Overexpression of CgbHLH001, a Positive Regulator to Adversity, Enhances the Photosynthetic Capacity of Maize Seedlings under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Maize Transgenic Lines and Treatments
2.2. Total RNA Isolation from Maize Seedlings
2.3. RNA-Sequencing Analysis
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.5. Measurement of Physiological Parameters
2.5.1. Determination of Photosynthetic Pigments
2.5.2. Measurements of Gas Exchange Parameters and Chlorophyll Fluorescence
2.5.3. Carbohydrate Fractions
2.5.4. Activity of Photosynthetic Enzymes
2.6. Statistical Analysis
3. Results
3.1. Overexpression of CgbHLH001 Increased the Root Biomass and Relieved the Reduction of Leaf Area under Drought Stress
3.2. Overexpression of CgbHLH001 Increased Chlorophyll Accumulation under Drought Stress
3.3. Overexpression of CgbHLH001 Promoted Photosynthetic Rate under Drought Stress
3.4. Overexpression of CgbHLH001 Increased Chlorophyll Fluorescence under Drought Stress
3.5. Overexpression of CgbHLH001 Positively Regulated Sugar Metabolism under Drought Stress
3.6. Overexpression of CgbHLH001 Increased Activities of Photosynthetic Enzymes under Drought Stress
3.7. CgbHLH001 Positively Regulated the Expressions of Multiple Photosynthesis-Related Genes
3.8. Comprehensive Responses at Transcriptional Level of CgbHLH001 Overexpression in Maize Seedlings Subjected to Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassmann, R.; Jagadish, S.V.K.; Sumfleth, K.; Pathak, H.; Heuer, S. Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Adv. Agron. 2009, 102, 99–133. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotech. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, J.M.; Ragot, M. Marker-assisted selection to improve drought adaptation in maize: The backcross approach, perspectives, limitations, and alternatives. J. Exp. Bot. 2007, 58, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.; Wu, X.; Zhang, H.; Chen, Y.; Wang, W. Making better maize plants for sustainable grain production in a changing climate. Front. Plant Sci. 2015, 6, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Liu, C.; Li, Z.; Ran, Q.; Xie, G.; Wang, B.; Fang, S.; Chu, J.; Zhang, J. ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol. 2018, 178, 753–770. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Li, Y.Y.; Zhou, Z.C.; Xiang, X.; Liu, X.; Wang, J.; Hu, Z.R.; Xiang, S.P.; Li, W.; Xiao, Q.Z. Tobacco transcription factor bHLH123 improves salt tolerance by activating NADPH oxidase NtRbohE expression. Plant Physiol. 2021, 3, 3. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Q.; Dai, Y.; Hu, W.; Huang, X. Genome-wide identification of PbrbHLH family genes, and expression analysis in response to drought and cold stresses in pear (Pyrus bretschneideri). BMC Plant Biol. 2021, 21, 86. [Google Scholar] [CrossRef]
- Ort, D.R.; Long, S.P. Limits on Yields in the Corn Belt. Science 2014, 344, 484. [Google Scholar] [CrossRef]
- Tausta, S.L.; Coyle, H.M.; Rothermel, B.; Stiefel, V.; Nelson, T. Maize C4 and non-C4 NADP-dependent malic enzymes are encoded by distinct genes derived from a plastid-localized ancestor. Plant Mol. Biol. 2002, 50, 635–652. [Google Scholar] [CrossRef]
- Edwards, G.; Walker, D. C3 C4: Mechanisms and cellular and environmental regulation of photosynthesis. Plant Cell Environ. 1983, 6, 747–748. [Google Scholar]
- Omoto, E.; Taniguchi, M.; Miyake, H. Adaptation responses in C4 photosynthesis of maize under salinity. J. Plant Physiol. 2012, 169, 469–477. [Google Scholar] [CrossRef]
- Peterhansel, C.; Horst, I.; Niessen, M.; Blume, C.; Kebeish, R.; Kürkcüoglu, S.; Kreuzaler, F. Photorespiration. Arab. Book 2010, 8, e0130. [Google Scholar] [CrossRef]
- Anderson, L.E. Chloroplast and cytoplasmic enzymes II. Pea leaf triose phosphate isomerases. BBA-Enzymol. 1971, 235, 237–244. [Google Scholar] [CrossRef]
- Maiti, R.K.; Maiti, L.E.; Sonia, M.; Maiti, A.M.; Maiti, M.; Maiti, H. Genotypic variability in maize cultivars (Zea mays L.) for resistance to drought and salinity at the seedling stage resistance to drought and salinity at the seedling stage. J. Plant Physiol. 1996, 148, 741–744. [Google Scholar] [CrossRef]
