC-induced ultrastructural damage of HL-7702 cells was indicated by changes on the rough endoplasmic reticulum (RER) and mitochondrial cristae (MC) (Figure 5B). HL-7702 cells showed swelling in MC and RER and a loss in the ribosomes in RER at 1000 μg L−1 of TC (Figure 5B).
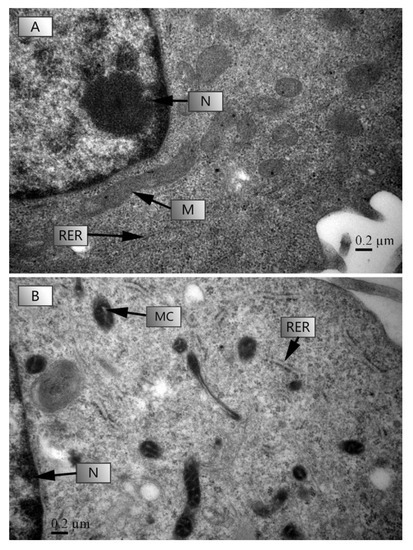
Figure 5.
The effects of TC on the ultrastructural changes in HL-7707 cells. Note: (A): tetracycline 0 μg L−1; (B): tetracycline 1000 μg L−1; Arrows: N: nucleus; RER: rough endoplasmic reticulum; M: mitochondria; MC: mitochondrial cristae. Six to seven images were taken to get a representative image.
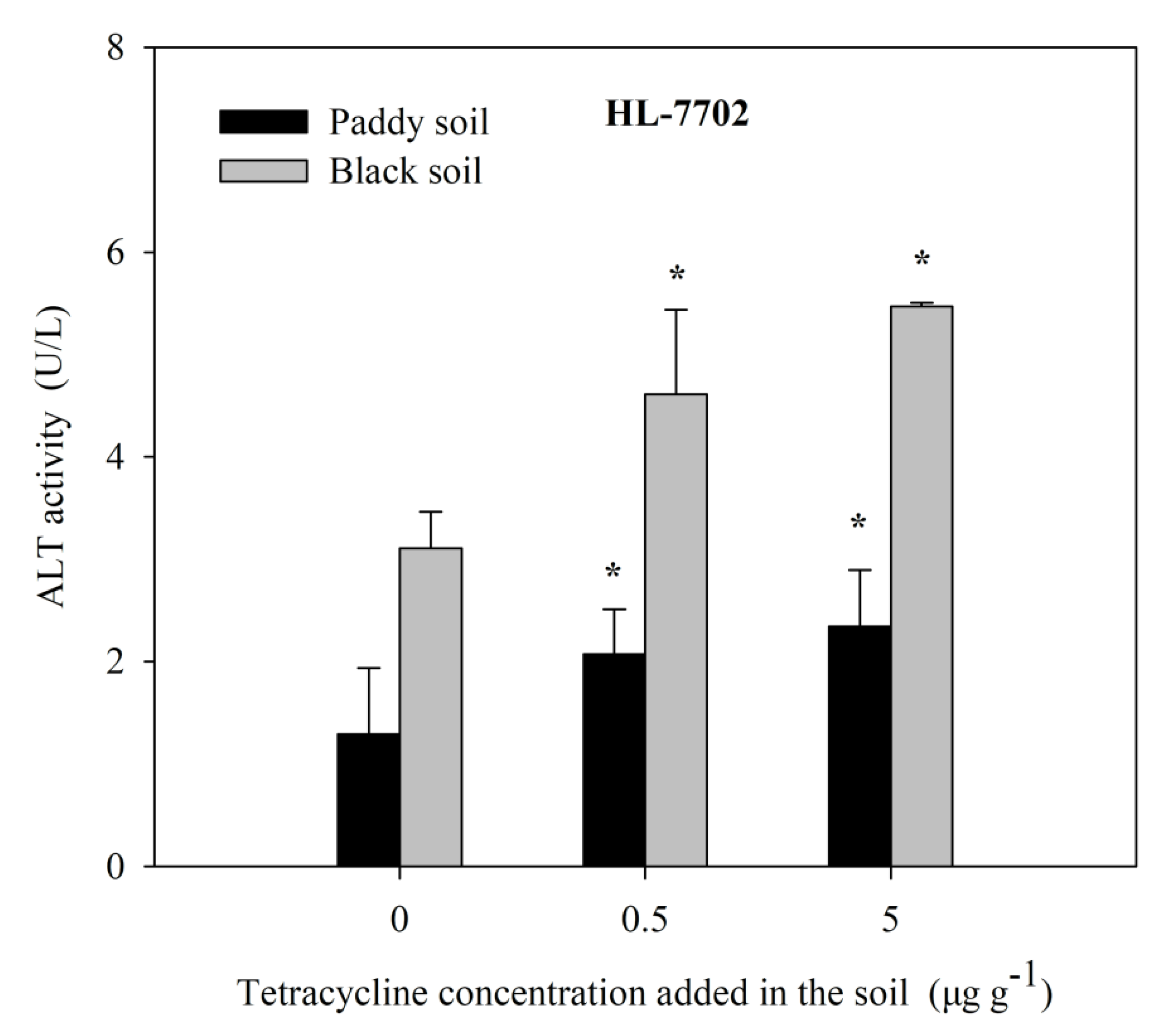
Farm paddy soil and black soil were selected and exogenously added, with different concentrations of TC, for aqueous solution extraction, and human cells HL-7702 were treated by TC solution extraction for 4 h. The activity of ALT, a cytotoxicity index obtained by screening, was detected, as shown in Figure 6. With the increase in exogenous tetracycline concentration, the soil solution treated with human cells HL-7702 can lead to a gradual increase in ALT activity, indicating that human cells HL-7702 can achieve a preliminary diagnosis of antibiotic contamination in the soil solution.
