Macroscopic and Microscopic Phenotyping Using Diverse Yellow Rust Races Increased the Resolution of Seedling and Adult Plant Resistance in Wheat Breeding Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Vernalization, and Growth Conditions
2.2. Pathogen Isolates, Multiplication and Growth Conditions
2.3. Macroscopic Phenotyping
2.3.1. Seedling Stage
2.3.2. Adult Plants
2.4. Microscopic Phenotyping
2.4.1. Sample Collection
2.4.2. Fixation, Staining, and Microscopy
2.5. Statistical Analysis
3. Results
3.1. Macroscopic Phenotyping
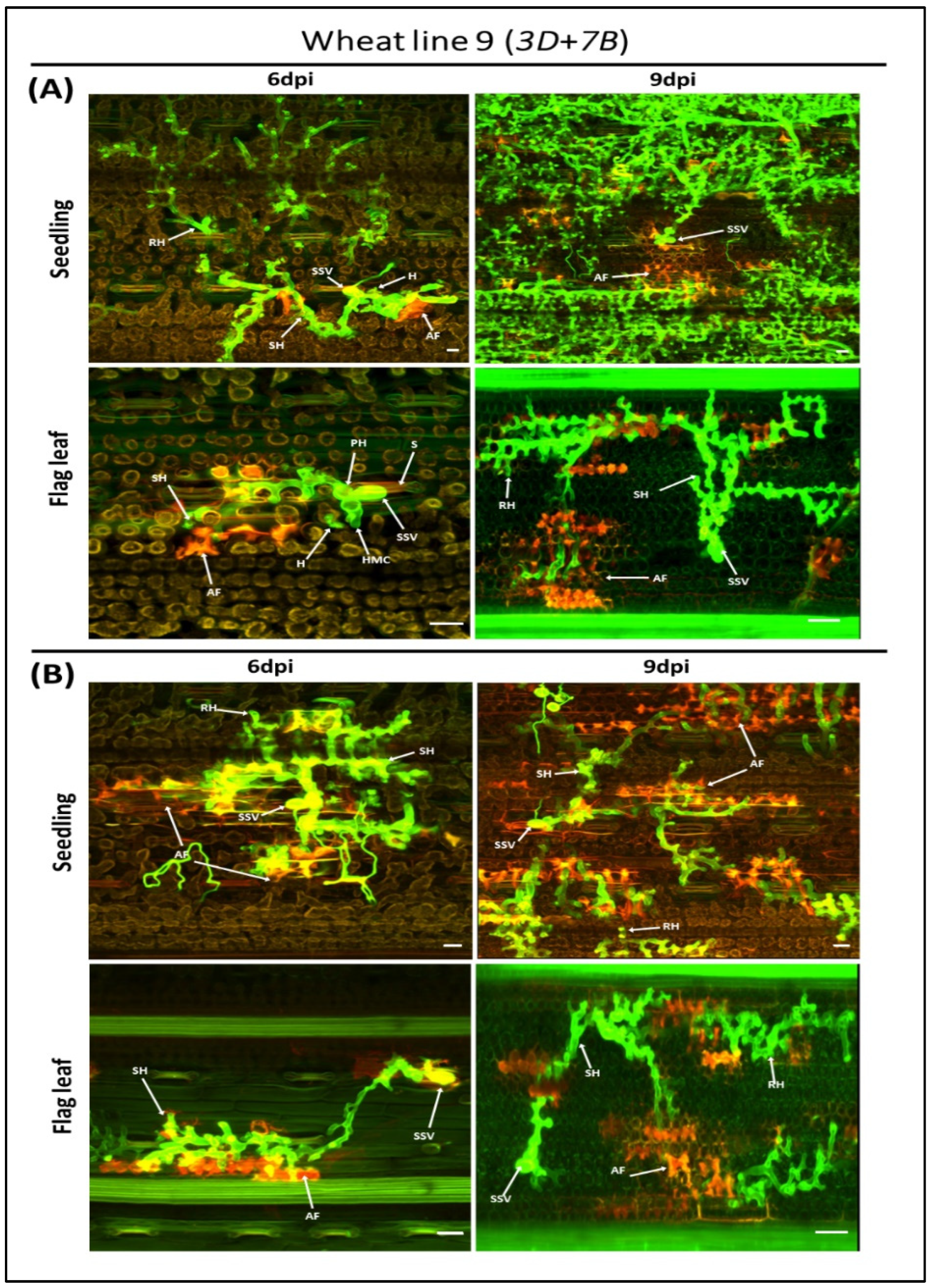
3.2. Microscopic Phenotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beddow, J.M.; Pardey, P.G.; Chai, Y.; Hurley, T.M.; Kriticos, D.J.; Braun, H.-J.; Park, R.F.; Cuddy, W.S.; Yonow, T. Research investment implications of shifts in the global geography of wheat stripe rust. Nat. Plants 2015, 1, 15132. [Google Scholar] [CrossRef] [PubMed]
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Walter, S.; Justesen, A.F. Escalating threat of wheat rusts. Science 2010, 329, 369. [Google Scholar] [CrossRef] [Green Version]
- Milus, E.A.; Kristensen, K.; Hovmøller, M.S. Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology 2009, 99, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.; Ali, S.; Kemen, E.; Nazari, K.; Bahri, B.A.; Enjalbert, J.; Hansen, J.G.; Brown, J.K.M.; Sicheritz-Pontén, T.; Jones, J.; et al. Molecular markers for tracking the origin and worldwide distribution of invasive strains of Puccinia striiformis. Ecol. Evol. 2016, 6, 2790–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat stripe (Yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.N.; Hovmøller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Goral, T.; et al. IPM strategies and their dilemmas including an introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281. [Google Scholar] [CrossRef]
- Lázaro, E.; Makowski, D.; Vicent, A. Decision support systems halve fungicide use compared to calendar-based strategies without increasing disease risk. Commun. Earth Environ. 2021, 2, 224. [Google Scholar] [CrossRef]
- Lowe, I.; Cantu, D.; Dubcovsky, J. Durable resistance to the wheat rusts: Integrating systems biology and traditional phenotype-based research methods to guide the deployment of resistance genes. Euphytica 2011, 179, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef] [Green Version]
