Abstract
Coconut is widely used as a food source in producing countries, and during consumption, the waste that is generated needs to be reduced through by-products processing to ensure environmental sustainability. This study aimed to assess the functionality of by-products (endo- and mesocarp) of coconuts at early and mature stages. The aqueous and ethanolic (50 and 100% ethanol in water) extracts of coconut by-products were evaluated for the DPPH radical scavenging activity and subjected to linoleic acid-β-carotene system assay in contrast with synthetic antioxidants. Ultrasound-producing extract of young coconut mesocarp provided the highest antioxidant activity with a lower IC50 value (117 µg mL−1) than butylhydroxytoluene (BHT, 170 µg mL−1). Based on the linoleic acid-β-carotene system assay, the extract exhibited a higher antioxidant activity (1.25×) than tertiary butylhydroquinone (TBHQ, 200 µg mL−1); and comparable with butylhydroxyanisole (BHA, 250 µg mL−1). Therefore, extracts of coconut by-products, particularly the young mesocarp, can be an alternative natural antioxidant.
1. Introduction
An increased awareness of a healthy lifestyle linked to food preference and consumption has been highlighted in the global market. Currently, food manufacturers are trying to utilize natural sources because consumers prefer natural over synthetic antioxidants. Evidence suggests that agricultural products rich in phenolic compounds, including coconut (Cocos nucifera) fruit, provide significantly positive antioxidant effects, [1,2,3,4].
Phenolic compounds described in coconut fruit are catechins and phenolic acids, such as protocatechuic, chlorogenic, and vanillic acid [5,6,7,8,9,10]. The level and composition of the phenolic compounds in coconut fruits may differ among the varieties [11,12,13,14]. However, frequently consumed young coconut fruit provides considerable antioxidant effects. The green coconut water mitigates the oxidative stress in hypoglycemia and hypertensive rat model [15,16,17,18]. Additionally, the maturation level is also essential in characterizing the antioxidant compounds in the fruit [12,13]. Henceforth, the aforementioned variables affecting the antioxidant activities need to be studied to optimize the use of coconut fruit.
Indonesia is one of the largest coconut producers, contributing up to 27% of world production of coconuts [19]. Within the country, the fruit is typically processed by food industries into oil, copra, virgin coconut oil (VCO), coconut milk, and desiccated coconut. These coconut-derived products require a specific type of fruit as the raw material, mainly based on the maturity levels: young (6-month-old) and mature (12- month-old) fruit. Young coconut flesh is most suited as an ingredient for beverage products. In contrast, mature fruit is frequently processed into several products, such as coconut milk, dried shredded coconut flesh, and VCO. Due to the coconut processing, some by-products were generated, including the meso- and endocarp of the fruits.
Furthermore, in keeping with the zero waste strategy, the utilization of by-products generated from food and agricultural industries is recently favorable due to the availability of advanced extraction technologies [20,21]. Earlier reports have disclosed a considerable amount of phenolic compounds and the antioxidant effects contained in extracts from tomato pomace, grape peel, coffee spent, and other agro-industrial by-products [22,23]. As many parts of the coconut have proven to contain phenolic compounds providing antioxidant activities [4,24,25,26], it is reasonable to suppose that the extract of coconut processing by-products may deliver similar benefits.
The coconut mesocarp is the fibrous mid-part contributing 85% of the whole fruit, whilst the endocarp is the hardest part accounting for 10% of the coconut fruit. Some previous studies have reported that both meso- and endocarp contain a considerable amount of antioxidant compounds. Young coconut mesocarp has been reported to provide radical scavenging activities (DPPH) ranging from 5.72 [27] to 0.032 mg mL−1 [7], whereas the endocarp exerts 10.89 µg mL−1 in a cell line [10,28]. To earn these advantages, effective extraction of the antioxidant compounds from coconut by-products is therefore essential.
The conventional methods that have been utilized to extract antioxidant compounds are Soxhlet, maceration, mechanical agitation, and hot water extraction [6,8,9,29,30]. These methods are time-consuming (2 to 144 h) and operate at high temperatures (up to 100 °C) to increase the extraction rates, leading to the degradation of thermal labile phenolic compounds. To overcome the problem described, advanced methods are proposed with the aid of sonication.
The pulse-duty cycle of an ultrasound-assisted extraction defines the release of cavitation that passes through an elastic medium. The extraction mechanism is based on cavitation bubbles that can grow during rarefaction phases and decrease in size during compression cycles. When the size of these bubbles reaches a critical point, the bubbles collapse during a compression cycle and destroy the cell walls of the plant matrix [21,31,32,33]. This approach facilitates a faster extraction. Additionally, applying low to moderate extraction temperature in the sonication process tends to increase antioxidant compounds recovered from the matrices [34].
Ultrasounds have been applied to improve the extraction of several different kinds of both vegetables and fruits [35]. Some of the most interesting compounds extracted using ultrasound-assisted extraction are powerful antioxidant compounds [36] including simple flavonols from onion [37], anthocyanins from blackcurrant [38], stilbenes from grape canes, and simple phenolics from red algae [39]. Ultrasound-assisted extraction has proved to be more efficient than the conventional extraction methods [40,41] and with similar or even better recovery rates than other green extraction techniques [42,43].
Therefore, this research aimed to evaluate the antioxidant activities of ultrasound-producing extracts from coconut by-products (meso- and endocarp) with different maturation levels (6 and 12 months). Ultimately, the antioxidant activities of the studied extracts were compared with the commercial synthetic antioxidants.
2. Materials and Methods
2.1. Materials
Coconut fruits at two different maturation stages, i.e., young (6 months old) and mature (12 month-old), originated from local farmers in Bantul Region, Yogyakarta, Indonesia. Chemicals such as ethanol (ethanol (gradient grade for liquid chromatography with ≥99.9% (GC) purity), methanol, water, sodium carbonate, 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), Folin–Ciocalteu’s reactive, gallic acid, β-carotene tween-20, and synthetic antioxidants, including butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and tertiary butylhydroquinone (TBHQ), were acquired from Sigma-Aldrich, Germany.
