Nitrogen Critical Level in Leaves in ‘Chardonnay’ and ‘Pinot Noir’ Grapevines to Adequate Yield and Quality Must
Abstract
:1. Introduction
2. Materials and Methods
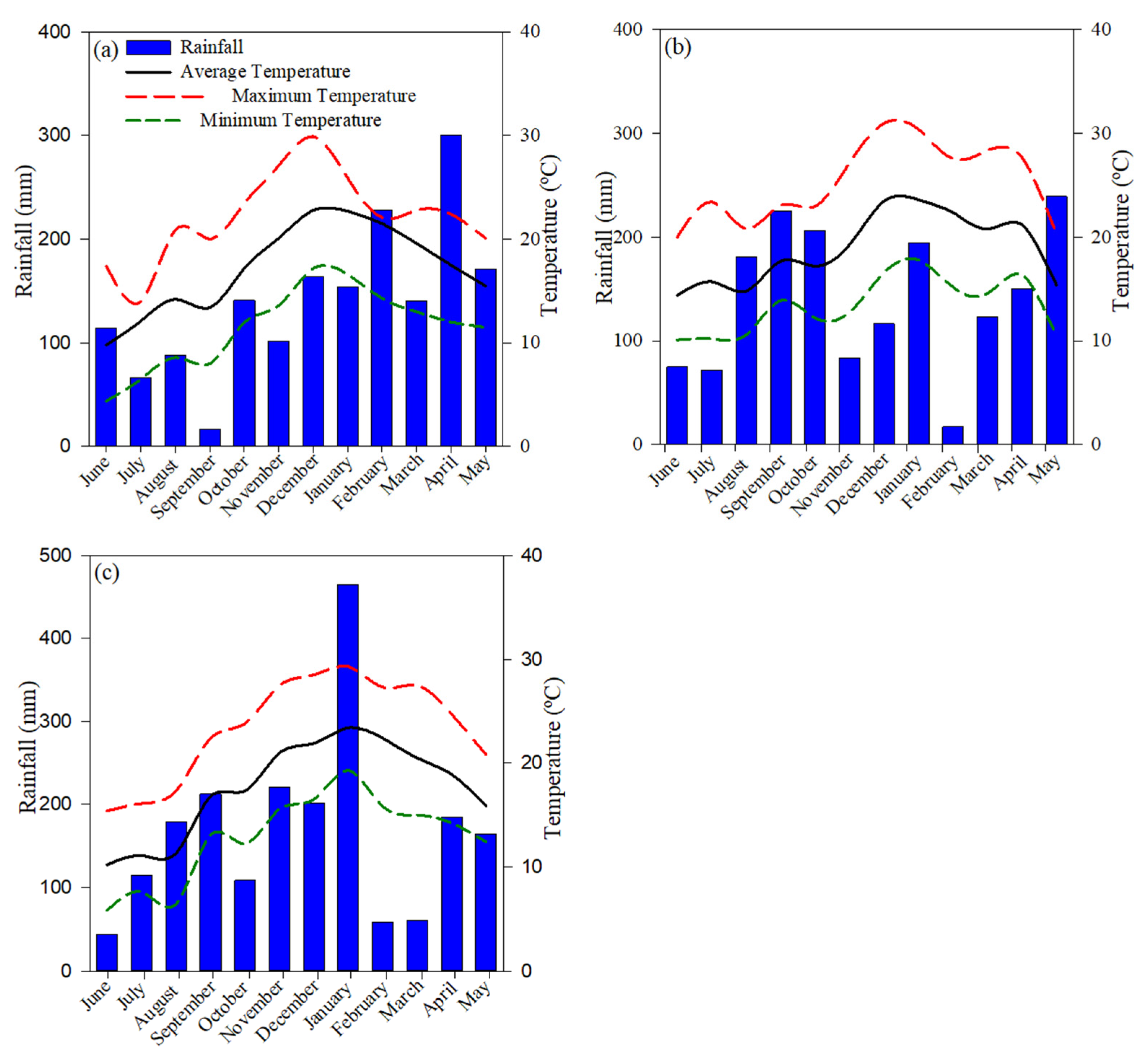
2.1. Experiment Description
2.2. Leaf Collection and N Analysis
2.3. Grape Yield
2.4. Grape Must Composition
2.5. Statistical Analysis
3. Results
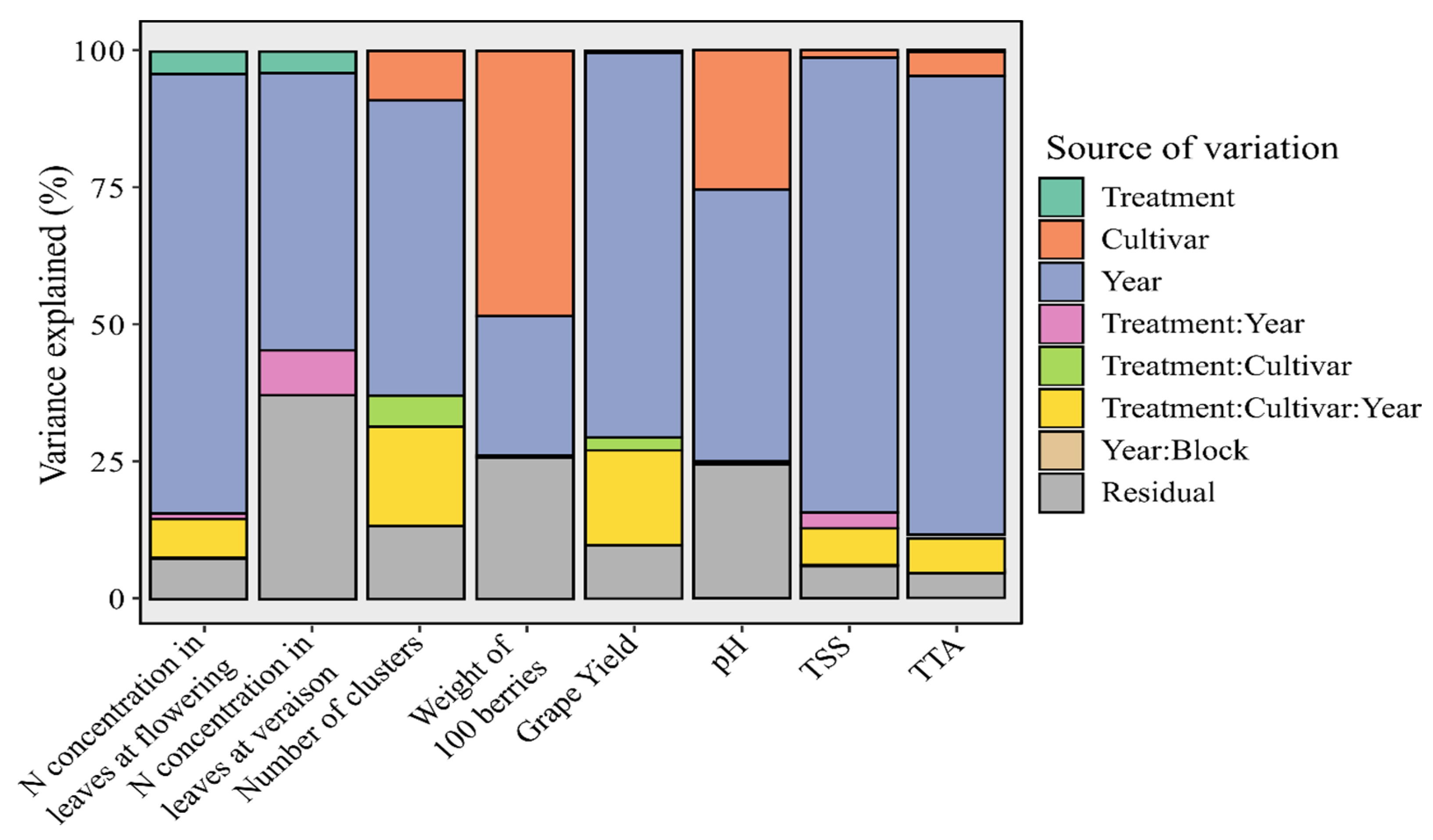
3.1. Variance Components
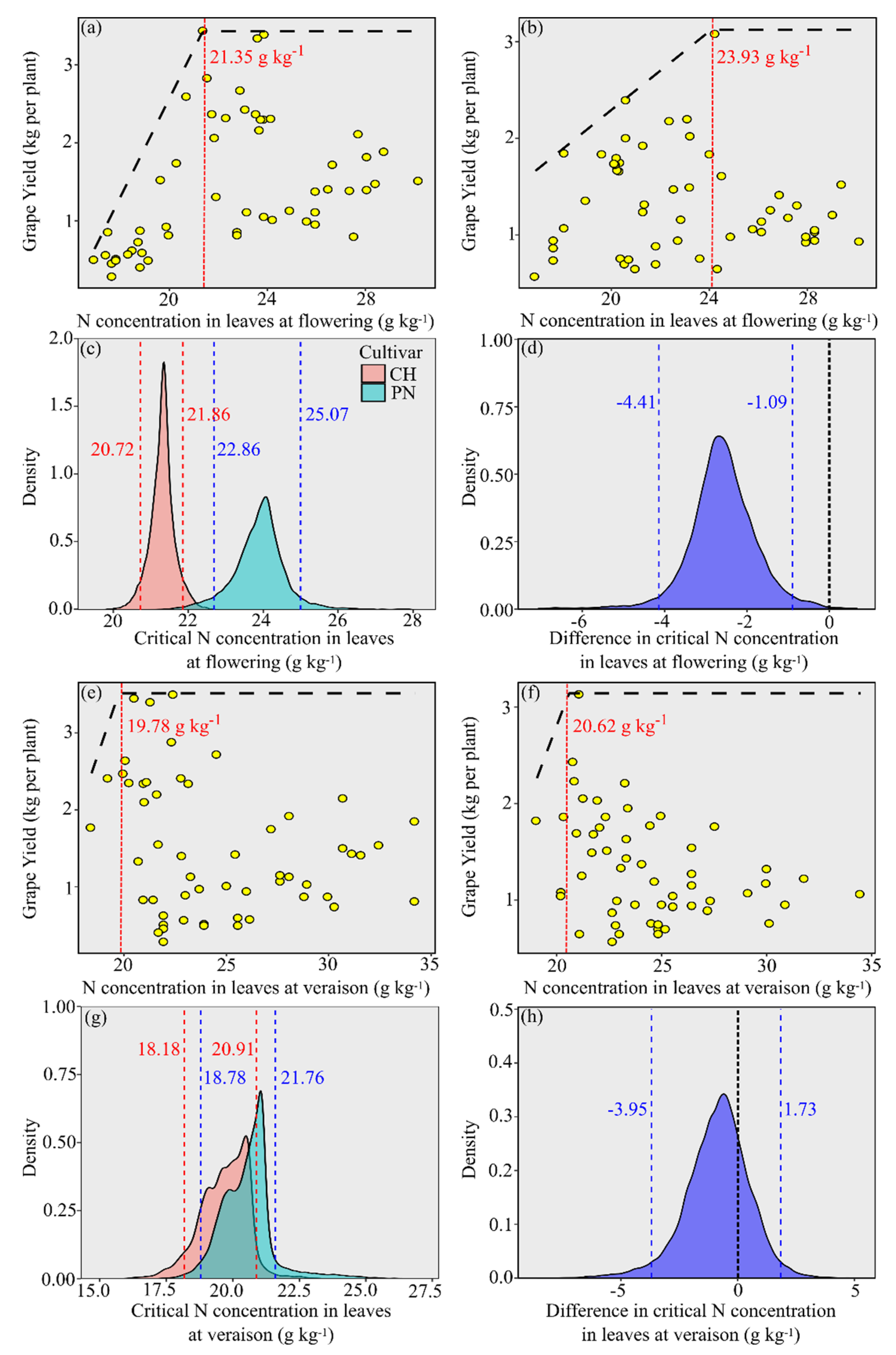
3.2. N Critical Levels in Leaves
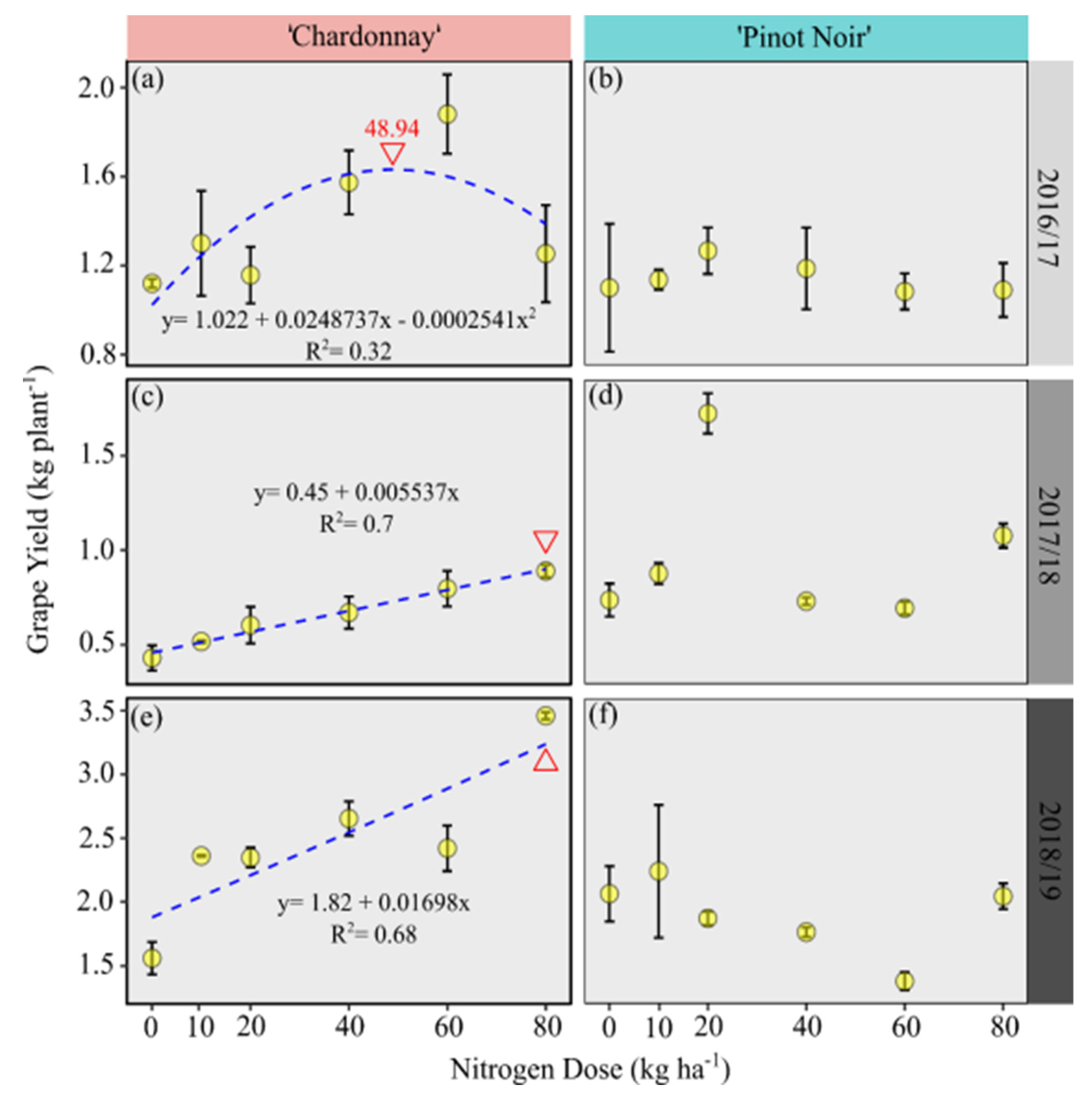
3.3. Maximum Technical Efficiency (MTE) Doses
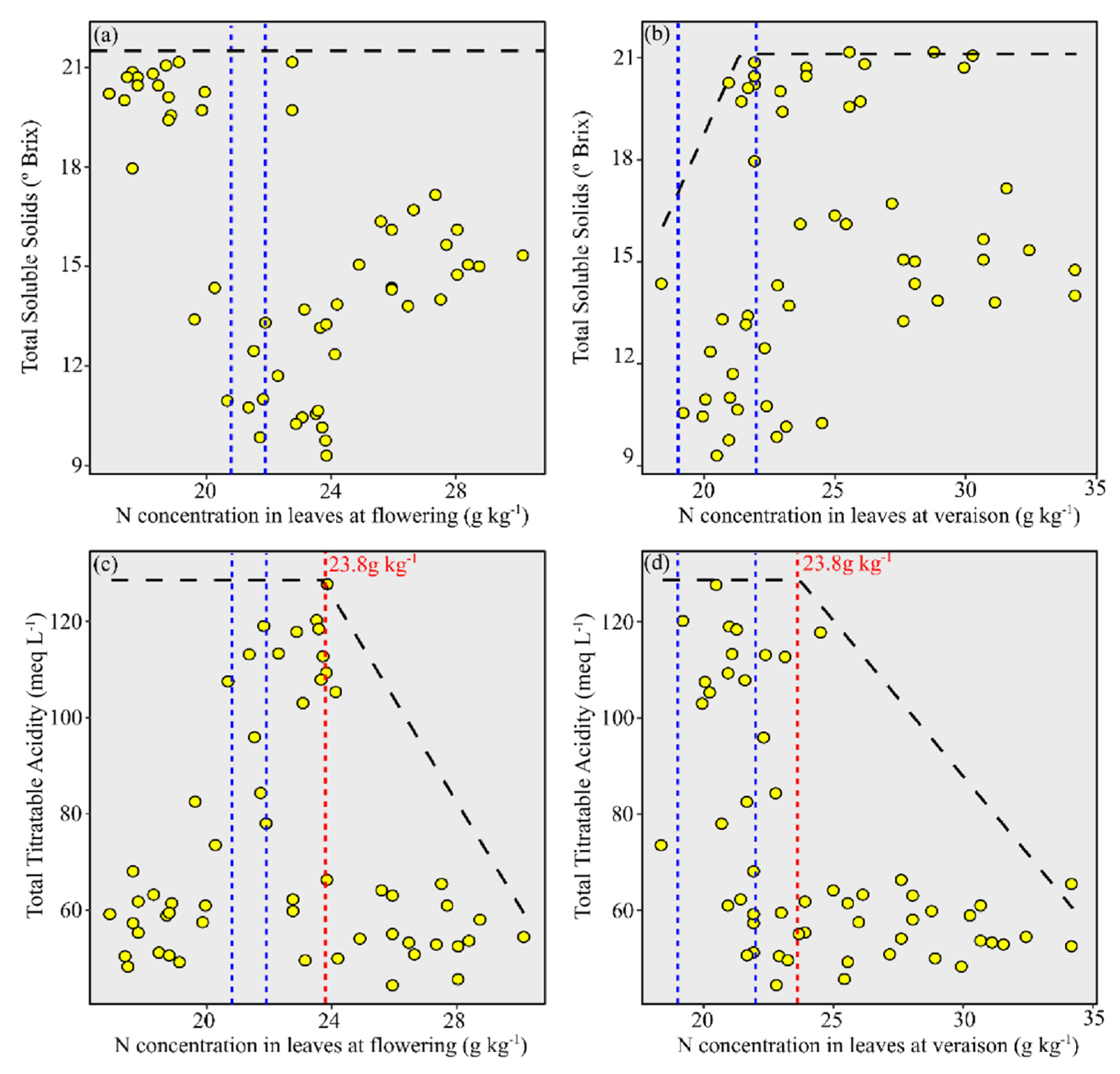
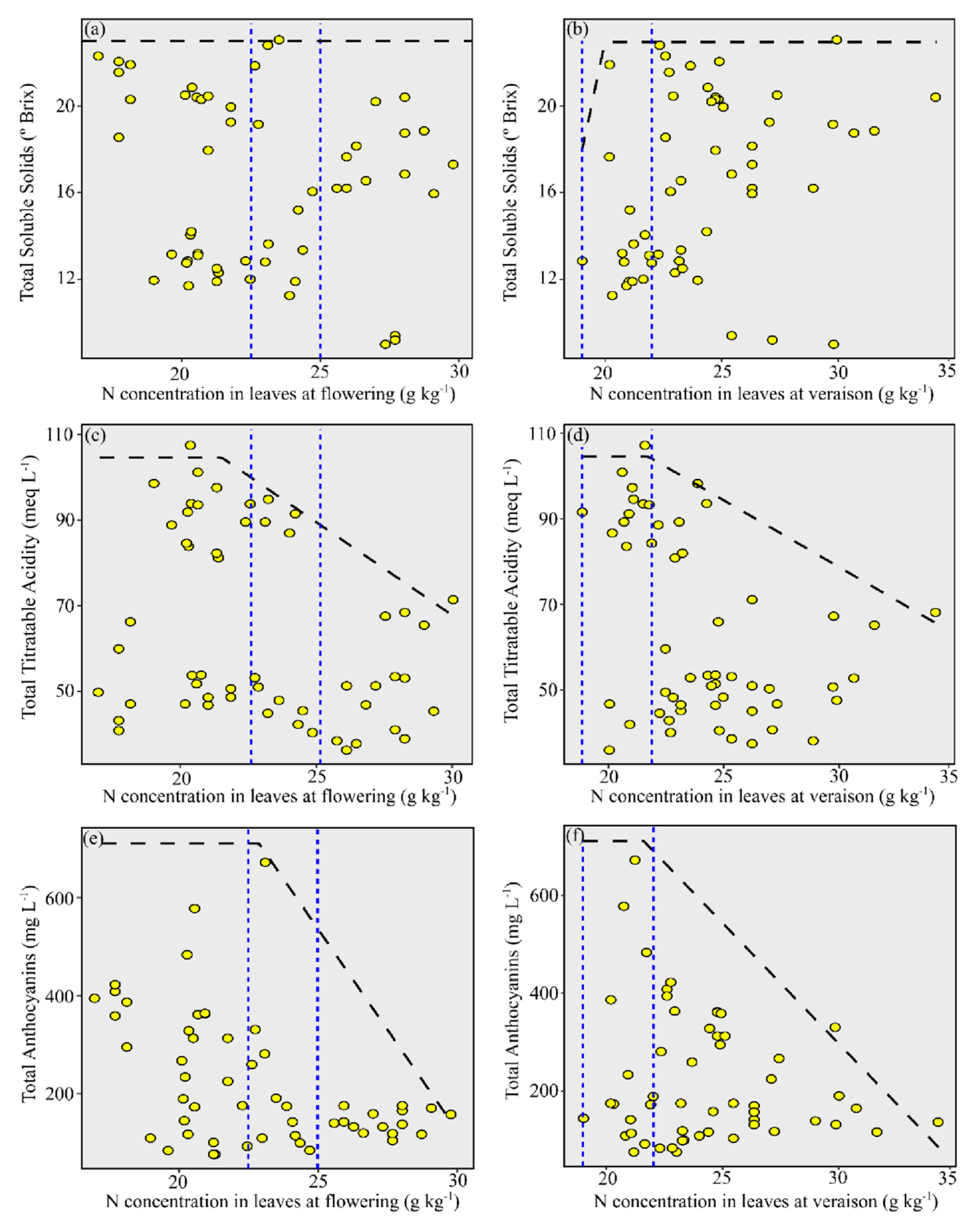
3.4. Relationship between N Concentration in Leaves and Grape Must Composition for ‘Chardonnay’
3.5. Relationship between N Concentration in Leaves and Grape Must Composition for ‘Pinot Noir’
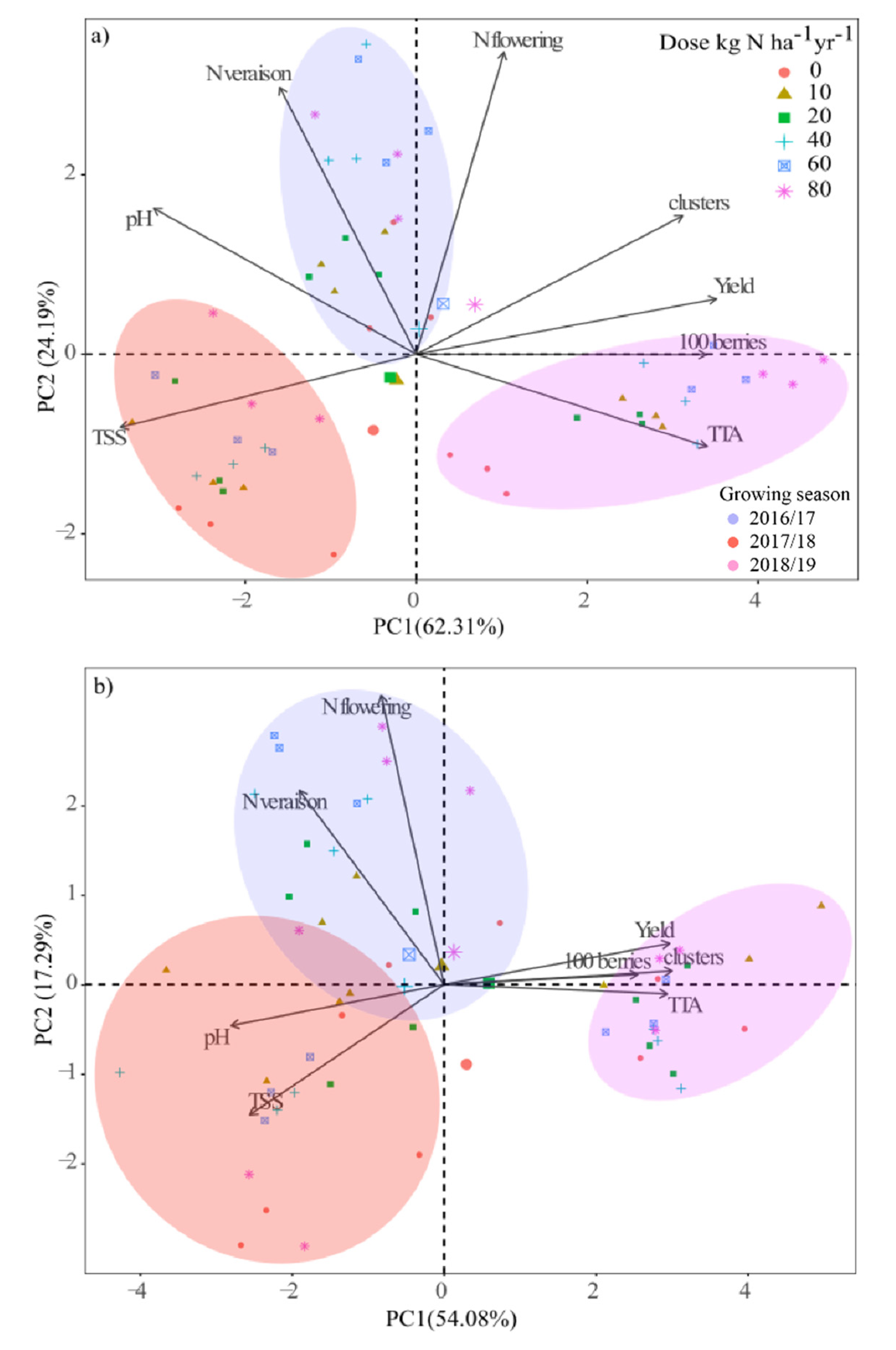
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metay, A.; Magnier, J.; Guilpart, N.; Christophe, A. Nitrogen supply controls vegetative growth, biomass and nitrogen allocation for grapevine (cv. Shiraz) grown in pots. Funct. Plant Biol. 2015, 42, 105–114. [Google Scholar] [CrossRef]
- García-Díaz, A.; Bienes, R.; Sastre, B.; Novara, A.; Gristina, L.; Cerdà, A. Nitrogen losses in vineyards under different types of soil groundcover. A field runoff simulator approach in central Spain. Agric. Ecosyst. Environ. 2017, 236, 256–267. [Google Scholar] [CrossRef]