- Hovmøller, M.S. Sources of seedling and adult plant resistance to Puccinia striiformis f.sp. tritici in European wheats. Plant Breed. 2007, 126, 225–233. [Google Scholar] [CrossRef]
- Niks, R.E.; Qi, X.; Marcel, T.C. Quantitative resistance to biotrophic filamentous plant pathogens: Concepts, misconceptions, and mechanisms. Annu. Rev. Phytopathol. 2015, 53, 445–470. [Google Scholar] [CrossRef] [Green Version]
- Boukhatem, N.; Baret, P.V.; Mingeot, D.; Jacquemin, J.M. Quantitative trait loci for resistance against yellow rust in two wheat-derived recombinant inbred line populations. Theor. Appl. Genet. 2002, 104, 111–118. [Google Scholar] [CrossRef]
- Johnson, R. Past, present and future opportunities in breeding for disease resistance, with examples from wheat. Euphytica 1992, 63, 3–22. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Wellings, C.R.; Park, R.F. Wheat Rusts: An Atlas of Resistance Genes; CSIRO Publications: East Melbourne, Australia, 1995. [Google Scholar]
- Sørensen, C.K.; Hovmøller, M.S.; Leconte, M.; Dedryver, F.; de Vallavieille-Pope, C. New races of Puccinia striiformis found in Europe reveal race specificity of long-term effective adult plant resistance in wheat. Phytopathology 2014, 104, 1042–1051. [Google Scholar] [CrossRef] [Green Version]
- Milus, E.A.; Moon, D.E.; Lee, K.D.; Mason, R.E. Race-specific adult-plant resistance in winter wheat to stripe rust and characterization of pathogen virulence patterns. Phytopathology 2015, 105, 1114–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R. A critical analysis of durable resistance. Annu. Rev. Phytopathol. 1984, 22, 309–330. [Google Scholar] [CrossRef]
- Johnson, R. Reflections of a plant pathologist on breeding for disease resistance, with emphasis on yellow rust and eyespot of wheat. Plant Pathol. 1992, 41, 239–254. [Google Scholar] [CrossRef]
- Boyd, L.A. Can robigus defeat an old enemy?—Yellow rust of wheat. J. Agric. Sci. 2005, 143, 233–243. [Google Scholar] [CrossRef]
- Kanwal, M.; Qureshi, N.; Gessese, M.; Forrest, K.; Babu, P.; Bariana, H.; Bansal, U. An adult plant stripe rust resistance gene maps on chromosome 7A of Australian wheat cultivar Axe. Theor. Appl. Genet. 2021, 134, 2213–2220. