Metabolomic Profile of Citrus limon Leaves (‘Verna’ Variety) by 1H-NMR and Multivariate Analysis Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling Preparation
2.2. Leaves Metabolomic Profile by 1H-NMR
2.3. Statistical Analysis
3. Results and Discussion
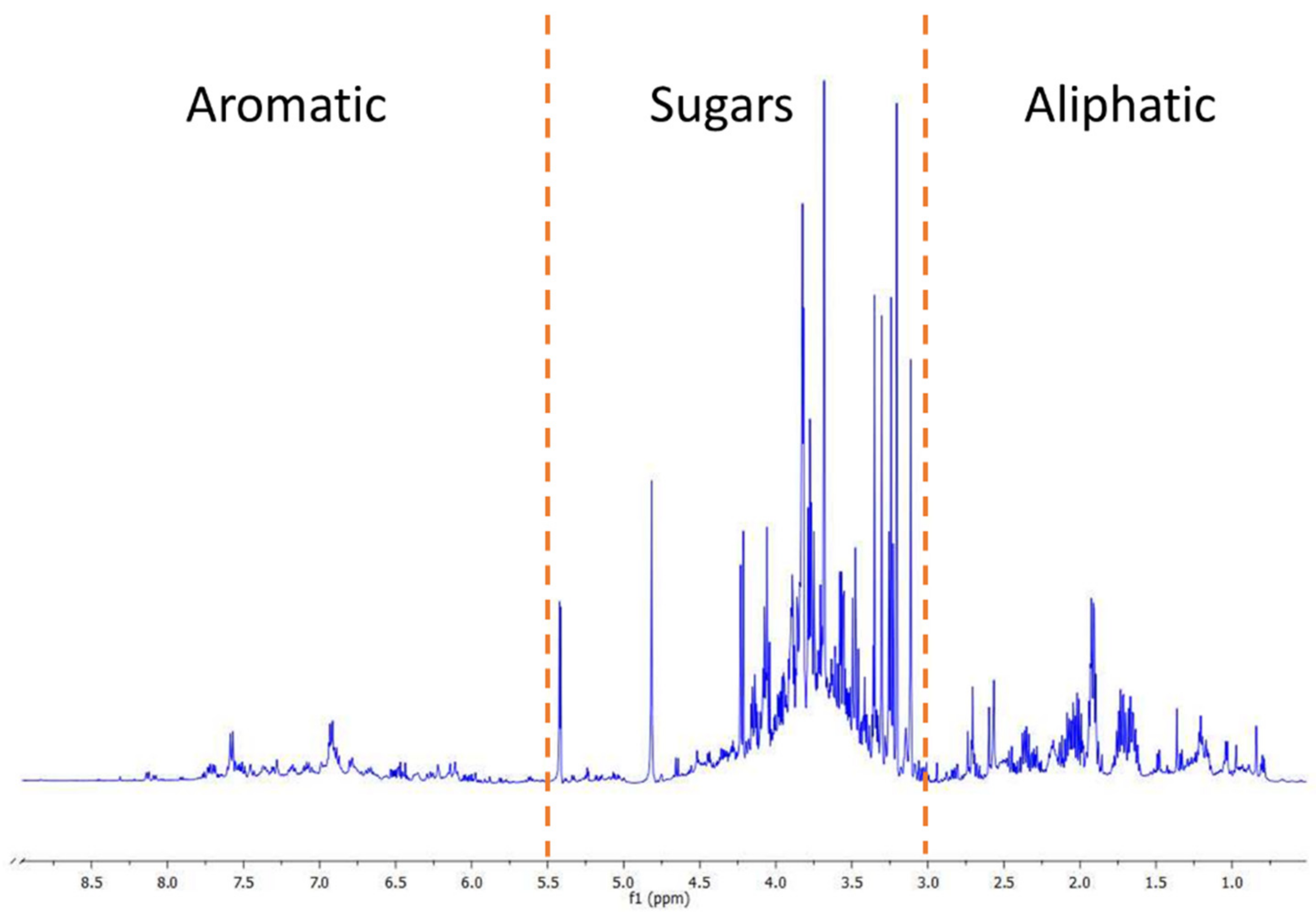
3.1. Metabolomic Fingerprint
3.2. Multivariate Analysis
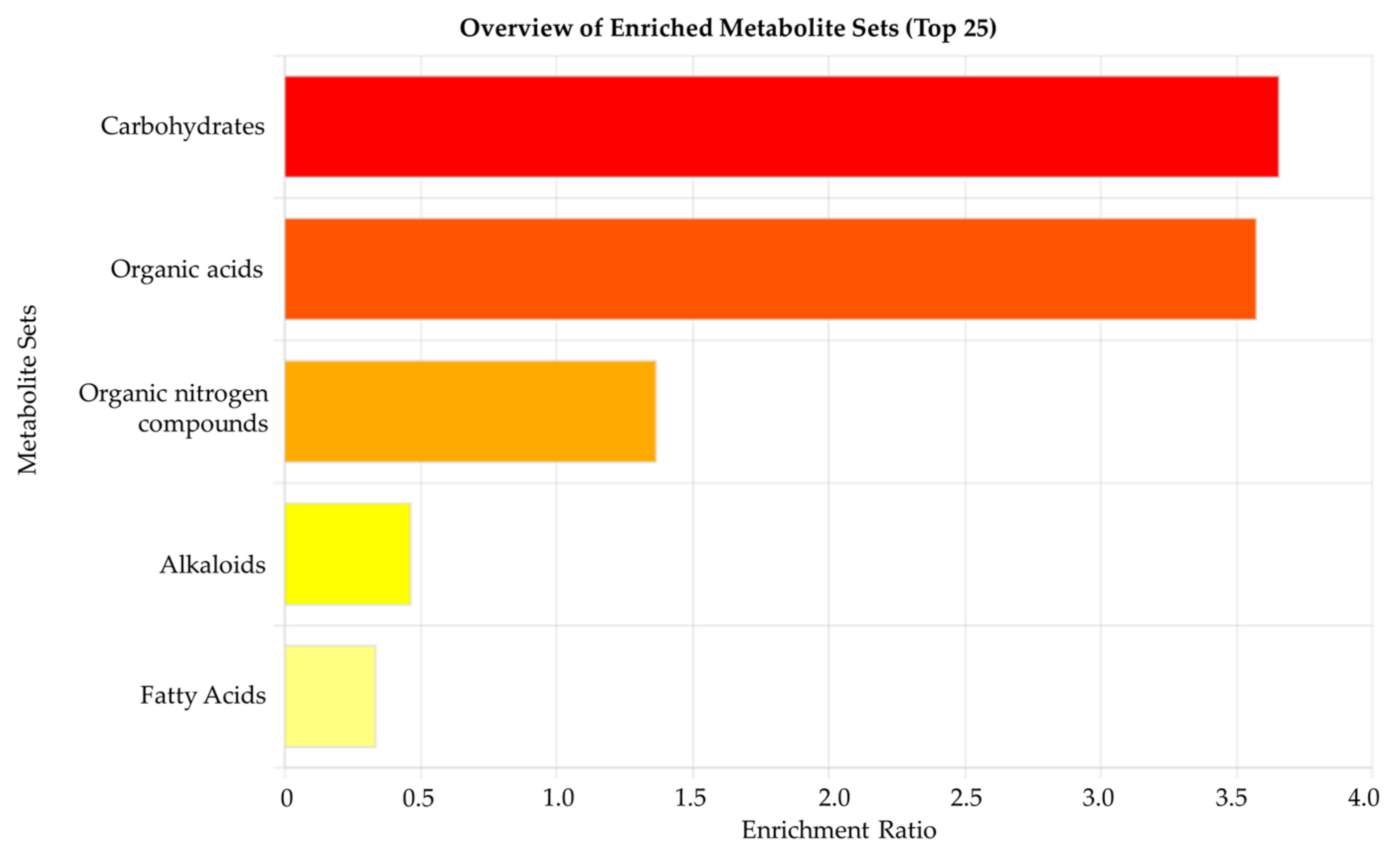
3.3. Metabolomic Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Dean, L.L. Targeted and Non-Targeted Analyses of Secondary Metabolites in Nut and Seed Processing. Eur. J. Lipid Sci. Technol. 2018, 120, 1700479. [Google Scholar] [CrossRef]
- Mucci, A.; Parenti, F.; Righi, V.; Schenetti, L. Citron and lemon under the lens of HR-MAS NMR spectroscopy. Food Chem. 2013, 141, 3167–3176. [Google Scholar] [CrossRef]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, M.; Núñez-Gómez, D.; Forner-Giner, M.-Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality Parameters of Spanish Lemons with Commercial Interest. Foods 2021, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Hojjati, M.; Barzegar, H. Chemical Composition and Biological Activities of Lemon (Citrus limon) Leaf Essential Oil. Nutr. Food Sci. Res. 2017, 4, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Kamiyama, S. Studies on the Leaf Oils of Citrus Species. Biosci. Biotechnol. Biochem. 2014, 31, 1091–1096. [Google Scholar] [CrossRef]