- Lorensini, F.; Ceretta, C.A.; Brunetto, G.; Cerini, J.B.; Lourenzi, C.R.; De Conti, L.; Tiecher, T.L.; Schapanski, D.E. Disponibilidade de nitrogênio de fontes minerais e orgânicas aplicadas em um Argissolo cultivado com videira. Rev. Ceres 2014, 61, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Tecchio, M.A.; Paioli-pires, E.J.; Terra, M.M.; Moura, M.F. Yield and nutrients levels of Niagara grapevine in Jundiai and Louveira. Biosci. J. 2007, 23, 48–58. [Google Scholar]
- Brunetto, G.; Bongiorno, C.L.; Mattias, J.L.; Deon, M.; De Melo, G.W.; Kaminski, J.; Ceretta, C.A. Produção, composição da uva e teores de nitrogênio na folha e no pecíolo em videiras submetidas à adubação nitrogenada. Cienc. Rural 2008, 38, 2622–2625. [Google Scholar] [CrossRef]
- Picariello, L.; Rinaldi, A.; Forino, M.; Petracca, F.; Moio, L.; Gambuti, A. Modification of the organic acid profile of grapes due to climate changes alters the stability of red wine phenolics during controlled oxidation. Vitis J. Grapevine Res. 2019, 58, 127–133. [Google Scholar] [CrossRef]
- Piccardo, D.; González-Neves, G.; Favre, G.; Pascual, O.; Canals, J.M.; Zamora, F. Impact of must replacement and hot pre-fermentative maceration on the color of uruguayan tannat red wines. Fermentation 2019, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Schelezki, O.J.; Antalick, G.; Šuklje, K.; Jeffery, D.W. Pre-fermentation approaches to producing lower alcohol wines from Cabernet Sauvignon and Shiraz: Implications for wine quality based on chemical and sensory analysis. Food Chem. 2020, 309, 125698. [Google Scholar] [CrossRef] [PubMed]