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, C.; Cheng, Y.; Yao, F.; Long, L.; Wu, Y.; Li, J.; Li, H.; Wang, J.; Jiang, Q.; et al. Genome-wide association mapping reveals potential novel loci controlling stripe rust resistance in a Chinese wheat landrace diversity panel from the southern autumn-sown spring wheat zone. BMC Genom. 2021, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Blake, V.; Woodhouse, M.; Lazo, G.; Odell, S.; Wight, C.; Tinker, N.; Wang, Y.; Gu, Y.; Birkett, C.; Jannink, J.; et al. GrainGenes: Centralized small grain resources and digital platform for geneticists and breeders. Database 2019, 2019, baz065. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, C.Y.; Wang, M.N.; See, D.R.; Zemetra, R.S.; Chen, X.M. QTL analysis of durable stripe rust resistance in the North American winter wheat cultivar Skiles. Theor. Appl. Genet. 2019, 132, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Maree, G.J.; Prins, R.; Boyd, L.A.; Castelyn, H.D.; Bender, C.M.; Boshoff, W.H.; Pretorius, Z.A. Assessing the individual and combined effects of QTL for adult plant stripe rust resistance derived from cappelle-desprez. Agronomy 2019, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Hu, T.; Li, X.; Yu, M.; Li, Y.; Yang, S.; Huang, K.; Han, D.; Kang, Z. Genome-wide mapping of adult plant stripe rust resistance in wheat cultivar Toni. Theor. Appl. Genet. 2019, 132, 1693–1704. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, Z.; Ren, J.D.; Du, Z.Y.; Quan, W.; Zhang, Y.B.; Zhang, Z.J. A QTL with major effect on reducing stripe rust severity detected from a Chinese wheat landrace. Plant Dis. 2017, 101, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Huerta-Espino, J.; Bhavani, S.; Herrera-Foessel, S.A.; Singh, D.; Singh, P.K.; Velu, G.; Mason, R.E.; Jin, Y.; Njau, P.; et al. Race non-specific resistance to rust diseases in CIMMYT spring wheats. Euphytica 2011, 179, 175–186. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.; Crespo-Herrera, L.A.; Villaseñor-Mir, H.E.; Rodriguez-Garcia, M.F.; Dreisigacker, S.; Barcenas-Santana, D.; Lagudah, E. Adult plant slow rusting genes confer high levels of resistance to rusts in bread wheat cultivars from Mexico. Front. Plant Sci. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Huerta-Espino, J.; Rajaram, S. Achieving near-immunity to leaf and stripe rusts in wheat by combining slow rusting resistance genes. Acta Phytopathol. Entomol. Hung. 2000, 35, 133–139. [Google Scholar]