- Jha, M.; Ansari, S.; Shimpi, N.G. Ultrasonic assisted green synthesis of Ag:CdO nanocubes and nanospheres using Citrus limon leaves for efficient degradation of organic dyes. J. Ind. Eng. Chem. 2019, 69, 269–284. [Google Scholar] [CrossRef]
- Vankar, P.S.; Shukla, D. Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci. 2012, 2, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Melgarejo, P.; Legua, P.; Pérez-Sarmiento, F.; Martínez-Font, R.; José Martínez-Nicolás, J.; Hernández, F. Effect of a New Remediated Substrate on Fruit Quality and Bioactive Compounds in Two Strawberry Cultivars Effect of a New Remediated Substrate on Fruit Quality and Bioactive Compounds in Two Strawberry Cultivars. J. Food Nutr. Res. 2017, 5, 579–586. [Google Scholar] [CrossRef]
- Martínez-Nicolás, J.J.; Legua, P.; Núñez-Gómez, D.; Martínez-Font, R.; Hernández, F.; Giordani, E.; Melgarejo, P. Potential of dredged bioremediated marine sediment for strawberry cultivation. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Tozzi, F.; Pecchioli, S.; Renella, G.; Melgarejo, P.; Legua, P.; Macci, C.; Doni, S.; Masciandaro, G.; Giordani, E.; Lenzi, A. Remediated marine sediment as growing medium for lettuce production: Assessment of agronomic performance and food safety in a pilot experiment. J. Sci. Food Agric. 2019, 99, 5624–5630. [Google Scholar] [CrossRef]
- Van der Sar, S.; Kim, H.K.; Meissner, A.; Verpoorte, R.; Choi, Y.H. Nuclear Magnetic Resonance Spectroscopy for Plant Metabolite Profiling. Handb. Plant Metab. 2013, 57–76. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Broadhurst, D.I.; Kell, D.B. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006, 2, 171–196. [Google Scholar] [CrossRef] [Green Version]
