Describing Lettuce Growth Using Morphological Features Combined with Nonlinear Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Computer Vision Data Acquisition
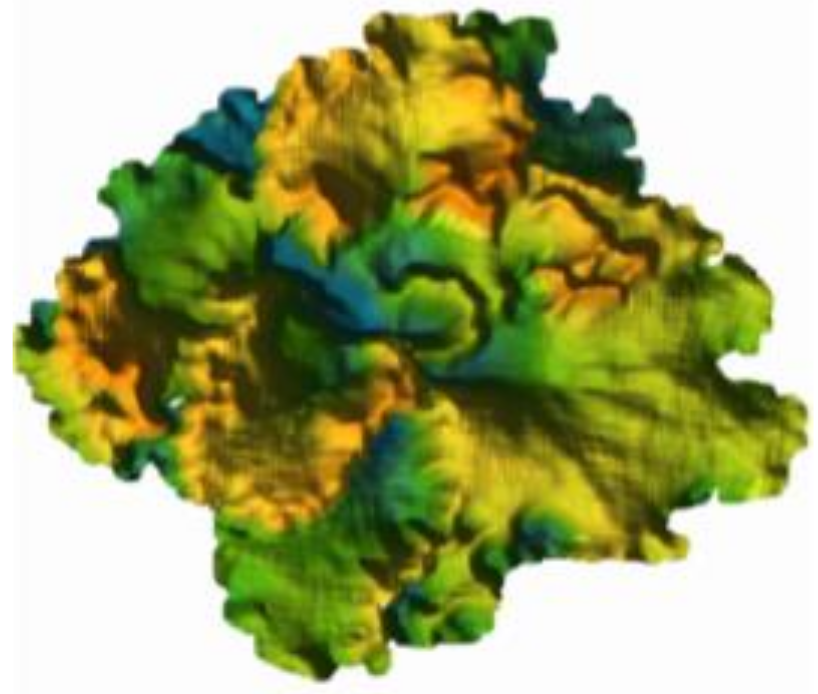
2.3. 3D Point Clouds Data Collection
2.4. Nonlinear Growth Models
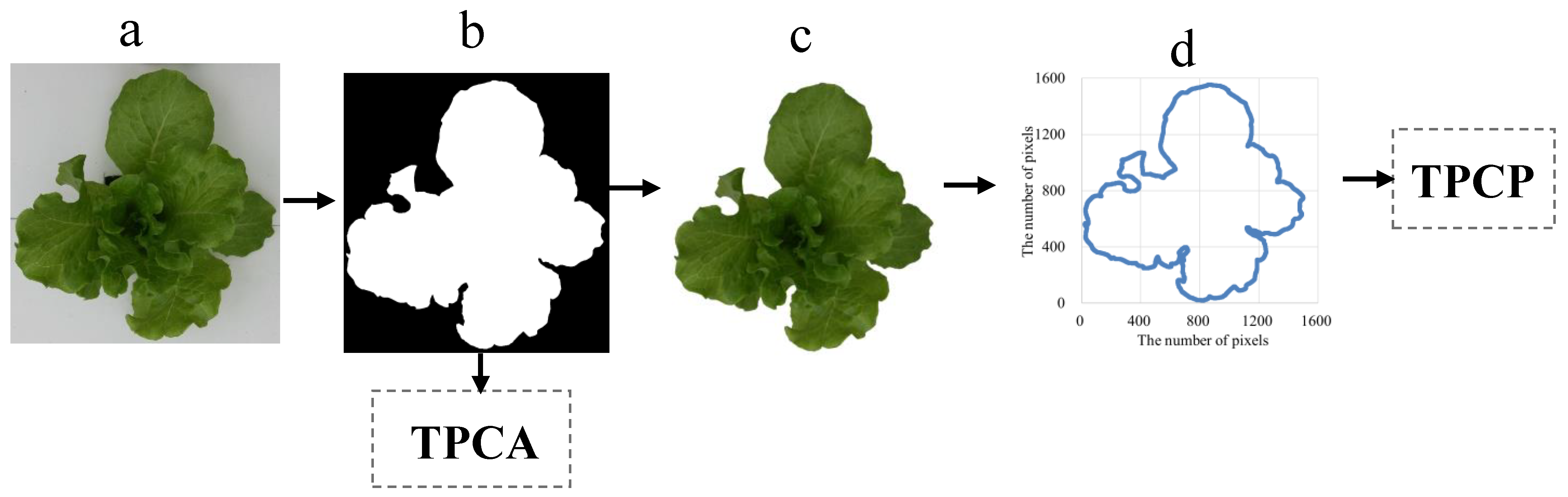
2.5. Extraction of Lettuce Canopy MFs from RGB Images
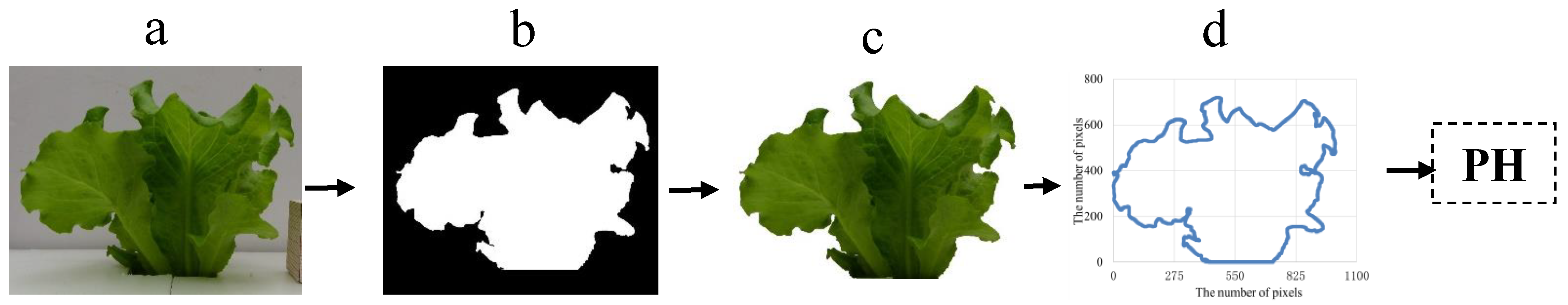
2.6. Extraction of Lettuce Canopy MFs from 3D Point Clouds Data
3. Results
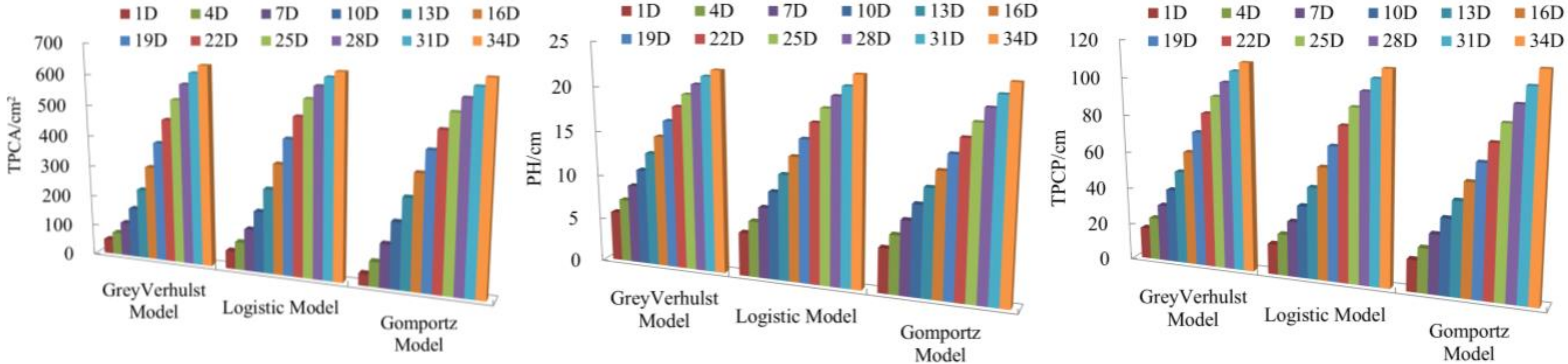
3.1. Establishment of Gompertz, Logistic and Grey Verhulst prediction Models
3.2. Model Selection
3.3. Determination of the Three Points
4. Discussion
4.1. Growth Models
4.2. Growth Points
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.F.; Yang, Q.C.; Li, T. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Chen, Y.C.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.C.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 11. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Zhang, Y.W.; Wang, L.; Liu, X.W.; Zhang, J.R.; He, Z.Y. Stereoselective metabolomic and lipidomic responses of lettuce (Lactuca sativa L.) exposing to chiral triazole fungicide tebuconazole. Food Chem. 2022, 371, 131209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Z.; Xu, D.; Ma, J.; Chen, Y.; Fu, Z. Growth monitoring of greenhouse lettuce based on a convolutional neural network. Hortic. Res 2020, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Jane, S.A.; Fernandes, F.A.; Silva, E.M.; Muniz, J.A.; Fernandes, T.J.; Pimentel, G.V. Adjusting the growth curve of sugarcane varieties using nonlinear models. Cienc. Rural 2020, 50, e20190408. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source-sink perspective on growth rate. JExB 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, H.C.; Zheng, J.Q.; Yin, Y.; Wang, G.S.; Chen, Y.; Yu, J.; Ge, Y.F. Comparative analysis of nonlinear growth curve models for Arabidopsis thaliana rosette leaves. Acta Physiol. Plant. 2018, 40, 114. [Google Scholar] [CrossRef]
- Ofir, M.; Kigel, J. Variation in onset of summer dormancy and flowering capacity along an aridity gradient in Poa bulbosa L., a geophytic perennial grass. Ann. Bot. 2003, 91, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Yadav, R.; Gupta, S.; Gaikwad, K.B.; Jain, N.; Ranjan, R.; Kumar, M. Identification of genotypes and marker validation for grain filling rate and grain filling duration in wheat under conservation agriculture. Indian J. Genet. Plant Breed. 2018, 78, 309–316. [Google Scholar]