- Dedryver, F.; Paillard, S.; Mallard, S.; Robert, O.; Trottet, M.; Nègre, S.; Verplancke, G.; Jahier, J. Characterization of Genetic Components Involved in Durable Resistance to Stripe Rust in the Bread Wheat “Renan”. Phytopathology 2009, 99, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, X. Chapter 5: Stripe Rust Resistance. In Stripe Rust; Chen, X., Kang, Z., Eds.; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar]
- Cobb, J.N.; DeClerck, G.; Greenberg, A.; Clark, R.; McCouch, S. Next-generation phenotyping: Requirements and strategies for enhancing our understanding of genotype–phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. 2013, 126, 867–887. [Google Scholar] [CrossRef] [Green Version]
- Willocquet, L.; Savary, S.; Yuen, J. Multiscale phenotyping and decision strategies in breeding for resistance. Trends Plant Sci. 2017, 22, 420–432. [Google Scholar] [CrossRef]
- Furbank, R.T.; Tester, M. Phenomics–Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldenhauer, J.; Moerschbacher, B.M.; Van Der Westhuizen, A.J. Histological investigation of stripe rust (Puccinia striiformis f.sp. tritici) development in resistant and susceptible wheat cultivars. Plant Pathol. 2006, 55, 469–474. [Google Scholar] [CrossRef]
- Jagger, L.J.; Newell, C.; Berry, S.T.; Maccormack, R.; Boyd, L.A. Histopathology provides a phenotype by which to characterize stripe rust resistance genes in wheat. Plant Pathol. 2011, 60, 640–648. [Google Scholar] [CrossRef]
- Sørensen, C.K.; Labouriau, R.; Hovmøller, M.S. Temporal and spatial variability of fungal structures and host responses in an incompatible rust–wheat interaction. Front. Plant Sci. 2017, 8, 484. [Google Scholar] [CrossRef]
- Maree, G.J.; Prins, R.; Bender, C.M.; Boshoff, W.H.P.; Negussie, T.G.; Pretorius, Z.A. Phenotyping Kariega × Avocet S doubled haploid lines containing individual and combined adult plant stripe rust resistance loci. Plant Pathol. 2019, 68, 659–668. [Google Scholar] [CrossRef]
- Saleem, K.; Sørensen, C.K.; Labouriau, R.; Hovmøller, M.S. Spatiotemporal changes in fungal growth and host responses of six yellow rust resistant near-isogenic lines of wheat. Plant Pathol. 2019, 68, 1320–1330. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Cheng, Y.; Chen, X.; Han, Q.; Huang, L.; Wei, G.; Kang, Z. Histological and cytological characterization of adult plant resistance to wheat stripe rust. Plant Cell Rep. 2012, 31, 2121–2137. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, J.; Pretorius, Z.A.; Moerschbacher, B.M.; Prins, R.; Van Der Westhuizen, A.J. Histopathology and PR-protein markers provide insight into adult plant resistance to stripe rust of wheat. Mol. Plant Pathol. 2008, 9, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Melichar, J.P.E.; Berry, S.; Newell, C.; MacCormack, R.; Boyd, L.A. QTL identification and microphenotype characterisation of the developmentally regulated yellow rust resistance in the UK wheat cultivar Guardian. Theor. Appl. Genet. 2008, 117, 391–399. [Google Scholar] [CrossRef]
- Cericola, F.; Jahoor, A.; Orabi, J.; Andersen, J.R.; Janss, L.L.; Jensen, J. Optimizing training population size and genotyping strategy for genomic prediction using association study results and pedigree information. A case of study in advanced wheat breeding lines. PLoS ONE 2017, 12, e0169606. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rodriguez-Algaba, J.; Thach, T.; Sørensen, C.K.; Hansen, J.G.; Lassen, P.; Nazari, K.; Hodson, D.P.; Justesen, A.F.; Hovmøller, M.S. Yellow rust epidemics worldwide were caused by pathogen races from divergent genetic lineages. Front. Plant Sci. 2017, 8, 1057. [Google Scholar] [CrossRef] [Green Version]
- Hovmøller, M.S.; Rodriguez-algaba, J.; Thach, T.; Sørensen, C.K. Wheat rust diseases. Methods Mol. Biol. 2017, 1659, 29–40. [Google Scholar] [CrossRef]
- McNeal, F.H.; Konzak, C.F.; Smith, E.P.; Tate, W.S.; Russell, T.S. A uniform system for recording and processing cereal research data. US Dept. Agric. Res. Serv. ARS 1971, 34, 121–143. [Google Scholar]
- Sørensen, C.K.; Thach, T.; Hovmøller, M.S. Evaluation of spray and point inoculation methods for the phenotyping of Puccinia striiformis on wheat. Plant Dis. 2016, 100, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal growth code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Baart, P.G.J.; Parlevliet, J.E.; Limburg, H. Effects of infection density on the size of barley and wheat leaf rust colonies before and on the size of uredia after the start of sporulation. J. Phytopathol. 1991, 131, 59–64. [Google Scholar] [CrossRef]