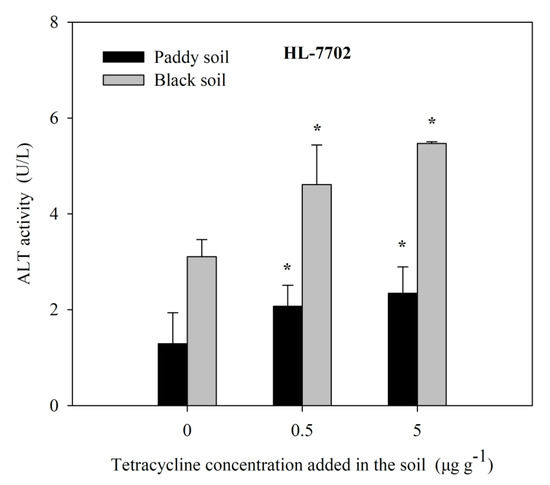
Figure 6.
The effects of soil TC on the ALT activity in HL-7707 cells. Data are given as mean ± S.E. (n = 3); “*” indicate significant (p < 0.05) differences between controls (0 μg L−1 TC) and treatments.
4. Discussion
Antibiotics, particularly TC, threatened the environmental safety of soils, crops and water [6,7,8,17], and have posed potential risks to human health, such as hepatotoxicity [31,32]. The liver is a vital organ that not only performs functions, such as plasma protein synthesis, but also works as a target organ for the metabolism and detoxification of drugs [33]. However, it is complex to study antibiotic hepatotoxicity mechanisms in vivo [27]. Hence, a cell line model can be a useful tool for conducting cell toxicity pre-research, because there is a relationship between in vitro and in vivo cell toxicity [27,34], which provides a theoretical basis for understanding the human hepatotoxicity. Moreover, it can also be a valuable tool for the ecological risk assessment of antibiotics [28]. Thus, establishing an appropriate in vitro cell line model is necessary to predict the relative risk posed by antibiotics in humans and understand their ecotoxicity in the environment.