2.2. Sample Preparation
Coconut samples were washed with clean water. Subsequently, the meso- and endocarp (Figure 1) were manually separated from other parts of the coconut fruit and cut into cuboid shapes (40 × 10 × 5 mm). Afterward, the two studied parts were dried in a cabinet dryer at 50 °C for 48 h. The dried samples were ground until 60 mesh size and stored in tight plastic containers at ambient temperature, which were labeled to identify young mesocarp (YM), young endocarp (YE), mature mesocarp (MM), and mature endocarp (ME).
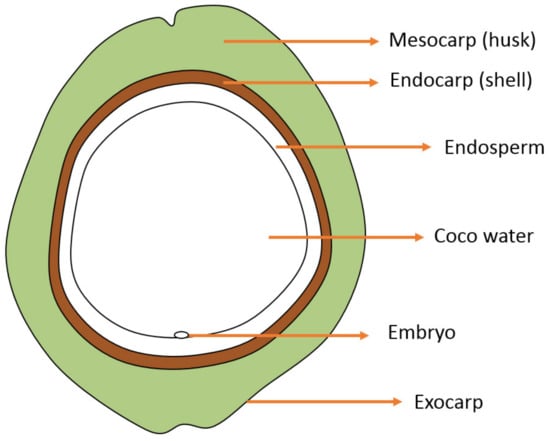
Figure 1.
Transverse structure of coconut fruit.
2.3. Ultrasound-Assisted Extraction
Extraction was conducted using an ultrasonic bath Transsonic Elma (Elma Schmidbauer GmbH, Gottlieb-Daimler-Str, Germany) with a frequency of 37 kHz, maximum power of 320 W, and a volume capacity of 2750 mL. A sample of 10 g was weighed and placed in a 250 mL flask. Different compositions of water and ethanol (0:100, 50:50, and 100:0) were used as the extraction solvent and poured into the flask containing the sample with a sample-to-solvent ratio of 1:20 (w/v). The sample was subjected to extraction for 1 h at 45 ± 5 °C. The resulting extract was then filtered using a Whatman No. 1 filter paper and evaporated under a vacuum at 45 °C to remove the solvent. Subsequently, the extract was weighed to calculate the extraction yield and stored in a refrigerator at 4 °C until analysis. The yield was expressed in weight (%, w/w) with respect to the dry basis of coconut by-products.
2.4. Antioxidant Activity
2.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)-Radical Scavenging Activity (RSA) Assays
The DPPH-RSA of coconut by-product extracts was determined according to the method of Brand-Williams, et al. [44] with minor modification. The extract was accurately weighed (0.01 g), dissolved into 10 mL methanol, and diluted 5× by methanol. Subsequently, 100 μL of the liquid extract at concentrations ranging from 0 to 250 μg mL−1 were mixed with a 1.9 mL DPPH solution (0.06 mM). The mixture was then homogenized and incubated at ambient temperature for 30 min in the dark. The absorbance of the mixture was measured by a UV–Vis spectrophotometer (UV-2450, Shimadzu Corporation, Kyoto, Japan) at 515 nm using DPPH solution as the blank. The DPPH-RSA was indicated by the IC50 value measuring the concentration of sample required to scavenge 50% of DPPH free radical. The IC50 of synthetic antioxidants, i.e., BHT, BHA, and TBHQ, were also determined with the same procedure. The scavenging capacity of DPPH was calculated according to the following formula:
2.4.2. β-Carotene-Linoleic-Acid System Assay
The β-carotene bleaching method was applied to determine the antioxidant activity of the aqueous and ethanolic extracts of coconut by-products. β-carotene (10 mg) was dissolved in 10 mL of chloroform. Subsequently, the solution (4 mL) was mixed with 40 mg of linoleic acid and 400 mg of Tween-20 in dark conditions. Chloroform was purged using nitrogen gas for 2 min. The remaining emulsion was diluted with 100 mL distilled water and then was agitated for 2 min. Thereafter, the β-carotene emulsion (200 μL) was transferred into a test tube containing the extract of coconut by-product to obtain a concentration of 20 μg mL−1. A control sample was prepared using distilled water instead of sample extract in the β-carotene-linoleic-acid system. BHA, BHT, and TBHQ with a concentration of 200 μg mL−1 were used for comparative purposes. The tubes were placed at room temperature. The oxidation of β-carotene emulsion was monitored spectrophotometrically (UV-2450, Shimadzu Corporation, Kyoto, Japan) by measuring at every 30 min for 4 h at 450 nm.
2.5. Total Phenolic Compounds Determination
Total phenolic compounds were determined by the Folin-Ciocalteu method. Up to 200 μL of diluted extract (200 μg mL−1) and 800 μL of 10% Folin–Ciocalteu reagent were mixed. After 2 min, 1 mL of 7.5% sodium carbonate was added to the mixture and the mixture stood for 2 h at room temperature. The absorbance values were measured by a UV/Vis spectrophotometer at 765 nm (UV-2450, Shimadzu Corporation, Kyoto, Japan). Subsequently, a calibration curve of gallic acid was prepared at concentrations ranging from 10 to 100 μg mL−1. The results were expressed as gallic acid equivalents in the dry matrix (mg GAE g−1 of dry matter).