- Labouriau, R. postHoc: Tools for Post-Hoc Analysis. R Package, v.0.1.1. Available online: https://CRAN.R-project.org/package=postHoc (accessed on 20 March 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ma, J.; Qin, N.; Cai, B.; Chen, G.; Ding, P.; Zhang, H.; Yang, C.; Huang, L.; Mu, Y.; Tang, H.; et al. Identification and validation of a novel major QTL for all-stage stripe rust resistance on 1BL in the winter wheat line 20828. Theor. Appl. Genet. 2019, 132, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Dedryver, F.; Paillard, S.; Mallard, S.; Robert, O.; Trottet, M.; Nègre, S.; Verplancke, G.; Jahier, J. Characterization of genetic components involved in durable resistance to stripe rust in the bread wheat ‘Renan’. Phytopathology 2009, 99, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Basnet, B.R.; Singh, R.P.; Ibrahim, A.M.H.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Lan, C.; Rudd, J.C. Characterization of Yr54 and other genes associated with adult plant resistance to yellow rust and leaf rust in common wheat Quaiu 3. Mol. Breed. 2014, 33, 385–399. [Google Scholar] [CrossRef]
- Yang, E.N.; Rosewarne, G.M.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Tang, Z.X.; Sun, C.F.; Ren, Z.L.; Singh, R.P. QTL analysis of the spring wheat “Chapio” identifies stable stripe rust resistance despite inter-continental genotype × environment interactions. Theor. Appl. Genet. 2013, 126, 1721–1732. [Google Scholar] [CrossRef]
- Lin, F.; Chen, X. Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor. Appl. Genet. 2007, 114, 1277–1287. [Google Scholar] [CrossRef]
- Ren, R.S.; Wang, M.N.; Chen, X.M.; Zhang, Z.J. Characterization and molecular mapping of Yr52 for high-temperature adult-plant resistance to stripe rust in spring wheat germplasm PI 183527. Theor. Appl. Genet. 2012, 125, 847–857. [Google Scholar] [CrossRef]
- Zegeye, H.; Rasheed, A.; Makdis, F.; Badebo, A.; Ogbonnaya, F.C. Genome-wide association mapping for seedling and adult plant resistance to stripe rust in synthetic hexaploid wheat. PLoS ONE 2014, 9, e105593. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.L.; Wang, M.N.; Chen, X.M.; Lu, Y.; Kang, Z.S.; Jing, J.X. Identification of Yr59 conferring high-temperature adult-plant resistance to stripe rust in wheat germplasm PI 178759. Theor. Appl. Genet. 2014, 127, 935–945. [Google Scholar] [CrossRef]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2017, 19, 21–33. [Google Scholar] [CrossRef]