- Min, H.; Chen, C.; Wei, S.; Shang, X.; Sun, M.; Xia, R.; Liu, X.; Hao, D.; Chen, H.; Qi, X. Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 7, e01080. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.N.; Wang, L.C. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Yeh, S.Y.; Lin, H.H.; Chang, Y.M.; Chang, Y.L.; Chang, C.K.; Huang, Y.C.; Ho, Y.W.; Lin, C.Y.; Zheng, J.Z.; Jane, C.K.; et al. Maize Golden2-like transcription factors boost rice chloroplast development, photosynthesis, and grain yield. Plant Physiol. 2021, 188, 442–459. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene. 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Hayano-Kanashiro, C.; Calderon-Vazquez, C.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Simpson, J. Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE 2009, 4, e7531. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot-Lond. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Martinstpaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 11. [Google Scholar] [CrossRef]
- Mitchel, P.L.; Sheehy, J.E. Supercharging rice photosynthesis to increase yield. New Phytol. 2006, 171, 688–693. [Google Scholar] [CrossRef]
- Gu, J.; Yin, X.; Stomph, T.J.; Wang, H.; Struik, P.C. Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well-watered conditions. J. Exp. Bot. 2012, 63, 5137–5153. [Google Scholar] [CrossRef] [Green Version]
- Almoguera, C.; Prieto-Dapena, P.; Personat, J.M.; Tejedor-Cano, J.; Lindahl, M.; Diaz- Espejo, A.; Jordano, J. Protection of the photosynthetic apparatus from extreme dehydration and oxidative stress in seedlings of transgenic tobacco. PLoS ONE 2012, 7, e51443. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Tang, S.; An, Y.; Zheng, D.C.; Xia, X.L.; Yin, W.L. Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J. Exp. Bot. 2013, 14, 4589–4601. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.; Wang, M.; Wei, Y.; Xia, Z. Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front. Plant Sci. 2016, 6, 1223. [Google Scholar] [CrossRef] [Green Version]
- Mbambalala, N.; Panda, S.K.; Vyver, C. Overexpression of AtBBX29 improves drought tolerance by maintaining photosynthesis and enhancing the antioxidant and osmolyte capacity of sugarcane plants. Plant Mol. Biol. Rep. 2021, 39, 419–433. [Google Scholar] [CrossRef]
- Zhao, H.J.; Wang, C.H.; Lan, H.Y. A bHLH transcription factor from Chenopodium glaucum confers drought tolerance to transgenic maize by positive regulation of morphological and physiological performances and stress-responsive genes’ expressions. Mol. Breed. 2021, 41, 47. [Google Scholar] [CrossRef]