- Comuzzo, P.; Battistutta, F. Chapter 2—Acidification and pH Control in Red Wines. In Red Wine Technology; Morata, A., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128144008. [Google Scholar]
- Bruwer, F.A.; du Toit, W.; Buica, A. Nitrogen and sulphur foliar fertilisation. S. Afr. J. Enol. Vitic. 2019, 40, 1–16. [Google Scholar] [CrossRef]
- Gago, P.; Conejero, G.; Martínez, M.C.; This, P.; Verdeil, J.L. Comparative anatomy and morphology of the leaves of grenache Noir and Syrah grapevine cultivars. S. Afr. J. Enol. Vitic. 2019, 40, 132–140. [Google Scholar] [CrossRef]
- Long, S.P. Making our plant modelling community more than the sum of its parts: A personal perspective. In Silico Plants 2019, 1, diy002. [Google Scholar] [CrossRef] [Green Version]
- Moran, E.V.; Clark, J.S. Estimating seed and pollen movement in a monoecious plant: A hierarchical Bayesian approach integrating genetic and ecological data. Mol. Ecol. 2011, 20, 1248–1262. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, Y.; Xu, Y.; Wagner, T. Bayesian change point quantile regression approach to enhance the understanding of shifting phytoplankton-dimethyl sulfide relationships in aquatic ecosystems. Water Res. 2021, 201, 117287. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Moyeed, R.A. Bayesian quantile regression. Stat. Probab. Lett. 2001, 54, 437–447. [Google Scholar] [CrossRef]
- Ekbic, H.B.; Ozdemir, G.; Sabir, A.; Tangolar, S. The effects of different nitrogen doses on yield, quality and leaf nitrogen content of some early grape cultivars (V. vinifera L.) grown in greenhouse. Afr. J. Biotechnol. 2010, 9, 5108–5112. [Google Scholar]