2.6. Individual Phenolic Compounds Identification
The identification of phenolic compounds was carried out on a Shimadzu HPLC system (Kyoto, Japan) equipped with a binary pump (LC-20AD), auto-sampler (SIL-HTC, Shimadzu, Japan), and UV-Vis SPD M-20A diode array detector (DAD). The detector was set for compound identification using a three-dimensional (3D) scan mode in the wavelength range from 200 to 400 nm. The individual phenolic compounds in the sample (10 µL) were separated on a reverse-phase C18 column Shim-Pac GIST Shimadzu (150 mm, 4.6 mm, 5 µm) at 30 °C. Mobile phase A (2% acetic acid and 5% methanol in water) and phase B (2% acetic acid and 88% methanol in water) were pumped at a flow rate of 1 mL min−1. The following gradient was applied (time, % solvent B): 0 min, 0%; 0.02 min, 18.3%; and 10−13 min, 100%. The identification was performed by comparing the retention time and UV-Vis 3D spectra of chromatographic peaks of the sample with standard compounds. Additionally, a spiking method was also conducted to confirm the identity of the compound.
2.7. Statistical Analysis
Significancy level of the studied variables (part of coconut by-products, meso- and endocarp; maturation levels, 6 and 12 months) were statistically calculated using analysis of variance (ANOVA). Provided that the variables affect the responses (antioxidant activities of the extracts from coconut by-products), a Duncan test with 95% confidence was performed to check the differences among the means using IBM SPSS Statistics software, version 20 (IBM Company, Armonk, NY, USA). All the experiments were conducted in triplicate.
3. Results and Discussion
The foremost study in this research was the evaluation of the sample matrices and extraction solvents on the level of phenolic compounds in the extract. Subsequently, the antioxidant activities of phenolic compounds extracted from the coconut by-products (meso- and endocarp) at two maturation levels were evaluated. Compounds responsible for the antioxidant activity were also identified.
3.1. Effect of the Sample Matrices and Solvent on the Extraction Yield
The presence of phenolic compounds in coconut by-products varies in composition and levels and usually forms complexes with other compounds in the matrices. Hence, ultrasound-assisted extraction was conducted to separate the phenolic compounds from the complex matrices to obtain a high extraction yield. The assistance of ultrasonic waves in this study probably degraded the sample matrix to promote extraction yield [31], as shown in Figure 2.
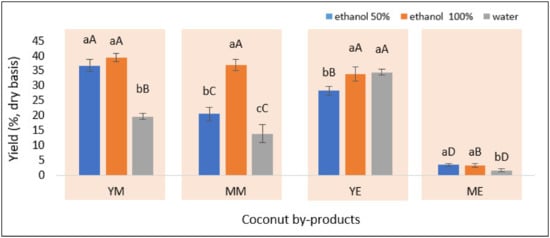
Figure 2.
The yield of phenolic compounds extracted from YM—young coconut mesocarp, YE—young coconut endocarp, MM—mature coconut mesocarp, and ME—mature coconut endocarp. Different letters within the same coconut by-products (lower case letters) and solvents (capital letters) indicate significant differences at a 5% significance level according to Duncan’s multiple range test (DMRT).
The different matrices of young mesocarp (YM), young endocarp (YE), mature mesocarp (MM), and mature endocarp (ME) are mainly due to the compositions of the cell wall that include lignin, cellulose, and hemicellulose [45,46,47]. The unique composition of the cell wall defines the hardness of each matrix [48,49,50,51]. The cavitation generated by the ultrasound wave easily degrades the matrix with a lower composition of lignin, cellulose, and hemicellulose [48,49], as occurred in the young coconut by-products (YM and YE). However, despite being produced by the mature coconut fruit, the mesocarp (MM) is relatively more tender than the endocarp (ME).
Mature coconut by-products (MM and ME) produced different extraction yields, i.e., 19.70–39.41% and 1.28–3.47%, respectively. However, the young coconut by-products (YM and YE) remain comparable, ranging from 13.93–36.94% and 24.53–39.21%. This finding explained that the tender matrix of the coconut mesocarp is more easily degraded by ultrasonic waves than the hard endocarp. Ultrasonic waves destroy the matrix to facilitate a quick release of phenolic compounds, leading to an increased extraction yield. Hence, the more tender the matrix subjected to ultrasonic waves, the more compounds are released into the extraction solvent, producing a higher yield.
In addition to the matrix effect, the extraction yield is also influenced by the organic solvent type used [52,53,54]. Figure 2 also reveals that the phenolic compounds extracted from different matrices of coconut by-products required specific extraction solvents. The mesocarp samples (YM and MM) produced a higher level of phenolic compounds in the extract by applying 100% ethanol as the extraction solvent. In contrast, water was comparable with the pure ethanol to extract phenolic compounds from the endocarp of the young coconut fruit, whereas 50% ethanol was also appropriate for the mature fruit. This result agreed with Arivalagan et al. [4], which reported different optimum solvents for each matrix because the composition of phenolic compounds in the matrix also varies according to their polarity.
3.2. Effect of the Sample Matrices and Solvent on the Antioxidant Activities
3.2.1. DPPH Radical Scavenging Activity
DPPH radical scavenging activity is expressed in IC50 value, i.e., the minimum concentration needed to inhibit 50% of DPPH free radicals. A lower IC50 value indicates high effectiveness in inhibiting free radicals. The IC50 values of phenolic compounds extracted from the coconut by-products (Figure 3) were measured and compared with commercial synthetic antioxidants (BHT, BHA, and TBHQ).
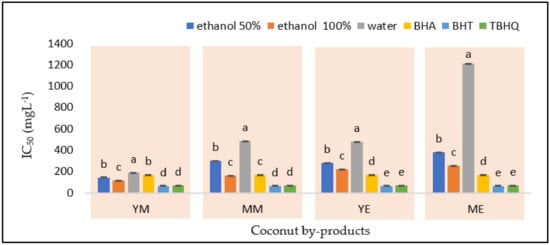
Figure 3.