- Ren, J.; Xie, T.; Wang, Y.; Li, H.; Liu, T.; Zhang, S.; Yin, L.; Wang, S.; Deng, X.; Ke, Q. Coordinated regulation of carbon and nitrogen assimilation confers drought tolerance in maize (Zea mays L.). Environ. Exp. Bot. 2020, 176, 104086. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Djangaopa, J.T.; Mambu, S.M.; Song, A.N. Variations in Leaf chlorophyll concentration in croton plants (Codiaeum variegatum L.) cultivar Gelatik at a different leaf age. J. Ilm. Sains 2020, 20, 78–87. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Genernal Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer Study edn; Springer: Berlin/Heidelberg, Germany, 1995; Volume 100, pp. 49–70. [Google Scholar] [CrossRef]
- Sujatha, E.; Anusha, C.; Taheniyat, S.; Lalitha, R. Effect of 28-Homobrassinolide on photosynthesis and carbohydrate content of Maize under salt and cadmium stress. Ann. Plant Sci. 2019, 8, 3517. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Advances in photosynthesis and respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 279–319. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wood, C.W.; Reeves, D.W.; Himelrick, D.G. Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: A review. Proc. Agron. Soc. N.Z. 1993, 23, 1–9. [Google Scholar]
- Athar, H.U.R.; Khan, A.; Ashraf, M. Inducing Salt Tolerance in Wheat by Exogenously Applied Ascorbic Acid through Different Modes. J. Plant Nutr. 2009, 32, 1799–1817. [Google Scholar] [CrossRef]
- Jabeen, F.; Shahbaz, M.; Ashraf, M. Discriminating some prospective cultivars of maize (Zea mays L.) for drought tolerance using gas exchange characteristics and proline contents as physiological markers. Pak. J. Bot. 2008, 40, 2329–2343. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.G.; Nielsen, D.C.; Vigil, M.F.; Mikha, M.M.; Calderon, F. Water Deficit Stress Effects on Corn (Zea mays L.) Root:Shoot Ratio. Open J. Soil Sci. 2014, 04, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, M.M.; Muchow, R.C. A critical evaluation of traits for improving crop yields in water-limited environments. Adv. Agron. 1990, 43, 107–153. [Google Scholar] [CrossRef]