- Stefanello, L.O.; Schwalbert, R.; Schwalbert, R.A.; Drescher, G.L.; De Conti, L.; Pott, L.P.; Tassinari, A.; Kulmann, M.S.S.; da Silva, I.C.B.; Brunetto, G.; et al. Ideal nitrogen concentration in leaves for the production of high-quality grapes cv ‘Alicante Bouschet’ (Vitis vinifera L.) subjected to modes of application and nitrogen doses. Eur. J. Agron. 2021, 123, 126200. [Google Scholar] [CrossRef]
- USDA—Soil Survey Staff. Keys to Soil Taxonomy; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Tedesco, M.; Gianello, C.; Bissani, C. Análises de Solo, Plantas e Outros Materiais; UFRGS: Porto Alegre, Brazil, 1995; p. 174.
- Stefanello, L.O.; Schwalbert, R.; Schwalbert, R.A.; De Conti, L.; Kulmann, M.S.S.; Garlet, L.P.; Silveira, M.L.R.; Sautter, C.K.; de Melo, G.W.B.; Rozane, D.E.; et al. Nitrogen supply method affects growth, yield and must composition of young grape vines (Vitis vinifera L. cv Alicante Bouschet) in southern Brazil. Sci. Hortic. 2020, 261, 108910. [Google Scholar] [CrossRef]
- Long, Q. varComp: Variance Component Models. R Package Version 0.1-396. 2015. Available online: https://cran.r-project.org/web/packages/VCA/vignettes/VCA_package_vignette.html (accessed on 13 March 2020).
- R Core Team. Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 13 March 2020).
- Kyveryga, P.M.; Caragea, P.C.; Kaiser, M.S.; Blackmer, T.M. Predicting risk from reducing nitrogen fertilization using hierarchical models and on-farm data. Agron. J. 2013, 105, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel Models. In Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007; p. 625. [Google Scholar]