- Carini, F.; Cargneltitti, A.; Pezzini, R.V.; de Souza, J.M.; Chaves, G.G.; Procedi, A. Nonlinear models for describing lettuce growth in autumn-winter. Cienc. Rural 2020, 50, 12. [Google Scholar] [CrossRef]
- Birch, C.P.D. A New Generalized Logistic Sigmoid Growth Equation Compared with the Richards Growth Equation. Ann. Bot. 1999, 83, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Archontoulis, S.V.; Miguez, F.E. Nonlinear Regression Models and Applications in Agricultural Research. Agron. J. 2015, 107, 786–798. [Google Scholar] [CrossRef] [Green Version]
- Karadavut, U.; Palta, C.; Kokten, K.; Bakoglu, A. Comparative Study on Some Non-linear Growth Models Describing Leaf Growth of Maize. Int. J. Agric. Biol. 2010, 12, 227–230. [Google Scholar]
- Tsoularis, A.; Wallace, J. Analysis of logistic growth models. Math. Biosci. 2002, 179, 21–55. [Google Scholar] [CrossRef] [Green Version]
- Veloso, R.C.; Winkelstroter, L.K.; Silva, M.T.P.; Pires, A.V.; Torres, R.A.; Pinheiro, S.R.F.; Costa, L.S.; Amaral, J.M. Selection and classification of multivariate nonlinear models for broilers. Arq. Bras. Med. Vet. Zootec. 2016, 68, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, S.; Qian, W.; Li, X. Forecasting New Product Diffusion Using Grey Time-Delayed Verhulst Model. J. Appl. Math. 2013, 625028. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, F.; Ji, Q.; Shaikh, P.H.; Mirjat, N.H.; Uqaili, M.A. Forecasting China’s natural gas demand based on optimised nonlinear grey models. Energy 2017, 140, 941–951. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Marthews, T.R.; Vogt, D.R.; Purves, D.; Rees, M.; Hector, A.; Turnbull, L.A. How to fit nonlinear plant growth models and calculate growth rates: An update for ecologists. Methods Ecol. Evol. 2012, 3, 245–256. [Google Scholar] [CrossRef]
- Wardhani, W.S.; Kusumastuti, P. Describing the height growth of corn using Logistic and Gompertz model. Agrivita J. Agric. Sci. 2013, 35, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Bem, C.M.; Cargnelutti Filho, A.; Carini, F.; Pezzini, R.V. Univariate and multivariate nonlinear models in productive traits of the sunn hemp. Rev. Cienc. Agron. 2020, 51, 10. [Google Scholar] [CrossRef]
- Bem, C.M.; Cargnelutti Filho, A.; Facco, G.; Schabarum, D.E.; Silveira, D.L.; Simões, F.M.; Uliana, D.B. Growth models for morphological traits of sunn hemp. Semin. Cienc. Agrar. 2017, 38, 2933–2943. [Google Scholar] [CrossRef] [Green Version]