The radical scavenging activity by DPPH of extracts from YM−young coconut mesocarp; YE−young coconut endocarp; MM−mature coconut mesocarp; ME−mature coconut endocarp. Different letters within the sample indicate significant differences among the means and control (BHA, BHT, and TBHQ) at a 5% significance level according to Duncan’s multiple range test.
The IC50 values of the extracts from coconut by-products ranged from 117.66 (YM) to 1209.87 mg L−1 (ME) and were in the same ranges as reported by a previous study for similar samples [14]. In comparison with synthetic antioxidants, the ethanolic extract from YM provided a lower IC50 value (p < 0.05) than BHA, whereas the ethanolic extract from MM exhibited a similar result to the BHA (170.37 mg L−1). On the contrary, the IC50 values of YE and ME were higher than all synthetic antioxidants. These results confirmed that two (YM and MM) out of four studied coconut by-product samples contain natural antioxidants that can produce similar or even higher antioxidant results than BHA. Earlier studies also supported the evidence of antioxidant activities exposed by the extracts obtained through conventional extractions (7 days maceration and 30 min agitation) using methanol (5720 mg L−1) and ethyl acetate (5970 mg L−1) from coconut by-products [27,55]. Hence, sonication in this work provides extract with higher antioxidant activities, whereas the extraction was more practical and performed in a shorter time [32].
3.2.2. Linoleic-Acid-β-Carotene Bleaching System
The measurement of antioxidant activities in the linoleic acid-β-carotene system was performed to determine the potential of natural antioxidants derived from coconut by-products. The measurement principle is that the free radical of linoleic acid attacks the highly unsaturated β-carotene system. The antioxidant activities of the four extracts of coconut by-products, positive control, BHT, BHA, and TBHQ, are presented in Figure 4.

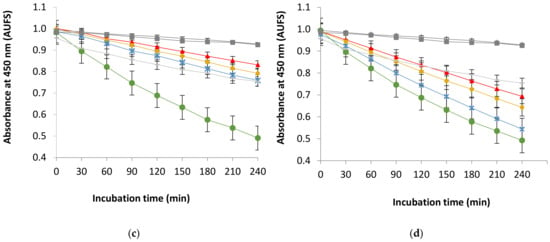
Figure 4.
The bleaching inhibition in the linoleic acid-β-carotene system by antioxidants in YM—young coconut mesocarp (a); YE—young coconut endocarp (b); MM—mature coconut mesocarp (c), and; ME—mature coconut mesocarp (d) extracted using different solvents compared with synthetic antioxidants (BHT, BHA, and TBHQ) and control.
The optical density (OD) that indicates the stability of the β-carotene system (control sample) will rapidly decrease during incubation in the absence of antioxidants. On the contrary, the presence of antioxidants prevents the degradation of the β-carotene system by two mechanisms: (i) by protecting the target substrate from the oxidation initiator (secondary antioxidant), particularly by scavenging the radical substance that is responsible for the oxidation initiation stage (O •−;(ii) by inhibiting the propagation or chain-breaking antioxidants which break the radical chain propagator (LOO •) [56]. The high antioxidant activity is indicated by the high trendline of bleaching inhibition compared with synthetic antioxidants.
All samples and synthetic antioxidants (BHT, BHA, and TBHQ) successfully inhibited β-carotene degradation. The ethanol 100% (OD240 min = 0.90) and 50% (OD240 min = 0.91) extracts of YM was found to have a similar inhibition pattern compared with BHA (OD240 min = 0.92) and BHT (OD240 min = 0.92), whereas it was higher than TBHQ (OD240 min = 0.76) (Figure 4a). On the other hand, aqueous extract of YM (OD240 min = 0.71) exhibited a lower inhibition compared with all synthetic antioxidants (Figure 4a). MM, which was extracted using ethanol 100% (OD240 min = 0.82), 50% (OD240 min = 0.87), exhibited higher inhibition compared with TBHQ (OD240 min = 0.76), whereas the water extract (OD240 min = 0.76) provided a similar result to TBHQ (Figure 4c). The antioxidant extracts from YM and MM exhibited more potent antioxidant activity than TBHQ; in particular, YM has the most similar trend of bleaching inhibition with BHA and BHT (Figure 4a). The results were consistent with reported preceding studies [57,58,59,60].
Conversely, the bleaching inhibition of endocarp from young (YE) and mature coconut (ME) was lower than synthetic antioxidants (Figure 4b,d). Endocarp from young (YE) and mature coconut (ME) that were extracted in ethanol 50% produced OD240 min 0.82 and 0.76, respectively, which were higher than TBHQ (OD240 min = 0.76). On the contrary, the ethanol 100% and water extracts from both YE and ME had lower OD240 min than all synthetic antioxidants (Figure 4c,d) as investigated by several studies [61,62,63,64]. These data implicate different results by DPPH measurement because of different mechanisms of antioxidant activities [56,65]. The activity of the antioxidant compound in the studied extracts defined by DPPH measurement acted as a radical scavenger, whilst, in the β-carotene system assay, the antioxidant compound worked as a chain initiation-blocker.
3.3. Effect of the Sample Matrices and Solvent on the Phenolic Compounds
The extractability of phenolic compounds was significantly defined by the type of matrix and solvent (p < 0.05), in which the highest phenolic compounds (395.97 ± 4.78 mg GAE g−1) were extracted from YM using 50% ethanol (Figure 5). Furthermore, the reported concentration in this study is higher than the result from former research on the extraction of phenolic compounds from coconut mesocarp by maceration using methanol (126.7 mg GAE g−1) and ethyl acetate (249.2 mg GAE g−1) [27].