The present study revealed that the HL-7702 cells were sensitive to TC toxicity, as indicated by the MTT assay, LDH release, liver function test (ALT, AST, ALP), and oxidative stress (CAT, MDA, H2O2) assay, when the cells were exposed to the different concentrations of TC treatments (Figure 1, Figure 2, Figure 3 and Figure 4). The cytotoxicity assays showed that after 4 h of exposure to increasing concentrations of TC in the culture medium, there was a significant decrease in cell viability (250 μg L−1) and an increase in LDH release at 1000 μg L−1, which were noted (Figure 1 and Figure 2). This evidence suggests that TC destroys membrane integrity, which is consistent with the report by Asha (2007) [35]. Clinically, abnormal liver function is usually tested by assessing serum liver enzyme (ALT, AST and ALP) activities, which are specifically used for liver function indicators. Elevated serum ALT and ALP activities have been attributed to hepatocellular damage and cholestasis, respectively [36,37]. In the present study, the increased activities of ALT and AST were observed in the HL-7702 cells upon exposure to TC (Figure 3A,B), compared with the control, as reported by Asha (2007) [35]. TC concentrations above 100 μg L−1, significantly induced liver cell toxicity. No changes in ALP activities indicated that the liver toxicity was not cholestatic (Figure 3C). These results supported that the category of liver injury might be merely hepatocellular, rather than cholestatic.
Tetracycline can cause oxidative stress in the normal human liver, usually by elevating the levels of lipid peroxide or reducing the levels of antioxidant enzymes [35]. As main products of oxidative stress, ROS can cause lipid oxidation and protein damage [38]. In the present study, the activities of CAT and the contents of H2O2 and MDA significantly increased under high TC exposure (Figure 4). This finding implies that the TC can cause oxidative stress in liver cells. Interestingly, the levels of MDA were elevated after TC exposure, while the high antioxidant enzyme CAT was induced. It is possible that the generation of ROS exceeded the capability of free radical scavengers (such as superoxide dismutase and CAT) during the administration of oxidative damage in cells. Then, the ultrastructural changes of HL-7702 cells were observed by TEM, after treatments with TC (Figure 5), to deeply study the mechanism of the cytotoxicity. HL-7702 cells after 4 h of incubation with TC exhibited both MC and RER swelling and ribosome loss (Figure 5B). These results were in agreement with Shen’s research [27]. ROS increase could result in the MC, RER and ribosome damage, because the mitochondria are implicated as an important source of ROS in most mammalian cells [39,40]; RER and ribosomes are involved in protein synthesis, which can also be attacked by ROS [41]. In the present study, TC cytotoxicity might be induced by the imbalance between the production of mitochondrial ROS and a mitochondrial radical detoxification system, or an inhibition of protein synthesis in HL-7702 cells, and Mitochondria and RER might be the target organelles of TC toxicity in liver cells. These results partially reflect the cellular toxic mechanism of TC in liver cells in vitro. Thus, the risk assessment of TC to human liver cells in vitro is necessary, to further study the mechanisms involved in the toxicity of TC to humans and animals.
Our objective was to find accurate, faster and more sensitive bioindicators of TC toxicity from the liver cytotoxicity bioassay. As shown in Figure 1, Figure 2, Figure 3 and Figure 4 and Table 1, there was no relationship between cell viability and ALP activity and the TC concentrations, and in addition, no significant increase in cell viability, LDH activity and H2O2 content at low TC concentrations was found. Considering the average increase, correlation coefficients, p value and the threshold levels that cause ecotoxic effects (≤100 μg kg−1), the cell viability, LDH release, ALP and H2O2 are not highly sensitive to TC exposure, while there was a significant positive linear relationship between the values of ALT, AST, CAT and MDA and the TC’s exposure levels. Additionally, ALT activity was most increased at low TC concentrations (≤100 μg L−1). This evidence implies that ALT was the most sensitive to TC toxicity in HL-7702 cells. Therefore, we chose ALT as an indicator to diagnose TC toxicity in the soil, and found that, with the tetracycline concentration increase, the soil solution treated with human cells HL-7702 can lead to a gradual increase in ALT activity (Figure 6), and according to our previous study, there was higher correlation between soil TC concentration and ALT activities [42], so human cells HL-7702 can achieve a preliminary diagnosis of TC contamination in the soil solution. Different from previous studies, the indicators in the liver cells for testing threshold concentrations of TC to induce human liver cytotoxicity were studied. Although we found another antibiotic, such as penicillin, has similar cytotoxicity as TC, the most sensitive indicator was not ALT activity in liver cells (data not shown), and further studies are required to explore the TC-specific indicator. Therefore, valuable information can be provided by the data from these indicators, for diagnosing the early effects of exposure to low concentrations of TC in the environment.