- Muianga, C.A.; Muniz, J.A.; Nascimento, M.D.; Fernandes, T.J.; Savian, T.V. Description of the Growth Curve of Cashew Fruits in Nonlinear Models. Rev. Bras. Frutic. 2016, 38, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Mello, A.C.; Toebe, M.; de Souza, R.R.; Paraginski, J.A.; Somavilla, J.C.; Martins, V.; Pinto, A.C.V. Nonlinear models in the height description of the Rhino sunflower cultivar. Cienc. Rural 2022, 52, 8. [Google Scholar] [CrossRef]
- Ribeiro, T.D.; Savian, T.V.; Fernandes, T.J.; Muniz, J.A. The use of the nonlinear models in the growth of pears of ‘Shinseiki’ cultivar. Cienc. Rural 2018, 48, 7. [Google Scholar] [CrossRef] [Green Version]
- Pezzini, R.V.; Cargnelutti, A.; de Bem, C.M.; de Souza, J.M.; Chaves, G.G.; Neu, I.M.M.; Procedi, A. Gompertz and Logistic models for morphological traits of sudangrass cultivars during sowing seasons. Semin. Cienc. Agrar. 2019, 40, 3399–3417. [Google Scholar] [CrossRef] [Green Version]
- Sari, B.G.; Lucio, A.D.; Santana, C.S.; Savian, T.V. Describing tomato plant production using growth models. Sci. Hortic. 2019, 246, 146–154. [Google Scholar] [CrossRef]
- Mischan, M.M.; de Pinho, S.Z.; de Carvalho, L.R. Determination of a point sufficiently close to the asymptote in nonlinear growth functions. Sci. Agric. 2011, 68, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Lucio, A.D.; Nunes, L.F.; Rego, F. Nonlinear models to describe production of fruit in Cucurbita pepo and Capiscum annuum. Sci. Hortic. 2015, 193, 286–293. [Google Scholar] [CrossRef]
- Maia, E.; de Siqueira, D.L.; Silva, F.F.E.; Peternelli, L.A.; Salomao, L.C.C. Method of comparison of models non-linear regression in bananas trees. Cienc. Rural 2009, 39, 1380–1386. [Google Scholar] [CrossRef]
- Gao, H.Y.; Mao, H.P.; Zhang, X.D. Determination of lettuce nitrogen content using spectroscopy with efficient wavelength selection and extreme learning machine. Zemdirb. Agric. 2015, 102, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Xu, Z. Analyzing the air quality of Beijing, Tianjin, and Shijiazhuang using grey Verhulst model. Air Qual. Atmos. Health 2019, 12, 1419–1426. [Google Scholar] [CrossRef]
- Fernandez-Pacheco, D.G.; Escarabajal-Henarejos, D.; Ruiz-Canales, A.; Conesa, J.; Molina-Martinez, J.M. A digital image-processing-based method for determining the crop coefficient of lettuce crops in the southeast of Spain. Biosyst. Eng. 2014, 117, 23–34. [Google Scholar] [CrossRef]