- Arbona, V.; Iglesias, D.J.; Gómez-Cadenas, A. Non-targeted metabolite profiling of citrus juices as a tool for variety discrimination and metabolite flow analysis. BMC Plant Biol. 2015, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Passarinho, J.A.P.; Lamosa, P.; Baeta, J.P.; Santos, H.; Ricardo, C.P.P. Annual changes in the concentration of minerals and organic compounds of Quercus suber leaves. Physiol. Plant. 2006, 127, 100–110. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Knill, T.; Schuster, J. Branched-chain amino acid metabolism in higher plants. Physiol. Plant. 2007, 129, 68–78. [Google Scholar] [CrossRef]
- Fowden, L. Aspects of Amino Acid Metabolism in Plants. Annu. Rev. Plant Physiol. 1967, 18, 85–106. [Google Scholar] [CrossRef]
- Wingler, A.; Roitsch, T. Metabolic regulation of leaf senescence: Interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol. 2008, 10, 50–62. [Google Scholar] [CrossRef]
- Van Dingenen, J.; de Milde, L.; Vermeersch, M.; Maleux, K.; de Rycke, R.; de Bruyne, M.; Storme, V.; Gonzalez, N.; Dhondt, S.; Inzé, D. Chloroplasts Are Central Players in Sugar-Induced Leaf Growth. Plant Physiol. 2016, 171, 590–605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Okubo, A.; Yamazaki, S. Measurement of Free Choline in Plant Leaves by Capillary Electrophoresis. Biosci. Biotechnol. Biochem. 2014, 65, 2573–2576. [Google Scholar] [CrossRef] [Green Version]