5. Conclusions
The in vitro study revealed that the cytotoxicity of TC to the HL-7702 cell line was mainly due to the impairment of liver function, oxidative stress, mitochondrial and rough endoplasmic reticulum lesions, in liver cells that were exposed to the TC, at concentrations ranging from 0 to 1000 μg L−1. In addition, high correlation between soil TC concentration and ALT activities was observed. The results also suggest that the liver cytotoxicity bioassay can serve as an efficient tool for monitoring and forecasting TC contamination. Specifically, extracellular ALT of HL-7702 cells can be used as indicators in ecotoxicological tests of TC pollution. Thus, the preliminary results, obtained in the present study, facilitate the possible development of in vitro models of human cell toxicity, as a rapid, sensitive and practical tool for assessing antibiotic pollutants in real environments.
6. Patents
Patent 1: Yang, X.E.; Liu, D.; Lu, L.L.; Feng, Y.; Li, T.Q. A Diagnostic Method of Soil Antibiotic Pollution. CN201410764095.4, 24 August 2016. (In Chinese).
Author Contributions
Conceptualization, X.Y. and L.L.; Methodology, Y.F., T.L. and R.A.; Software, M.T. and Z.W.; Validation, S.C.; Formal analysis, D.L.; Investigation, S.T.; Resources, D.L.; Data curation, Y.W.; Writing—original draft preparation, D.L.; Writing—review and editing, R.A., M.J.I.S. and M.W.; Supervision, X.Y.; Project administration, X.Y. and L.L.; Funding acquisition, X.Y., L.L. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 41977130 and No. 31872956) and Jiangxi Key Laboratory of Industrial Ecological Simulation and Environmental Health in Yangtze River Basin (No. JJ2021005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data obtained in this study are mentioned in the main text, further data will be provided on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moulin, G.; Cavalié, P.; Pellanne, I.; Chevance, A.; Laval, A.; Millemann, Y.; Colin, P.; Chauvin, C. A comparison of antimicrobial usage in human and veterinary medicine in France from 1999 to 2005. J. Antimicrob. Chemother. 2008, 62, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Mathers, J.J.; Flick, S.C.; Cox, L.A.J. Longer-duration uses of tetracyclines and penicillins in U.S. food-producing animals: Indications and microbiologic effects. Environ. Int. 2011, 37, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Hvistendahl, M. China takes aim at rampant antibiotic resistance. Science 2012, 336, 795. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, W.; Xu, W. Effects of tetracycline antibiotics in chicken manure on soil microbes and antibiotic resistance genes (ARGs). Environ. Geochem. Health 2022, 44, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, W.K. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, P.A.; Kay, P.; Boxall, B.A. The dissipation and transport of veterinary antibiotics in a sandy loam soil. Chemosphere 2007, 67, 292–299. [Google Scholar] [CrossRef]
- Murray, A.K.; Stanton, I.; Gaze, W.H.; Snape, J. Dawning of a new era: Environmental risk assessment of antibiotics and their potential to select for antimicrobial resistance. Water Res. 2021, 200, 117233. [Google Scholar] [CrossRef]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and accumulative pattern of TCs and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef]
- Li, Y.W.; Wu, X.L.; Mo, C.H.; Tai, Y.P.; Huang, X.P.; Xiang, L. Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta area, southern China. J. Agric. Food Chem. 2011, 59, 7268–7276. [Google Scholar] [CrossRef]