- Legay, S.; Lefèvre, I.; Lamoureux, D.; Barreda, C.; Tincopa, R.; Gutierrez, R.; Quiroz, R.; Hoffmann, L.; Hausman, J.F.; Bonierbale, M.; et al. Carbohydrate metabolism and cell protection mechanisms differentiate drought tolerance and sensitivity in advanced potato clones (Solanum tuberosum L.). Funct. Integr. Genom. 2011, 11, 275–291. [Google Scholar] [CrossRef]
- Bonos, S.A.; Rush, D.; Hignight, K.; Meyer, W.A. Selection for Deep Root Production in Tall Fescue and Perennial Ryegrass. Crop Sci. 2004, 44, 5. [Google Scholar] [CrossRef]
- Hynninen, P.H.; Mesilaakso, M. Synthesis and characterization of chlorophyll a enol derivatives: Chlorophyll a tert-butyldimethylsilyl-enol ether and 131-deoxo-131,132-didehydro-chlorophyll a. J. Porphyr. Phthalocya. 2016, 20, 590–601. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Sairam, R.K.; Srivastava, G.C. Physiological and Biochemical Responses of Hexaploid and Tetraploid Wheat to Drought Stress. J. Agron. Crop Sci. 2010, 185, 219–227. [Google Scholar] [CrossRef]
- Wang, Y.; Nii, N. Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotech. 2000, 75, 623–627. [Google Scholar] [CrossRef]
- Nyitrai, P.; Sárvári, É.; Keresztes, Á.; Láng, F. Organization of Thylakoid Membranes in Low-Light Grown Maize Seedlings. Effect of Lincomycin Treatmen. J. Plant Physiol. 1994, 144, 370–375. [Google Scholar] [CrossRef]
- Ohtsuka, O.; Ito, H.; Tanaka, A. Conversion of chlorophyll b to chlorophyll a and the assembly of chlorophyll with apoproteins by isolated chloroplasts. Plant Physiol. 1997, 113, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Falbel, T.G.; Staehelin, L.A. Partial blocks in the early steps of chlorophyll synthesis pathway: A common feature of chlorophyll b-deficient mutants. Physiol. Plant. 1996, 97, 311–320. [Google Scholar] [CrossRef]
- Çiçek, N.; Çakırlar, H. Effects of salt stress on some physiological and photosynthetic parameters at three different temperatures in six soya bean (Glycine max L. Merr.) cultivars. J. Agron. Crop Sci. 2008, 194, 34–46. [Google Scholar] [CrossRef]