- Plummer, M. rjags: Bayesian Graphical Models Using MCMC; R Package Version 3–13; R Foundation for Statistical Computing: Vienna, Austria, 2016; pp. 1–19. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.4; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://cran.r-project.org/package=factoextra (accessed on 13 March 2020).
- Moriwaki, T.; Falcioni, R.; Tanaka, F.A.O.; Cardoso, K.A.K.; Souza, L.A.; Benedito, E.; Nanni, M.R.; Bonato, C.M.; Antunes, W.C. Nitrogen-improved photosynthesis quantum yield is driven by increased thylakoid density, enhancing green light absorption. Plant Sci. 2019, 278, 1–11. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Franck, N.; Zamorano, D.; Sanhueza, C.; Carvajal, D.E.; Ibacache, A. Rootstock effect on irrigated grapevine yield under arid climate conditions are explained by changes in traits related to light absorption of the scion. Sci. Hortic. 2017, 218, 284–292. [Google Scholar] [CrossRef]
- Arrizabalaga, M.; Morales, F.; Oyarzun, M.; Delrot, S.; Gomès, E.; Irigoyen, J.J.; Hilbert, G.; Pascual, I. Tempranillo clones differ in the response of berry sugar and anthocyanin accumulation to elevated temperature. Plant Sci. 2018, 267, 74–83. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Santa María, E.; Torres-Pérez, R.; Royo, C.; Lijavetzky, D.; Bravo, G.; Aguirreolea, J.; Sánchez-Díaz, M.; Antolín, M.C.; Martínez-Zapater, J.M. Thermotolerance responses in ripening berries of Vitis vinifera L. cv muscat hamburg. Plant Cell Physiol. 