- Kim, S.; Aga, D.S. Potential ecological and human health impacts of antibiotics and antibiotic-resistant bacteria from wastewater treatment plants. J. Toxicol. Environ. Health. Part B 2007, 10, 559–573. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [Green Version]
- Boonsaner, M.; Hawker, D.W. Evaluation of food chain transfer of the antibiotic oxytetracycline and human risk assessment. Chemosphere 2013, 93, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Micallef, S.A.; Rosenberg, G.R.E.; George, A.; Ewing, L.; Tall, B.D.; Boyer, M.S.; Joseph, S.W.; Sapkota, A.R. Diversity, distribution and antibiotic resistance of Enterococcus spp. recovered from tomatoes, leaves, water and soil on U.S. Mid-Atlantic farms. Food Microbiol. 2013, 36, 465–474. [Google Scholar] [CrossRef]
- Liu, D.; Lu, L.; Wang, M.; Hussain, B.; Tian, S.; Luo, W.; Zhou, J.; Yang, X. Tetracycline uptake by pak choi grown on contaminated soils and its toxicity in human liver cell line HL-7702. Environ. Pollut. 2019, 253, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Prematta, T.; Shah, S.; Ishmael, F.T. Physician approaches to beta-lactam use in patients with penicillin hypersensitivity. Allergy. Asthma. Proc. 2012, 33, 145–151. [Google Scholar] [CrossRef]
- Xie, X.J.; Zhou, Q.X.; Lin, D.S.; Guo, J.M.; Bao, Y.Y. Toxic effect of tetracycline exposure on growth, antioxidative and genetic indices of wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2011, 18, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.X.; Gao, J.; Xie, X.J.; Zhou, Q.X. DNA damage and biochemical toxicity of antibiotics in soil on the earthworm Eisenia fetida. Chemosphere 2012, 89, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Shen, Q.H.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.L.; Wu, M.H. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai, China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kho, Y.L.; Park, Y.; Choi, K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants. Environ. Res. 2010, 110, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Dudka, I.; Kossowska, B.; Senhadri, H.; Latajka, R.; Hajek, J.; Andrzejak, R.; Antonowicz-Juchniewicz, J.; Gancarz, R. Metabonomic analysis of serum of workers occupationally exposed to arsenic, cadmium and lead for biomarker research: A preliminary study. Environ. Int. 2014, 68, 71–81. [Google Scholar] [CrossRef]
- Espín, S.; Martínez-López, E.; Jiménez, P.; María-Mojica, P.; García-Fernández, A.J. Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus). Environ. Res. 2014, 129, 59–68. [Google Scholar] [CrossRef]
- Tonomura, Y.; Kato, Y.; Hanafusa, H.; Morikawa, Y.; Matsuyama, K.; Uehara, T.; Ueno, M.; Torii, M. Diagnostic and predictive performance and standardized threshold of traditional biomarkers for drug-induced liver injury in rats. J. Appl. Toxicol. 2015, 35, 165–172. [Google Scholar] [CrossRef]
- Wei, K.Q.; Yang, J.X. Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish Procambarus clarkia. Ecotoxicol. Environ. Saf. 2015, 113, 446–453. [Google Scholar] [CrossRef]
- Eisenbrand, G.; Pool-Zobel, B.P.; Baker, V.; Balls, M.; Blaauboer, B.J.; Boobis, A.; Carere, A.; Kevekordes, S.; Lhuguenot, J.C.; Pieters, R.; et al. Methods of in vitro toxicology. Food Chem. Toxicol. 2002, 40, 193–236. [Google Scholar] [CrossRef]