- Rubiales, D.; Niks, R.E. Characterization of Lr34, a major gene conferring nonhypersensitive resistance to wheat leaf rust. Plant Dis. 1995, 79, 1208–1212. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; William, H.M.; Bouchet, S.; Cloutier, S.; McFadden, H.; Lagudah, E.S. Leaf tip necrosis, molecular markers and β1-proteasome subunits associated with the slow rusting resistance genes Lr46/Yr29. Theor. Appl. Genet. 2006, 112, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Ramburan, V.P.; Pretorius, Z.A.; Louw, J.H.; Boyd, L.A.; Smith, P.H.; Boshoff, W.H.P.; Prins, R. A genetic analysis of adult plant resistance to stripe rust in the wheat cultivar Kariega. Theor. Appl. Genet. 2004, 108, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.A.; Smith, P.H.; Wilson, A.H.; Minchin, P.N. Mutations in wheat showing altered field resistance to yellow and brown rust. Genome 2002, 45, 1035–1040. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Uauy, C.; Brevis, J.C.; Chen, X.; Khan, I.; Jackson, L.; Chicaiza, O.; Distelfeld, A.; Fahima, T.; Dubcovsky, J. High-temperature adult-plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor. Appl. Genet. 2005, 112, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.; Sela, H.; Fahima, T.; Dubcovsky, J. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef] [Green Version]
- Segovia, V.; Hubbard, A.; Craze, M.; Bowden, S.; Wallington, E.; Bryant, R.; Greenland, A.; Bayles, R.; Uauy, C. Yr36 confers partial resistance at temperatures below 18 °C to U.K. isolates of Puccinia striiformis. Phytopathology 2014, 104, 871–878. [Google Scholar] [CrossRef] [Green Version]
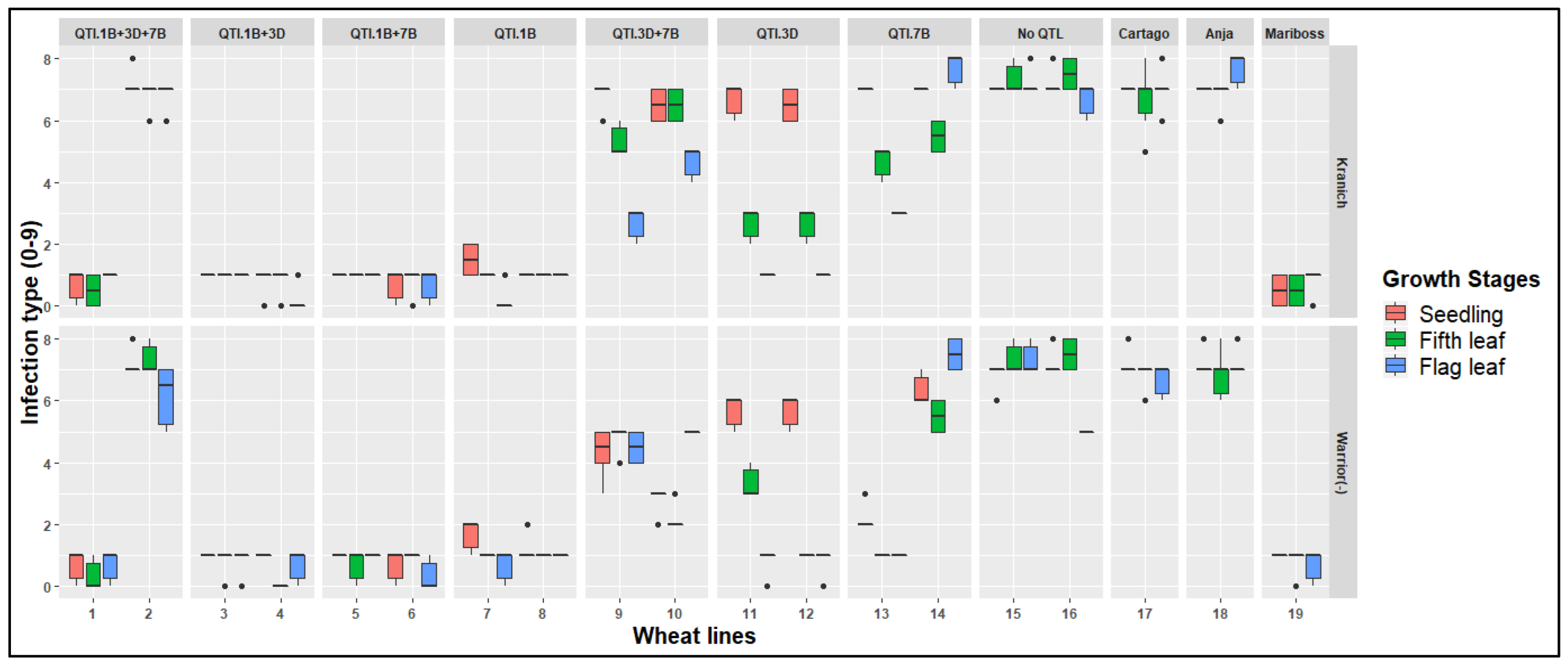
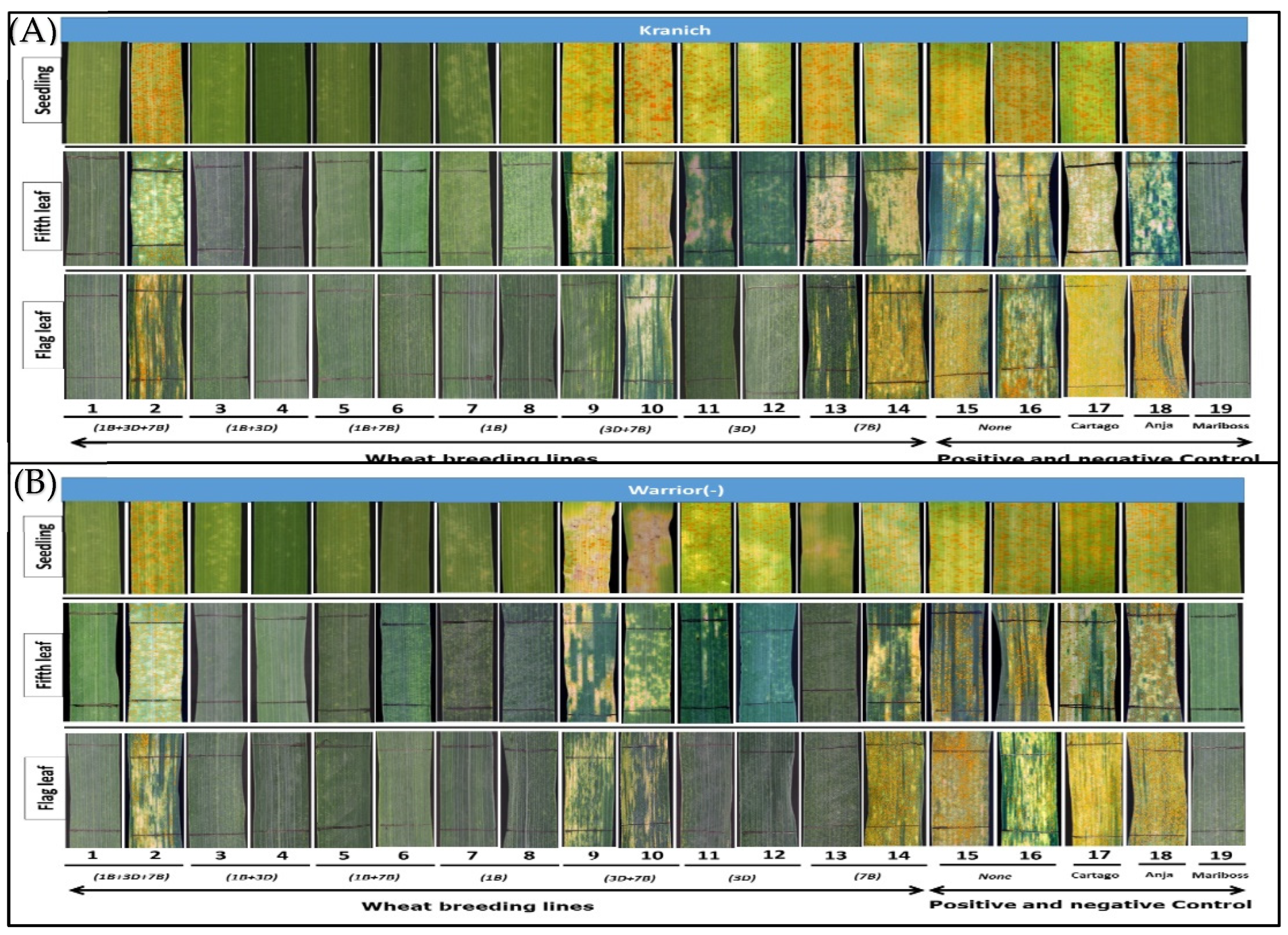
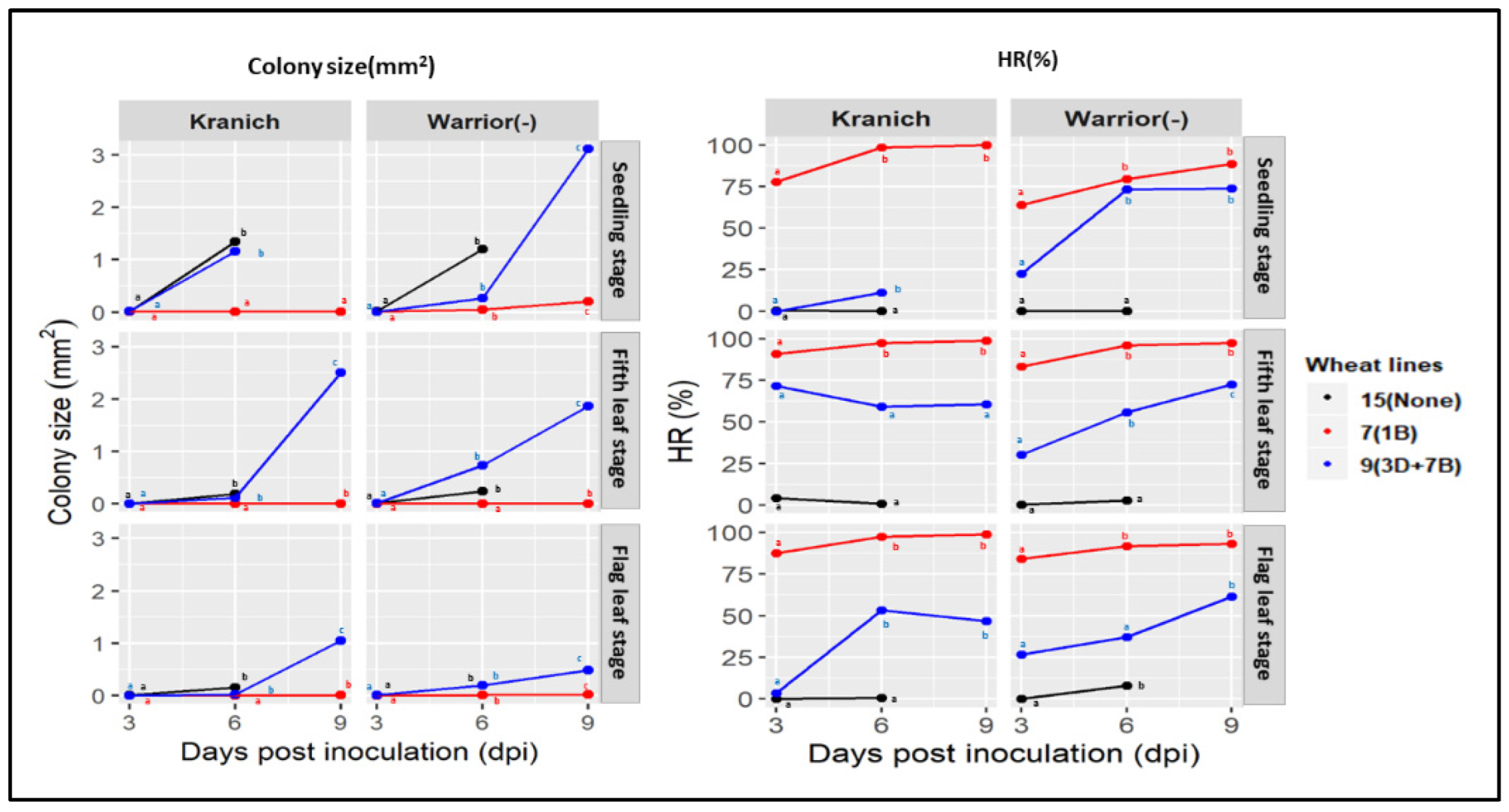
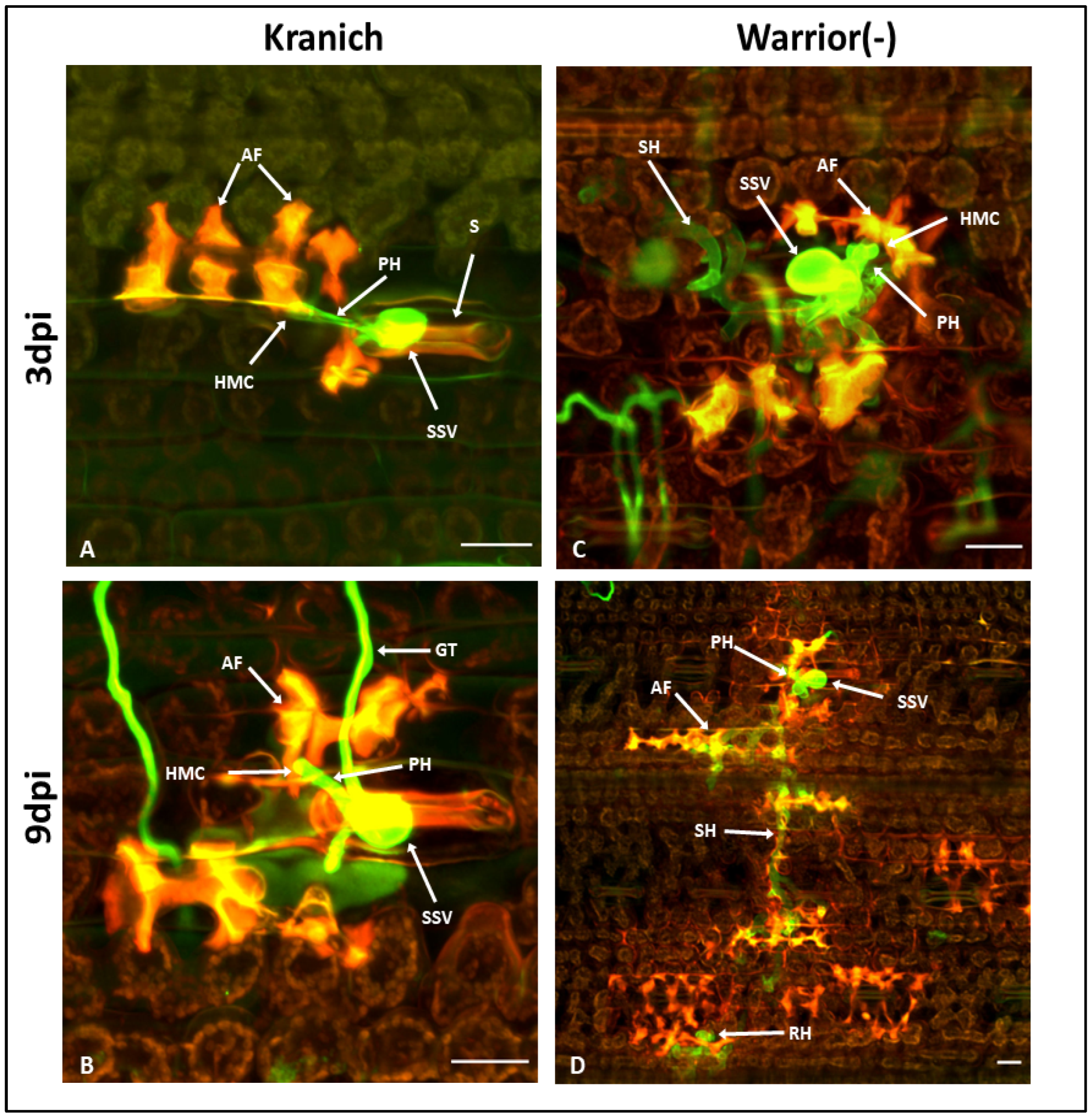
| Wheat Line | Line ID | No. QTL | QTL Chromosome Location a | Field Responses b | |||
|---|---|---|---|---|---|---|---|
| Year-I | Year-II | ||||||
| 1 | NOS-50904814 | 3 | 1B | 7B | 3D | 9 | 1 |
| 2 | NOS-50904823 | 3 | 1B | 7B | 3D | 9 | 5 |
| 3 | NOS-1800901 | 2 | 1B | - | 3D | 1 | 2 |
| 4 | NOS-1807218 | 2 | 1B | - | 3D | 2 | 2 |
| 5 | NOS-1705804 | 2 | 1B | 7B | - | 1 | 1 |
| 6 | NOS-1705808 | 2 | 1B | 7B | - | 1 | 1 |
| 7 | NOS-50906215 | 1 | 1B | - | - | 2 | 2 |
| 8 | NOS-50906216 | 1 | 1B | - | - | 1 | 1 |
| 9 | NOS-70140801 | 2 | - | 7B | 3D | 5 | 4 |
| 10 | NOS-70140808 | 2 | - | 7B | 3D | 6 | 5 |
| 11 | NOS-51014910 | 1 | - | - | 3D | 2 | 1 |