- Story, D.; Kacira, M.; Kubota, C.; Akoglu, A.; An, L.L. Lettuce calcium deficiency detection with machine vision computed plant features in controlled environments. Comput. Electron. Agric. 2010, 74, 238–243. [Google Scholar] [CrossRef]
- Mao, H.; Gao, H.; Zhang, X.; Kumi, F. Nondestructive measurement of total nitrogen in lettuce by integrating spectroscopy and computer vision. Sci. Hortic. 2015, 184, 1–7. [Google Scholar] [CrossRef]
- Bachofer, F.; Queneherve, G.; Zwiener, T.; Maerker, M.; Hochschild, V. Comparative analysis of Edge Detection techniques for SAR images. Eur. J. Remote Sens. 2016, 49, 205–224. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Xu, Q.J.; Yang, Y.Y.; Lu, H.; Li, H.; Feng, X.B.; Yin, W.Q. Classification of rice seed variety using point cloud data combined with deep learning. Int. J. Agric. Biol. Eng. 2021, 14, 206–212. [Google Scholar] [CrossRef]
- Kayacan, E.; Ulutas, B.; Kaynak, O. Grey system theory-based models in time series prediction. Expert Syst. Appl. 2010, 37, 1784–1789. [Google Scholar] [CrossRef]
- Chang, C.L.; Chung, S.C.; Fu, W.L.; Huang, C.C. Artificial intelligence approaches to predict growth, harvest day, and quality of lettuce (Lactuca sativa L.) in a IoT-enabled greenhouse system. Biosyst. Eng. 2021, 212, 77–105. [Google Scholar] [CrossRef]
- Fernandes, T.J.; Pereira, A.A.; Muniz, J.A. Double sigmoidal models describing the growth of coffee berries. Cienc. Rural 2017, 47, e20160646. [Google Scholar] [CrossRef] [Green Version]
- Lucio, A.D.C.; Nunes, L.F.; Rego, F. Nonlinear regression and plot size to estimate green beans production. Hortic. Bras. 2016, 34, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Lobo, T.F.; Grassi Filho, H.; Bull, L.T.; Kummer, A.C.B. Effect of sewage sludge and nitrogen on production factors of sunflower. Rev. Bras. Eng. Agric. Ambient. 2013, 17, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Valado, F.; Júnior, D.; Batista, R.F.; Paula, V.; Alves, T. Produtividade do girassol em funo do manejo da adubao nitrogenada/sunflower productivity in function of the management of nitrogen fertilization. Braz. J. Dev. 2020, 6, 84197–84213. [Google Scholar] [CrossRef]