- Achituv, M.; Bar-Akiva, A. Metabolic Pathway of a-Ketoglutarate in Citrus Leaves as Affected by Phosphorus Nutrition. Plant Physiol. 1978, 61, 703–705. [Google Scholar] [CrossRef] [Green Version]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef]
- Gao, H.J.; Yang, H.Q.; Wang, J.X. Arginine metabolism in roots and leaves of apple (Malus domestica Borkh.): The tissue-specific formation of both nitric oxide and polyamines. Sci. Hortic. Amsterdam 2009, 119, 147–152. [Google Scholar] [CrossRef]
- Lee, C.P.; Elsässer, M.; Fuchs, P.; Fenske, R.; Schwarzländer, M.; Millar, A.H. The versatility of plant organic acid metabolism in leaves is underpinned by mitochondrial malate–citrate exchange. Plant Cell 2021, 33, 3700–3720. [Google Scholar] [CrossRef]
- Ivanov, B.; Igamberdiev, A.; Zemlyanukhin, A. Nonphotosynthetic Utilization of Oxogenous C-14 Oxalate by Pea and Rice Seedlings Exposed in the Helium and Carbon-Dioxide Media. Fiziol. Biokhimiya Kult. Rastenii. 1989, 21, 329–333. [Google Scholar]
- Vallarino, J.G.; Osorio, S. Organic Acids. Postharvest Physiol. Biochem. Fruits Veg. 2019, 207–224. [Google Scholar] [CrossRef]

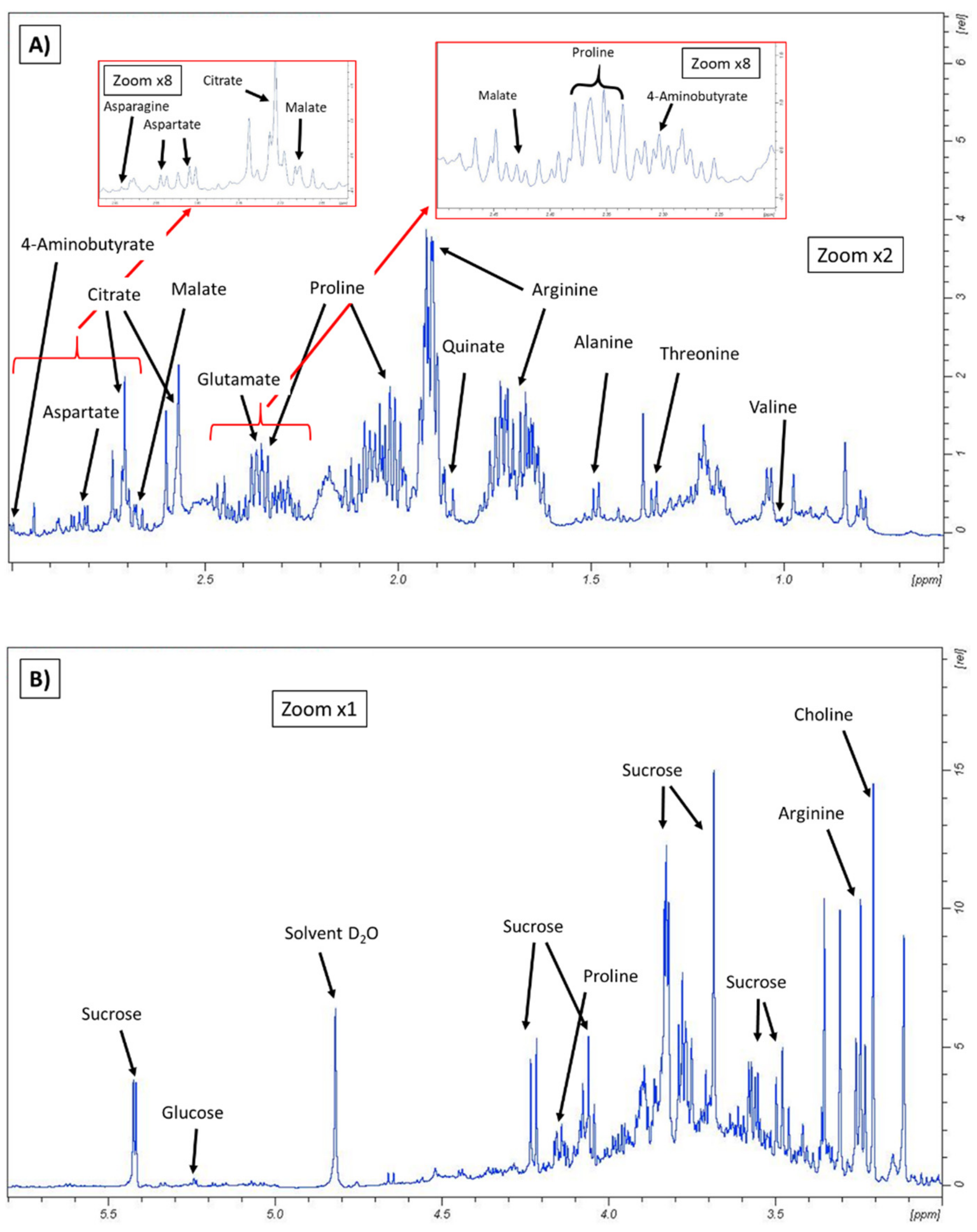



| Compound | Chemical Shift (ppm) 1 |
|---|---|
| Amino acids | |
| GABA | 3.01 (t) |
| Alanine | 1.46 (d) |
| Arginine | 1.6 and 1.7 (m) |
| Asparagine | 2.94 (dd) |
| Aspartate | 2.81 (dd) |
| Glutamate | 2.3 (m) |
| Proline | 2.00 (m) |
| Threonine | 1.32 (d) |
| Valine | 1.02 (d) |
| Organic acids | |
| Citrate | 2.79 (d) |
| Formate | 8.43 (s) |
| Malate | 2.7 (dd) |
| Quinate | 1.86 (dd) |