2013, 54, 1200–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.K.; He, F.; Wang, Y.X.; Liu, X.; Duan, C.Q.; Wang, J. Influences of Berry Size on Fruit Composition and Wine Quality of Vitis vinifera L. cv. ‘Cabernet Sauvignon’ Grapes. S. Afr. J. Enol. Vitic. 2018, 39, 67–76. [Google Scholar] [CrossRef] [Green Version]
- De Souza Kulmann, M.S.; Sete, P.B.; de Paula, B.V.; Stefanello, L.O.; Schwalbert, R.; Schwalbert, R.A.; Arruda, W.S.; Sans, G.A.; Parcianello, C.F.; Nicoloso, F.T.; et al. Kinetic parameters govern of the uptake of nitrogen forms in ‘Paulsen’ and ‘Magnolia’ grapevine rootstocks. Sci. Hortic. 2020, 264, 109174. [Google Scholar] [CrossRef]
- Schmitt, D.E.; Borghezan, M.; Ambrosini, V.G.; Comin, J.J.; Trapp, T.; Brunetto, G. Yield and must composition of grapevines subjected to phosphate fertilization in Southern Brazil. Pesq. Agropec. Bras. 2020, 55, e01167. [Google Scholar] [CrossRef]
- Brunetto, G.; Stefanello, L.O.S.; Ceretta, C.A.; Couto, R.R.; Ferreira, P.A.A.; Ambrosini, V.G.; Borghezan, M.; Comin, J.J.; De Melo, G.W.; Baldi, E.; et al. Nitrogen fertilization of ‘Chardonnay’ grapevines: Yield, must composition and their relationship with temperature and rainfall. Acta Hortic. 2018, 1228, 451–456. [Google Scholar] [CrossRef]
- Brunetto, G.; Ceretta, C.A.; Kaminski, J.; de Melo, G.W.; Girotto, E.; Trentin, E.E.; Lourenzi, C.R.; Vieira, R.C.B.; Gatiboni, L.C. Produção e composição química da uva de videiras Cabernet Sauvignon submetidas à adubação nitrogenada. Ciênc. Rural 2009, 39, 2035–2041. [Google Scholar] [CrossRef] [Green Version]