- Assmann, S.M.; Jegla, T. Guard cell sensory systems. Recent insights on stomatal responses to light, abscisic acid, and CO2. Curr. Opin. Plant Biol. 2016, 33, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot-Lond. 2009, 103, 635–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, T.; Gu, W.; Yao, M.; Li, J.; Wang, Y.; Qu, D.; Wei, S. Exogenous DCPTA ameliorates simulated drought conditions by improving the growth and photosynthetic capacity of maize seedlings. Sci. Rep. 2017, 7, 12684. [Google Scholar] [CrossRef] [Green Version]
- Picorel, R.; Miguel, A.; Maya, V. Editorial: Molecular basis of the response of photosynthetic apparatus to light and temperature stress. Front. Plant Sci. 2017, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Amaro, T.; Voltas, J.; Nakkoul, H.; Nachit, M.M. Chlorophyll fluorescence as a selection criterion for grain yield in durum wheat under Mediterranean conditions. Field Crop Res. 1998, 55, 209–223. [Google Scholar] [CrossRef]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of Photosystem II. BBA-Bioenerg. 2012, 1817, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Graßes, T.; Pesaresi, P.; Schiavon, F.; Varotto, C.; Slamini, F.; Jahns, P.; Leister, D. The role of ΔpH-dependent dissipation of excitation energy in protecting photosystem II against light-induced damage in Arabidopsis thaliana. Plant Physiol. Biochem. 2002, 40, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.C. Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Annu. Rev. Plant Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Du, Y.; Lu, R.; Xia, J. Impacts of global environmental change drivers on non-structural carbohydrates in terrestrial plants. Funct. Ecol. 2020, 34, 1525–1536. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Gianfagna, T.; Huang, B. Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J. Exp. Bot. 2011, 62, 5311–5333. [Google Scholar] [CrossRef] [Green Version]
- Hatch, M.D. C4 photosynthesis, a unique blend of modified biocheistry, anatomy, and ultrastructure. Biochim. Biophys. Acta 1987, 895, 81–106. [Google Scholar] [CrossRef]
- Jiao, D.; Huang, X.; Li, X.; Chi, W.; Kuang, T.; Zhang, Q.; Ku, M.S.; Cho, D. Photosynthetic characteristics and tolerance to photo-oxidation of transgenic rice expressing C4 photosynthesis enzymes. Photosynth. Res. 2002, 72, 85–93. [Google Scholar] [CrossRef]