| Succinate | 2.39 (s) |
| Sugars | |
| Glucose (α and β forms) | 5.22 (d) |
| Maltose | 5.42 (d) |
| Sucrose | 5.40 (d) |
| Other metabolites | |
| Choline | 3.19 (s) |
| Trigonelline | 9.11 (s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melgarejo, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Martínez-Font, R.; Lidón, V.; García-Sánchez, F.; Legua, P. Metabolomic Profile of Citrus limon Leaves (‘Verna’ Variety) by 1H-NMR and Multivariate Analysis Technique. Agronomy 2022, 12, 1060. https://doi.org/10.3390/agronomy12051060
Melgarejo P, Núñez-Gómez D, Martínez-Nicolás JJ, Hernández F, Martínez-Font R, Lidón V, García-Sánchez F, Legua P. Metabolomic Profile of Citrus limon Leaves (‘Verna’ Variety) by 1H-NMR and Multivariate Analysis Technique. Agronomy. 2022; 12(5):1060. https://doi.org/10.3390/agronomy12051060
Chicago/Turabian StyleMelgarejo, Pablo, Dámaris Núñez-Gómez, Juan José Martínez-Nicolás, Francisca Hernández, Rafael Martínez-Font, Vicente Lidón, Francisco García-Sánchez, and Pilar Legua. 2022. "Metabolomic Profile of Citrus limon Leaves (‘Verna’ Variety) by 1H-NMR and Multivariate Analysis Technique" Agronomy 12, no. 5: 1060. https://doi.org/10.3390/agronomy12051060
APA StyleMelgarejo, P., Núñez-Gómez, D., Martínez-Nicolás, J. J., Hernández, F., Martínez-Font, R., Lidón, V., García-Sánchez, F., & Legua, P. (2022). Metabolomic Profile of Citrus limon Leaves (‘Verna’ Variety) by 1H-NMR and Multivariate Analysis Technique. Agronomy, 12(5), 1060. https://doi.org/10.3390/agronomy12051060