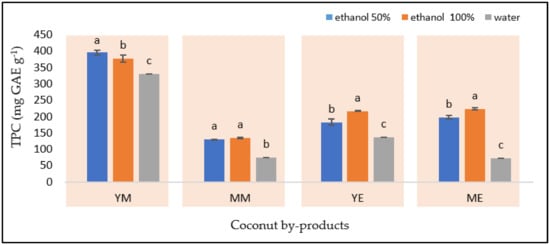
Figure 5.
The total phenolic compounds in YM−young coconut mesocarp, YE−young coconut endocarp, MM−mature coconut mesocarp; and ME−mature coconut endocarp. Different letters within the sample indicate significant differences among the means at a 5% significance level according to Duncan’s multiple range test (DMRT).
The solvent composition of ethanol:water (1:1) was also an appropriate solvent for extracting phenolic compounds from MM (129.37 mg GAE g−1). In contrast, 100% ethanol was suitable for endocarps, viz., YE (223.25 ± 3.54 mg GAE g−1) and ME (216.65 ± 1.19 mg GAE g−1). However, the phenolic compounds of coconut by-products were scarcely recovered by water. The results suggest that the polarity of the phenolic compounds in coconut by-products was lower in water and thus more soluble in organic solvents. These findings agree with the previous studies on the maceration of endocarp of coconut fruit using different extraction solvents in which water (6.96 GAE g−1) recovered the lowest concentration of phenolic compounds compared with methanol (10.56 GAE g−1) and ethanol (8.18 GAE g−1) [66].
The addition of water in organic solvent plays a role in polarity changes of the extraction solvent that can alter the solubility of phenolic compounds. The aqueous solvent system normally increases the solubility of organic matrices such as protein and carbohydrates that can interfere with phenolic compounds during the extraction.
Among the coconut by-products, the young mesocarp was selected as the highest natural antioxidant source according to the level of total phenolic compounds, DPPH radical scavenging activity, and the ability to prevent the oxidation of the linoleic-acid-β-carotene system. Therefore, the individual phenolic compounds were identified in the ethanolic extract from the young coconut mesocarp (Figure 6).
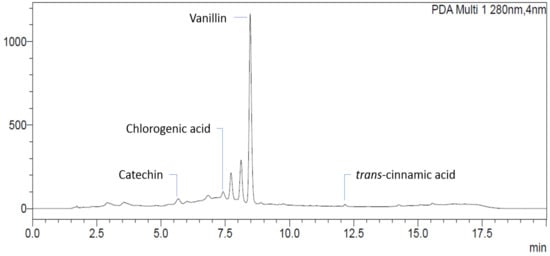
Figure 6.
Identification of phenolic compounds in young mesocarp extracted by 50% ethanol.
The identification was performed by comparing the spectra and retention time of the sample peak with the corresponding standards and further confirmed by the spiking method. The compounds identified were catechin, chlorogenic acid, vanillin, and trans-cinnamic acid, as reported by some former studies [9,67,68].
3.4. Correlation between Total Phenolic Compounds and Antioxidant Activities
The Pearson correlation analysis was performed to determine the correlation between total phenolic compounds and antioxidant activities of the extracts from coconut by-products (Figure 7). A strong positive correlation (0.87) between total phenolic content and antioxidant activity of the DPPH (IC50) measurement was found. This result was supported by previous work that revealed the correlation between the antioxidant activities and phenolic compounds in several herbs [69]. Hence, the phenolic compounds notably contributed to the antioxidant properties of the extract of coconut by-products by scavenging the free radicals. In addition, the phenolic compounds contained in the extract that work as chain initiation-blockers resulted in an intermediate positive correlation with linoleic acid-β-carotene (0.55). There are some previously published results also showing correlation between the level of phenolic compounds and the antioxidant results for the extracts from coconut mesocarp and exocarp [55].
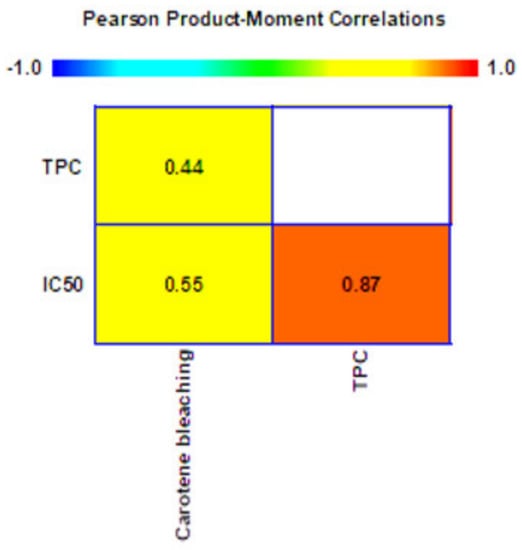
Figure 7.
Pearson correlation of total phenolic content, DPPH radical scavenging activity, and linoleic−acid−β−carotene bleaching system.
4. Conclusions
High antioxidant levels were found for some extracts produced with different solvents and from different coconut by-products. Both the specific conditions of the coconut by-products, i.e., young or mature, and the solvents used for the extraction, ethanol or water, determine the antioxidant levels found for the extracts. Specifically the mesocarp from a young fruit, extracted with pure ethanol or a 50/50 mixture ethanol water, produced as many antioxidant effects as some synthetic antioxidants, including BHA. Two different antioxidant activities were confirmed for the extracts, radical scavenger in DPPH method and chain initiation-blocker in the β-carotene system assay. It has been also demonstrated that the phenolic composition of the extracts affects the antioxidant levels as they showed a very high correlation. This study offers a new opportunity to use coconut mesocarp as a source of natural antioxidants that can produce as many antioxidant effects as some synthetic antioxidants.