- Rossouw, G.C.; Smith, J.P.; Barril, C.; Deloire, A.; Holzapfel, B.P. Carbohydrate distribution during berry ripening of potted grapevines: Impact of water availability and leaf-to-fruit ratio. Sci. Hortic. 2017, 216, 215–225. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Sofo, A.; Nuzzo, V.; Tataranni, G.; Manfra, M.; De Nisco, M.; Scopa, A. Berry morphology and composition in irrigated and non-irrigated grapevine (Vitis vinifera L.). J. Plant Physiol. 2012, 169, 1023–1031. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Zhang, C.; Xu, W.; He, J.; Zhu, L.; Wang, S. Effect of root restriction on nitrogen levels and glutamine synthetase activity in “Kyoho” grapevines. Sci. Hortic. 2012, 137, 156–163. [Google Scholar] [CrossRef]
- García, J.; Zheng, W.; Balda, P.; Martinez De Toda, F. Varietal differences in the sugar content of red grapes at the onset of anthocyanin synthesis. Vitis J. Grapevine Res. 2017, 56, 15–18. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aranjuelo, I.; Pascual, I.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Araus, J.L.; Morales, F. Is vegetative area, photosynthesis, or grape C uploading involved in the climate change-related grape sugar/anthocyanin decoupling in Tempranillo? Photosynth. Res. 2018, 138, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yang, B.H.; He, S.; Tu, T.Y.; Min, Z.; Fang, Y.L.; Sun, X.Y. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon (Vitis Vinifera L.) grapes and wines. Plant Physiol. Biochem. 