- Pinho, S.Z.; Carvalho, L.R.; Mischan, M.M.; Passos, J.R.S. Critical points on growth curves in autoregressive and mixed models. Sci. Agric. 2014, 71, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Wubs, A.M.; Ma, Y.T.; Heuvelink, E.; Hemerik, L.; Marcelis, L.F.M. Model Selection for Nondestructive Quantification of Fruit Growth in Pepper. J. Am. Soc. Hortic. Sci. 2012, 137, 71–79. [Google Scholar] [CrossRef] [Green Version]


| MFs | Gompertz Model | Logistic Model | ||
|---|---|---|---|---|
| TPCA | (9) | (12) | ||
| PH | (10) | (13) | ||
| TPCP | (11) | (14) |
| MFs | a | b | Grey Verhulst Models | |
|---|---|---|---|---|
| TPCA | −0.4777 | −0.0006895 | (15) | |
| PH | −0.3259 | −0.1133 | (16) | |
| TPCP | −0.3664 | −0.002972 | (17) |
| MFs | Comparison Criteria | Gompertz | Logistic | Grey Verhulst |
|---|---|---|---|---|
| TPCA | R2 | 0.8496 | 0.8746 | 0.9097 |
| MAPE | 0.0841 | 0.0393 | 0.0284 | |
| PH | R2 | 0.8535 | 0.8991 | 0.8978 |
| MAPE | 0.0472 | 0.0344 | 0.0349 | |
| TPCP | R2 | 0.8099 | 0.8272 | 0.8536 |
| MAPE | 0.0919 | 0.0801 | 0.0794 |
| MFs | Maximum Growth Rate Point * | |
|---|---|---|
| TPCA | (19) | |
| PH | (20) | |
| TPCP | (21) | |
| MFs | Starting point of rapid growth stage | |
| TPCA | (22) | |
| PH | (23) | |
| TPCP | (24) | |
| MFs | Ending point of rapid growth stage | |
| TPCA | (25) | |
| PH | (26) | |
| TPCP | (27) |
| MFs | Maximum Growth Rate Point (days) | Starting Point of Rapid Growth Stage (Days) | Ending Point of Rapid Growth Stage (Days) | Rapid Growth Stage (Days) |
|---|---|---|---|---|
| TPCA | 16.4 | 8.2 | 24.7 | 16.5 |
| PH | 16.1 | 4.0 | 28.2 | 24.2 |
| TPCP | 18.0 | 4.2 | 25.8 | 21.6 |
| Average | 16.8 | 5.5 | 26.2 | 20.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Gao, H.; Zhang, X.; Ni, J.; Mao, H. Describing Lettuce Growth Using Morphological Features Combined with Nonlinear Models. Agronomy 2022, 12, 860. https://doi.org/10.3390/agronomy12040860
Li Q, Gao H, Zhang X, Ni J, Mao H. Describing Lettuce Growth Using Morphological Features Combined with Nonlinear Models. Agronomy. 2022; 12(4):860. https://doi.org/10.3390/agronomy12040860
Chicago/Turabian StyleLi, Qinglin, Hongyan Gao, Xiaodong Zhang, Jiheng Ni, and Hanping Mao. 2022. "Describing Lettuce Growth Using Morphological Features Combined with Nonlinear Models" Agronomy 12, no. 4: 860. https://doi.org/10.3390/agronomy12040860
APA StyleLi, Q., Gao, H., Zhang, X., Ni, J., & Mao, H. (2022). Describing Lettuce Growth Using Morphological Features Combined with Nonlinear Models. Agronomy, 12(4), 860. https://doi.org/10.3390/agronomy12040860