Author Contributions
Conceptualization, L.L., U.S., and W.S.; methodology, L.L., U.S., and W.S.; software L.L.; validation, W.S., U.S., and M.P.; formal analysis, L.L. and W.S.; investigation, L.L.; resources, L.L. and U.S.; data curation, L.L. and S.; writing—original draft preparation, L.L. and W.S; writing—review and editing, M.P., S. and U.S.; visualization, W.S., and M.P.; supervision, U.S., W.S., S. and M.P.; project administration, U.S.; funding acquisition, L.L. and U.S. All authors have read and agreed to the published version of the manuscript.
Funding
L.L. acknowledges the financial support through a research grant of “Peningkatan Kualitas Publikasi Internasional (PKPI)/Sandwich like 2021” and “Pendidikan Magister menuju Doktor untuk Sarjana Unggul (PMDSU)” with a contract number of 6/E1/KP.PTNBH/2021 and 2354/UN1/DITLIT/DIT-LIT/PT/2021 awarded by the Directorate General of Higher Education (DIKTI), Ministry of Research, Technology and Higher Education of the Republic of Indonesia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
This report forms part of the research activity carried out by L.L. at Laboratories facilitated by the Department of Food and Agricultural Product Technology, Universitas Gadjah Mada, Indonesia and Department of Analytical Chemistry, University of Cadiz, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adekola, K.A.; Salleh, A.B.; Zaidan, U.H.; Azlan, A.; Chiavaro, E.; Paciulli, M. Total phenolic Content, Antioxidative and Antidiabetic Properties of Coconut (Cocos Nucifera L.) Testa and Selected Bean Seed Coats. Ital. J. Food Sci. 2017, 29, 741–753. [Google Scholar] [CrossRef]
- Akhter, A.; Zaman, S.; Ali, U.; Ali, Y.; Miah, M.A.J. Isolation of Polyphenolic Compounds from the Green Coconut (Cocos Nucifera) Shell and Characterization of Their Benzoyl Ester Derivatives. J. Sci. Res. 2009, 2, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Appaiah, P.; L., S.; A. G., G.K.; G., S.K. Phytochemicals and Antioxidant Activity of Testa Extracts of Commercial Wet and Dry Coconuts and Cakes. Int. Res. J. Pharm. 2016, 7, 9–13. [Google Scholar] [CrossRef]
- Arivalagan, M.; Roy, T.K.; Yasmeen, A.M.; Pavithra, K.C.; Jwala, P.N.; Shivasankara, K.S.; Manikantan, M.R.; Hebbar, K.B.; Kanade, S.R. Extraction of Phenolic Compounds with Antioxidant Potential from Coconut (Cocos Nucifera L.) Testa and Identification of Phenolic Acids and Flavonoids Using UPLC Coupled with TQD-MS/MS. LWT-Food Sci. Technol. 2018, 92, 116–126. [Google Scholar] [CrossRef]
- Onyechi, O.; Elijah, P.; Nkechi, J. Available Online through Phytochemical Analysis of Cocos Nucifera L. J. Pharm. Res. 2010, 3, 280–286. [Google Scholar]
- Kibria, A.A.; Nessa, K.; Rahman, M.M. Extraction and Evaluation of Phytochemicals From Green Coconut (Cocos Nucifera) Shell. Malays. J. Halal Res. J. 2018, 1, 19–22. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mitra, A. The Antioxidant and Antimicrobial Properties of the Methanolic Extract from Cocos Nucifera Mesocarp. Food Chem. 2008, 107, 994–999. [Google Scholar] [CrossRef]
- Silva, R.R.; e Silva, D.O.; Fontes, H.R.; Alviano, C.S.; Fernandes, P.D.; Alviano, D.S. Anti-Inflammatory, Antioxidant, and Antimicrobial Activities of Cocos Nucifera Var. Typica. BMC Complementary Altern. Med. 2013, 13, 107. [Google Scholar] [CrossRef] [Green Version]
- Valadez-carmona, L.; Cortez-Garcia, R.M.; Plazola-Jacinto, C.P.; Ortiz-Moreno, A.; Necoechea-Mondrago, H. Effect of Microwave Drying and Oven Drying on the Water Activity, Color, Phenolic Compounds Content and Antioxidant Activity of Coconut Husk (Cocos nucifera L.). J. Food Sci. Technol. 2016, 53, 3495–3501. [Google Scholar] [CrossRef] [Green Version]
- Elsbaey, M.; Abdel, B.F.M. Coconut Waste as a Potential Source for Cytotoxic and Antioxidant Compounds. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Solangi, A.H.; Iqbal, M.Z. Chemical Composition of Meat (Kernel) and Nut Water of Major Coconut (Cocos nucifera L.) Cultivars at Coastal Area of Pakistan. Pak. J. Bot. 2011, 43, 357–363. [Google Scholar]
- Dey, G.; Sachan, A.; Ghosh, S.; Mitra, A. Detection of Major Phenolic Acids from Dried Mesocarpic Husk of Mature Coconut by Thin Layer Chromatography. Ind. Crop. Prod. 2003, 18, 171–176. [Google Scholar] [CrossRef]
- Perera, C.; Meegahakumbura, M.K.; Dissanayaka, A.C.; Perera, L. Quantitative Characterization of Nut Yield and Fruit Components in Quantitative Characterization of Nut Yield and Fruit Components in Indigenous Coconut Germplasm in Sri Lanka. Int. J. Biodivers. 2014. [Google Scholar] [CrossRef] [Green Version]
- Kalina, S.; Navaratne, S.B. Analysis of Antioxidant Activity and Texture Profile of Tender-Young and King Coconut (Cocos Nucifera) Mesocarps under Different Treatments and the Possibility to Develop a Food Product. Int. J. Food Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Figueira, N.T.; Santos, R.M.; Campesatto, E.A.; Lúcio, I.M.L.; De Araújo, E.C.; Bastos, M.L.D.A. Review Article Biological Activity of the Cocos Nucifera L. and Its Profile in the Treatment of Diseases: A Review. J. Chem. Pharm. Res. 2013, 5, 297–302. [Google Scholar]
- Bhagya, D.; Prema, L.; Rajamohan, T. Therapeutic Effects of Tender Coconut Water on Oxidative Stress in Fructose Fed Insulin Resistant Hypertensive Rats. Asian Pac. J. Trop. Med. 2012, 5, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Preetha, P.P.; Devi, V.G.; Rajamohan, T. Hypoglycemic and Antioxidant Potential of Coconut Water in Experimental Diabetes. Food Funct. 2012, 3, 753–757. [Google Scholar] [CrossRef]
- Geetha, V.; Bhavana, K.P. Studies on the Composition and In-Vitro Antioxidant Activities of Concentrates from Coconut Testa and Tender Coconut Water. J. Food Process. Technol. 2016, 7. [Google Scholar] [CrossRef]
- FAOSTAT Title. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 31 March 2022).