| 12 | NOS-51014911 | 1 | - | - | 3D | 2 | 1 |
| 13 | NOS-51010312 | 1 | - | 7B | - | 5 | 3 |
| 14 | NOS-51010313 | 1 | - | 7B | - | 4 | 3 |
| 15 | NOS-51005012 | None | - | - | - | 4 | 5 |
| 16 | NOS-51005019 | None | - | - | - | 4 | 4 |
| 17 | Cartago | Susceptible Control | |||||
| 18 | Anja | Susceptible Control | |||||
| 19 | Mariboss | Resistant Control | |||||
| 20 | AvocetYr15 (seedling test) | Resistant Control | |||||
| Isolates ID | Common Race Name | Origin | Virulence for Yr Genes a | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 15 | 17 | 25 | 27 | 32 | AvS | Amb | Sp | |||
| DK02d/12 | Kranich | Denmark | 1 | 2 | 3 | - | - | 6 | 7 | 8 | 9 | - | - | 17 | 25 | - | 32 | AvS | Amb | - |
| DK110/15 | Warrior (−) | Denmark | 1 | 2 | 3 | 4 | - | 6 | 7 | - | 9 | - | - | 17 | 25 | - | 32 | AvS | - | Sp |
| DK92/02 | Boston | Denmark | 1 | 2 | 3 | - | - | - | - | - | 9 | - | 15 | (17) | 25 | - | - | AvS | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, K.; Hovmøller, M.S.; Labouriau, R.; Justesen, A.F.; Orabi, J.; Andersen, J.R.; Sørensen, C.K. Macroscopic and Microscopic Phenotyping Using Diverse Yellow Rust Races Increased the Resolution of Seedling and Adult Plant Resistance in Wheat Breeding Lines. Agronomy 2022, 12, 1062. https://doi.org/10.3390/agronomy12051062
Saleem K, Hovmøller MS, Labouriau R, Justesen AF, Orabi J, Andersen JR, Sørensen CK. Macroscopic and Microscopic Phenotyping Using Diverse Yellow Rust Races Increased the Resolution of Seedling and Adult Plant Resistance in Wheat Breeding Lines. Agronomy. 2022; 12(5):1062. https://doi.org/10.3390/agronomy12051062
Chicago/Turabian StyleSaleem, Kamran, Mogens Støvring Hovmøller, Rodrigo Labouriau, Annemarie Fejer Justesen, Jihad Orabi, Jeppe Reitan Andersen, and Chris Khadgi Sørensen. 2022. "Macroscopic and Microscopic Phenotyping Using Diverse Yellow Rust Races Increased the Resolution of Seedling and Adult Plant Resistance in Wheat Breeding Lines" Agronomy 12, no. 5: 1062. https://doi.org/10.3390/agronomy12051062
APA StyleSaleem, K., Hovmøller, M. S., Labouriau, R., Justesen, A. F., Orabi, J., Andersen, J. R., & Sørensen, C. K. (2022). Macroscopic and Microscopic Phenotyping Using Diverse Yellow Rust Races Increased the Resolution of Seedling and Adult Plant Resistance in Wheat Breeding Lines. Agronomy, 12(5), 1062. https://doi.org/10.3390/agronomy12051062