2019, 135, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Wine Chemistry, 3rd ed.; Science Press: Beijing, China, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassinari, A.; Stefanello, L.O.; Schwalbert, R.A.; Vitto, B.B.; Kulmann, M.S.d.S.; Santos, J.P.J.; Arruda, W.S.; Schwalbert, R.; Tiecher, T.L.; Ceretta, C.A.; et al. Nitrogen Critical Level in Leaves in ‘Chardonnay’ and ‘Pinot Noir’ Grapevines to Adequate Yield and Quality Must. Agronomy 2022, 12, 1132. https://doi.org/10.3390/agronomy12051132
Tassinari A, Stefanello LO, Schwalbert RA, Vitto BB, Kulmann MSdS, Santos JPJ, Arruda WS, Schwalbert R, Tiecher TL, Ceretta CA, et al. Nitrogen Critical Level in Leaves in ‘Chardonnay’ and ‘Pinot Noir’ Grapevines to Adequate Yield and Quality Must. Agronomy. 2022; 12(5):1132. https://doi.org/10.3390/agronomy12051132
Chicago/Turabian StyleTassinari, Adriele, Lincon Oliveira Stefanello, Rai Augusto Schwalbert, Beatriz Baticini Vitto, Matheus Severo de Souza Kulmann, João Pedro Jung Santos, Wagner Squizani Arruda, Raissa Schwalbert, Tadeu Luis Tiecher, Carlos Alberto Ceretta, and et al. 2022. "Nitrogen Critical Level in Leaves in ‘Chardonnay’ and ‘Pinot Noir’ Grapevines to Adequate Yield and Quality Must" Agronomy 12, no. 5: 1132. https://doi.org/10.3390/agronomy12051132
APA StyleTassinari, A., Stefanello, L. O., Schwalbert, R. A., Vitto, B. B., Kulmann, M. S. d. S., Santos, J. P. J., Arruda, W. S., Schwalbert, R., Tiecher, T. L., Ceretta, C. A., De Conti, L., Schumacher, R. L., & Brunetto, G. (2022). Nitrogen Critical Level in Leaves in ‘Chardonnay’ and ‘Pinot Noir’ Grapevines to Adequate Yield and Quality Must. Agronomy, 12(5), 1132. https://doi.org/10.3390/agronomy12051132