- Rodríguez García, S.L.; Raghavan, V. Green Extraction Techniques from Fruit and Vegetable Waste to Obtain Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Baysal, T.; Ersus, S.; Starmans, D.A.J. Supercritical CO2 Extraction of β-Carotene and Lycopene from Tomato Paste Waste. J. Agric. Food Chem. 2000, 48, 5507–5511. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochemistry 2017, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Agostini-costa, T.S. Bioactive Compounds and Health Bene Fi Ts of Some Palm Species Traditionally Used in Africa and the Americas–A Review. J. Ethnopharmacol. 2018, 224, 202–229. [Google Scholar] [CrossRef] [PubMed]
- Bezerra dos Santos Oliveira, M.; Barros Valentim, I.; Calado de Vasconcelos, C.; Maria Bazílio Omena, C.; José Henriques Bechara, E.; Gomes da Costa, J.; de Lima Freitas, M.; Euzébio Goulart Sant’Ana, A.; Oliveira Fonseca Goulart, M. Cocos Nucifera Linn.(Palmae) Husk Fiber Ethanolic Extract: Antioxidant Capacity and Electrochemical Investigation. Comb. Chem. High Throughput Screen. 2013, 16, 121–129. [Google Scholar] [CrossRef]
- Singla, R.K.; Jaiswal, N.; Bhat, V.; Jagani, H. Antioxidant & Antimicrobial Activities of Cocos Nucifera Linn (Arecaceae) Endocarp Extracts. Indo Glob. J. Pharm. Sci. 2011, 4, 354–361. [Google Scholar]
- Muritala, H.F.; Akolade, J.O.; Akande, S.A.; Abdulazeez, A.T.; Aladodo, R.A.; Bello, A.B. Antioxidant and Alpha-Amylase Inhibitory Potentials of Cocos Nucifera Husk. Food Sci. Nutr. 2018, 6, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Elsbaey, M.; Jie, B.; Tanaka, C.; Kato, H.; Tsukamoto, S.; Usui, K.; Hirai, G.; Miyamoto, T. Nuciferols A and B: Novel Sesquineolignans from Cocos Nucifera. Tetrahedron Lett. 2019, 60, 150948. [Google Scholar] [CrossRef]
- Ajay, S.; Madhan, S.; Vadivel, V.; Brindha, P. Research Article Recovery of Polyphenols from Agro-Food Byproducts: Coconut Shell and Groundnut Hull. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 161–167. [Google Scholar]
- Khalid Thebo, N.; Ahmed Simair, A.; Sughra Mangrio, G.; Ansari, K.; Ali Bhutto, A.; Lu, C.; Ali Sheikh, W. Antifungal Potential and Antioxidant Efficacy in the Shell Extract of Cocos Nucifera L. (Arecaceae) against Pathogenic Dermal Mycosis. Medicines 2016, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Abert-Vian, M. Ultrasonics Sonochemistry Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.B.; Guo, M.; Pu, Y.; Wang, W.; Ye, X. Valorisation of Baobab (Adansonia Digitata) Seeds by Ultrasound Assisted Extraction of Polyphenolics. Optimisation and Comparison with Conventional Methods. Ultrason. Sonochem. 2018, 52, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Barbero, G.F.; PiñEiro, Z.; Liazid, A.; Barroso, C.G.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Extraction of Natural Products: Principles and Fundamental Aspects. In RSC Green Chemistry; Royal Society of Chemistry: London, UK, 2013; pp. 58–88. ISBN 9781849736060. [Google Scholar]
- Ferreira, I.J.B.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Green Emerging Extraction Technologies to Obtain High-Quality Vegetable Oils from Nuts: A Review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- González-De-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Pablos, P.A. Flavonol Composition and Antioxidant Activity of Onions (Allium Cepa L) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- José Aliaño González, M.; Carrera, C.; Barbero, G.F.; Palma, M. A Comparison Study between Ultrasound–Assisted and Enzyme–Assisted Extraction of Anthocyanins from Blackcurrant (Ribes Nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef]
- Putra, V.G.P.; Mutiarahma, S.; Chaniago, W.; Rahmadi, P.; Kurnianto, D.; Hidayat, C.; Carrera, C.; Palma, M.; Setyaningsih, W. An Ultrasound-Based Technique for the Analytical Extraction of Phenolic Compounds in Red Algae. Arab. J. Chem. 2022, 15, 103597. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products-A Review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the Potential of Antioxidants from Fruits and Vegetables and Strategies for Their Recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of Ultrasound-Assisted Extraction on Physicochemical Properties, Bioactive Compounds, and Antioxidant Capacity for the Valorization of Hybrid Mandarin Peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, M.; Sun, Q.; Mujumdar, A.S.; Yu, D. Extraction of Functional Extracts from Berries and Their High Quality Processing: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Avelino, F.; Silva, K.T.; Mazzetto, S.E.; Lomonaco, D. Tailor-Made Organosolv Lignins from Coconut Wastes Effects of Green Solvents in Microwave-Assisted Processes upon Their Structure and Antioxidant Activities. Bioresour. Technol. Rep. 2019, 7, 100219. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Kim, H.; John, R. Hydroxystilbenes Are Monomers in Palm Fruit Endocarp Lignins. Plant Physiol. 2017, 174, 2072–2082. [Google Scholar] [CrossRef]