- Bousmaha-Marroki, L.; Boutillier, D.; Marroki, A.; Grangette, C. In vitro anti-staphylococcal and anti-inflammatory abilities of lacticaseibacillus rhamnosus from infant gut microbiota as potential probiotic against infectious women mastitis. Probiotics Antimicro. 2021, 13, 970–981. [Google Scholar] [CrossRef]
- Shen, C.; Meng, Q.; Schmelzer, E.; Bader, A. Gel entrapment culture of rat hepatocytes for investigation of tetracycline-induced toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 178–187. [Google Scholar] [CrossRef]
- Mater, N.; Geret, F.; Castillo, L.; Faucet-Marquis, V.; Albasi, C.; Pfohl-Leszkowicz, A. In vitro tests aiding ecological risk assessment of ciprofloxacin, tamoxifen and cyclophosphamide in range of concentrations released in hospital wastewater and surface water. Environ. Int. 2014, 63, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.; Russell, T. The Status of Alternative Methods in Toxicology; Royal Society of Chemistry: Cambridge, UK, 1995. [Google Scholar]
- Wang, L.; Liu, H.F.; Cai, Z.W.; Gan, Y.Q.; Han, C.Q. The effects of NDP and DDP on liver and kidney cells injure. Guangdong Med. J. 2012, 33, 736–738. (In Chinese) [Google Scholar]
- Leitner, J.M.; Graninger, W.; Thalhammer, F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical data. Infection 2010, 38, 3–11. [Google Scholar] [CrossRef]
- Simon, V.V.S.K.; Sasikumar, R.; Kanthlal, S.K. In vitro protective effect of ascorbic acid against antibiotic-induced hepatotoxicity. Curr. Drug Discov. Technol. 2020, 17, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ganong, W.F. Gastrointestinal Tract Functions. Review of Medical Physiology, 22th ed.; The McGraw-Hill Companies: New York, NY, USA, 2006; pp. 210–231. [Google Scholar]
- Ford, S.M.; Laska, D.A.; Hottendorf, G.H.; Williams, P.D. Correlation between the in-vitro and in-vivo nephrotoxicity of parenteral antibiotics in the rabbit. Toxicol. Method. 1993, 3, 1–17. [Google Scholar] [CrossRef]
- Asha, K.K.; Sankar, T.V.; Viswanathan, N.P.G. Effect of tetracycline on pancreas and liver function of adult male albino rats. J. Pharm. Pharmacol. 2007, 59, 1241–1248. [Google Scholar] [CrossRef]
- Björnsson, E.; Lindberg, J.; Olsson, R. Liver reactions to low-dose tetracyclines. Scand. J. Gastroenterol. 1997, 32, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.S.; Kaplan, M.M. Laboratory tests. In Schiff’s Diseases of the Liver, 8th ed.; Schiff, E.R., Sorrell, M.F., Maddrey, W.C., Eds.; Lippincott–Raven: Philadelphia, PA, USA, 1999; Volume 1, pp. 205–244. [Google Scholar]
- Al-Shaibani, E.A.S.; Alarami, A.M.J.; Al-Awar, M.S.A.; Salih, E.M.A.; Al-Eryani, M.A.Y. Antioxidant protective effect of vitamin E in penicillin and streptomycin-induced hepatotoxicity in guinea pig. J. Agric. Biol. Sci. 2013, 8, 546–554. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Briedé, J.J.; Jennen, D.G.J.; Summeren, A.V.; Saritas-Brauers, K.; Schaart, G.; Kleinjans, J.C.S.; de Kok, T.M.C.M. Increased mitochondrial ROS formation by acetaminophen in human hepatic cells is associated with gene expression changes suggesting disruption of the mitochondrial electron transport chain. Toxicol. Lett. 2015, 234, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lewen, A.; Matz, P.; Chan, P.H. Free radical pathways in CNS injury. Neurotrauma 2000, 17, 871–890. [Google Scholar] [CrossRef]
- Yang, X.E.; Liu, D.; Lu, L.L.; Feng, Y.; Li, T.Q. A Diagnostic Method of Soil Antibiotic Pollution. CN201410764095.4, 24 August 2016. (In Chinese). [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).