- Johar, M.F.; Ariff, T.F. Mechanical and Microstructural Properties of Hybrid Bio-Composites Using Microwaved Coconut Fibre and Rice Husk. J. Phys. Conf. Ser. 2022, 2199, 012015. [Google Scholar] [CrossRef]
- Nikhontha, K.; Krisanapook, K.; Imsabai, W. Fruit Growth, Endocarp Lignification, and Boron and Calcium Concentrations in Nam Hom (Aromatic) Coconut During Fruit Development. J. ISSAAS 2019, 25, 21–31. [Google Scholar]
- Emojevwe, V. Cocos Nucifera (Coconut) Fruit: A Review of Its Medical Properties. Adv. Agric. Sci. Eng. Res. 2013, 3, 718–723. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Barley Husk and Coconut Shell Reinforced Polypropylene Composites: The Effect of Fibre Physical, Chemical and Surface Properties. Compos. Sci. Technol. 2010, 70, 840–846. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of Activated Carbon Production from Coconut Shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Nugraheni, M.; Santoso, U.; Windarwati, W. Phytochemical Compounds and Antioxidant Activity of Coleus Tuberosus Flesh and Peel on Different Solvent. Food Reseach 2018, 2, 460–467. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulekbache-makhlouf, L.; Medouni, L.; Medouni-adrar, S.; Arkoub, L.; Madani, K. Effect of Solvents Extraction on Phenolic Content and Antioxidant Activity of the By-product of Eggplant. Ind. Crop. Prod. 2013, 49, 668–674. [Google Scholar] [CrossRef]
- Hassan, R.M.; Fhadhila, A.; Kawasaki, N. Antioxidant Activity of Natural Pigment from Husk of Coconut. Trop. Agriciltural Sci. 2018, 41, 441–452. [Google Scholar]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural Antioxidants against Lipid–Protein Oxidative Deterioration in Meat and Meat Products: A Review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Minh, T.N.; Khang, D.T.; Tuyen, P.T.; Minh, L.T.; Anh, L.H.; van Quan, N.; Ha, P.T.T.; Quan, N.T.; Toan, N.P.; Elzaawely, A.A.; et al. Phenolic Compounds and Antioxidant Activity of Phalaenopsis Orchid Hybrids. Antioxidants 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Navanesan, S.; Wahab, A.N.; Manickam, S.; Sim, K.S. Evaluation of Selected Biological Capacities of Baeckea Frutescens. BMC Complementary Altern. Med. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Atik-Bekkara, F. Evaluation of Antioxidant Activity of Hydromethanolic Extracts of Some Medicinal Species from South Algeria. J. Chin. Med. Assoc. 2014, 77, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh, T.N.; Tuyen, P.T.; Khang, D.T.; van Quan, N.; Ha, P.T.T.; Quan, N.T.; Andriana, Y.; Fan, X.; Van, T.M.; Khanh, T.D.; et al. Potential Use of Plant Waste from the Moth Orchid (Phalaenopsis Sogo Yukidian “V3”) as an Antioxidant Source. Foods 2017, 6, 85. [Google Scholar] [CrossRef] [Green Version]
- Doʇan, H.H.; Arslan, E. Biological Activities and DNA Interactions of Amanita Ovoidea. Pharm. Biol. 2015, 53, 1386–1390. [Google Scholar] [CrossRef] [Green Version]
- Kchaou, M.; ben Salah, H.; Mhiri, R.; Allouche, N. Anti-Oxidant and Anti-Acetylcholinesterase Activities of Zygophyllum Album. Bangladesh J. Pharmacol. 2016, 11, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.; Najmi, A.K.; Glabra, S. In-Vitro Antioxidant Activity of Ethanolic Extract of Stephania Glabra (Roxb.) Miers Tubers. Pharma Res. 2014, 12, 1–11. [Google Scholar]
- Ammar, A.-F.; Siddeeg, A.; Zhang, H. In Vitro Antioxidant Activity and Total Phenolic and Flavonoid Contents of Alhydwan (Boerhavia Elegana Choisy) Seeds. J. Food Nutr. Res. 2014, 2, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.S.; José, M.; Cordeiro, M.; Kauly, L.; Silva, R.; Carla, L.; Pereira, L.; Alves, I.; Caetano, S.; Viana, M. Natural Antioxidants Used in Meat Products: A Brief Review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Vadivel, V.; Banu, S.F.; Nithyanand, P.; Lalitha, C.; Brindha, P. Evaluation of Antioxidant and Antimicrobial Properties of Solvent Extracts of Agro-Food By-Products (Cashew Nut Shell, Coconut Shell and Groundnut Hull). Agric. Nat. Resour. 2018, 52, 451–459. [Google Scholar] [CrossRef]
- Oliveira, D.; Martins, G.R.; Jorge, A.; Alviano, S.; Nascimento, R.P.; Auxiliadora, M.; Kaplan, C. Chemical and Antimicrobial Analysis of Husk Fiber Aqueous Extract from Cocos Nucifera L. Afr. J. Biotechnol. 2013, 12, 2478–2483. [Google Scholar] [CrossRef]
- Lima, E.B.C.; Sousa, C.N.S.; Meneses, L.N.; Ximenes, N.C.; Júnior, M.A.S. Cocos Nucifera L. (Arecaceae): A Phytochemical and Pharmacological Review. Braz. J. Med. Biol. Res. 2015, 48, 953–964. [Google Scholar]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).