- Yoon, D.K.; Ishiyama, K.; Suganami, M.; Tazoe, Y.; Watanabe, M.; Imaruoka, S.; Ogura, M.; Ishida, H.; Suzuki, Y.; Obara, M.; et al. Transgenic rice overproducing Rubisco exhibits increased yields with improved nitrogen-use efficiency in an experimental paddy field. Nat. Food 2020, 1, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ohkubo, M.; Hatakeyama, H.; Ohashi, K.K.; Yoshizawa, R.; Kojima, S.; Hayakawa, T.; Yamaya, T.; Mae, T.; Makino, A. Increased Rubisco content in transgenic rice transformed with the ‘sense’ rbcS gene. Plant Cell Physiol. 2007, 48, 626–637. [Google Scholar] [CrossRef]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef]
- Jia, S.; Lv, J.; Jiang, S.; Liang, T.; Liu, C.; Jing, Z. Response of wheat ear photosynthesis and photosynthate carbon distribution to water deficit. Photosynthetica 2015, 53, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Peng, P.; Tang, Y.; Zhang, L.X.; Lv, J.Y. C4 photosynthetic enzymes play a key role in wheat spike bracts primary carbon metabolism response under water defcit. Plant Physiol. Biochem. 2019, 142, 163–172. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Jiao, Y.; Qin, Y.; Liu, X.; He, K. Global genome expression analysis of rice in response to drought and high-salinity stresses in shoot, flag leaf, and panicle. Plant Mol. Biol. 2007, 63, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, H.; Wu, X.; Wang, W. Identification of drought tolerant mechanisms in a drought-tolerant maize mutant based on physiological, biochemical and transcriptomic analyses. BMC Plant Biol. 2020, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Gereziher, T.; Xu, Y.; Magwanga, R.O.; Kirungu, J.N.; Cai, X.; Hou, Y.; Wang, Y.; Wang, K.; Zhou, Z.; Liu, F. Genome wide identification and characterization of light-harvesting Chloro a/b binding (LHC) genes reveals their potential role in enhancing drought tolerance in Gossypium hirsutum. J. Cotton Res. 2021, 4, 13. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liu, R.; Yan, L.; Liu, Z.Q.; Jiang, S.C.; Shen, Y.Y.; Wang, X.F.; Zhang, D.P. Light-harvesting chlorophyll a/b binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
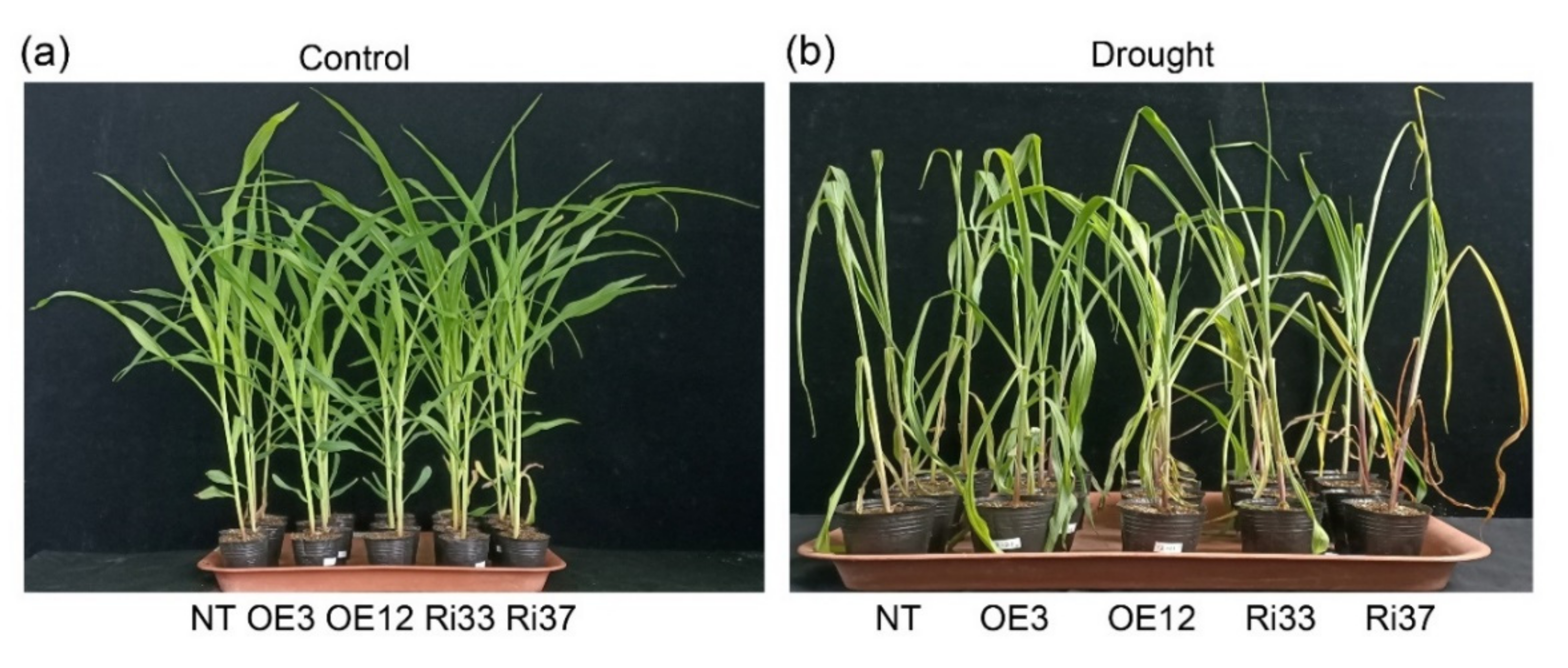
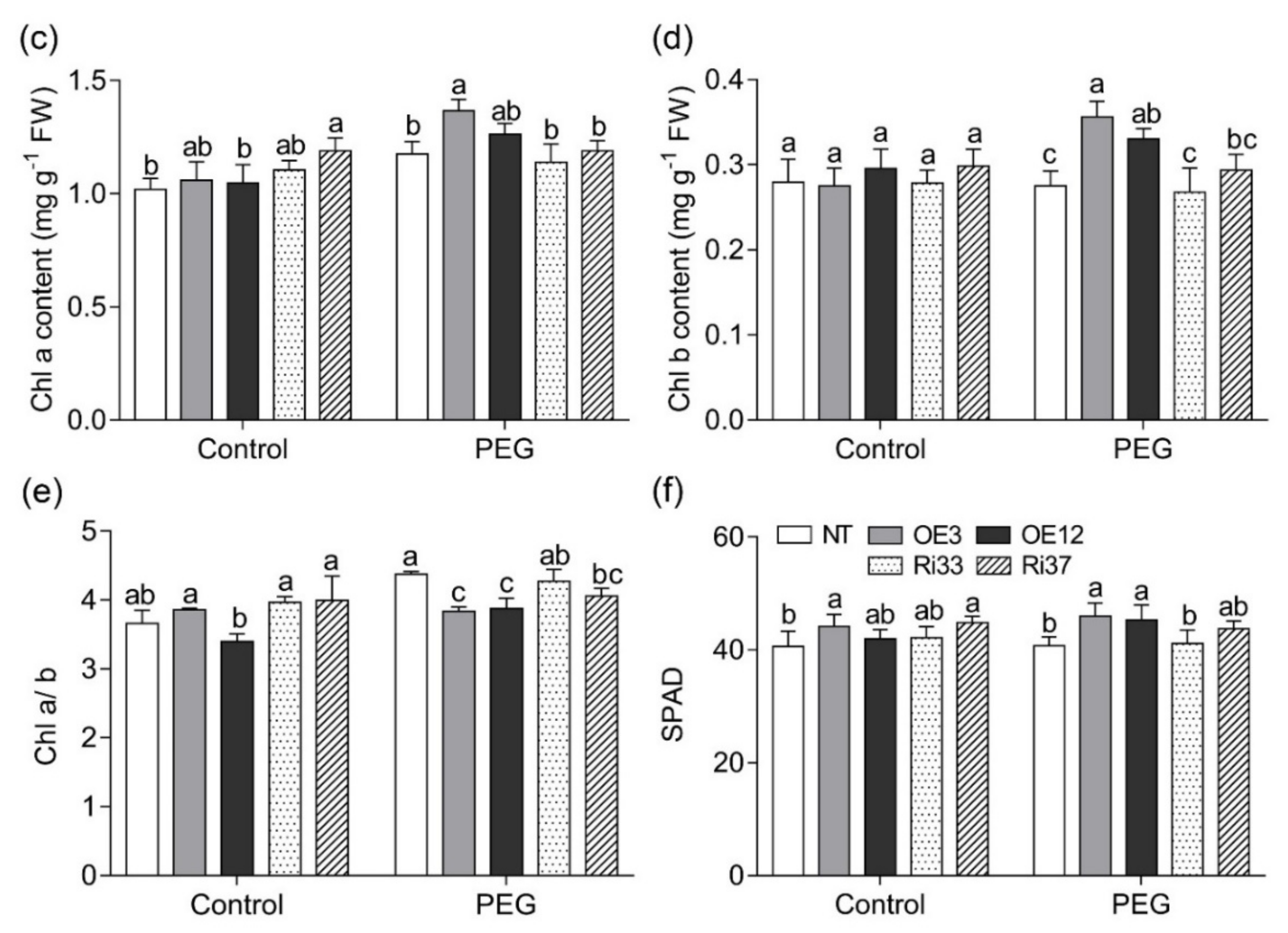
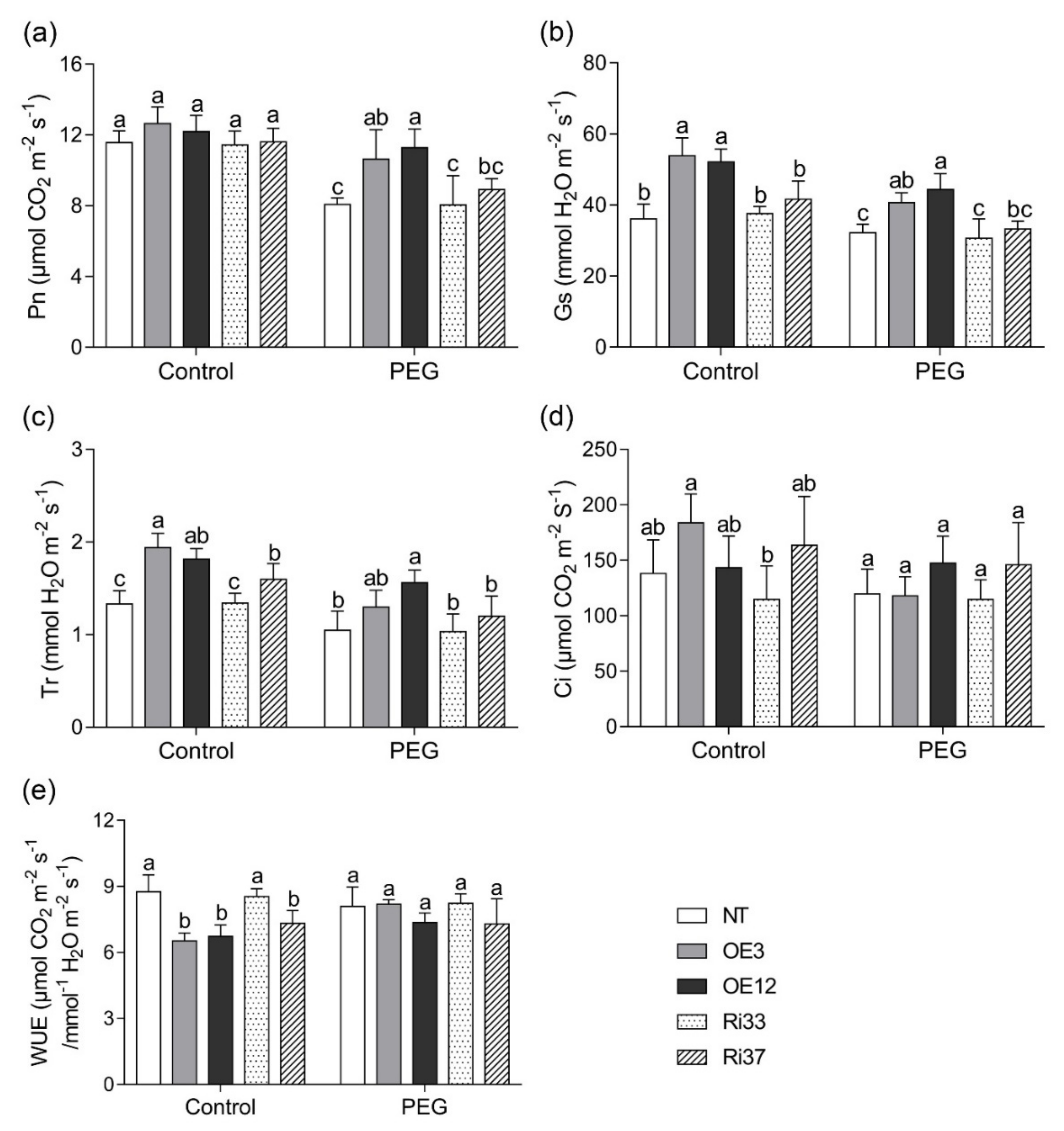

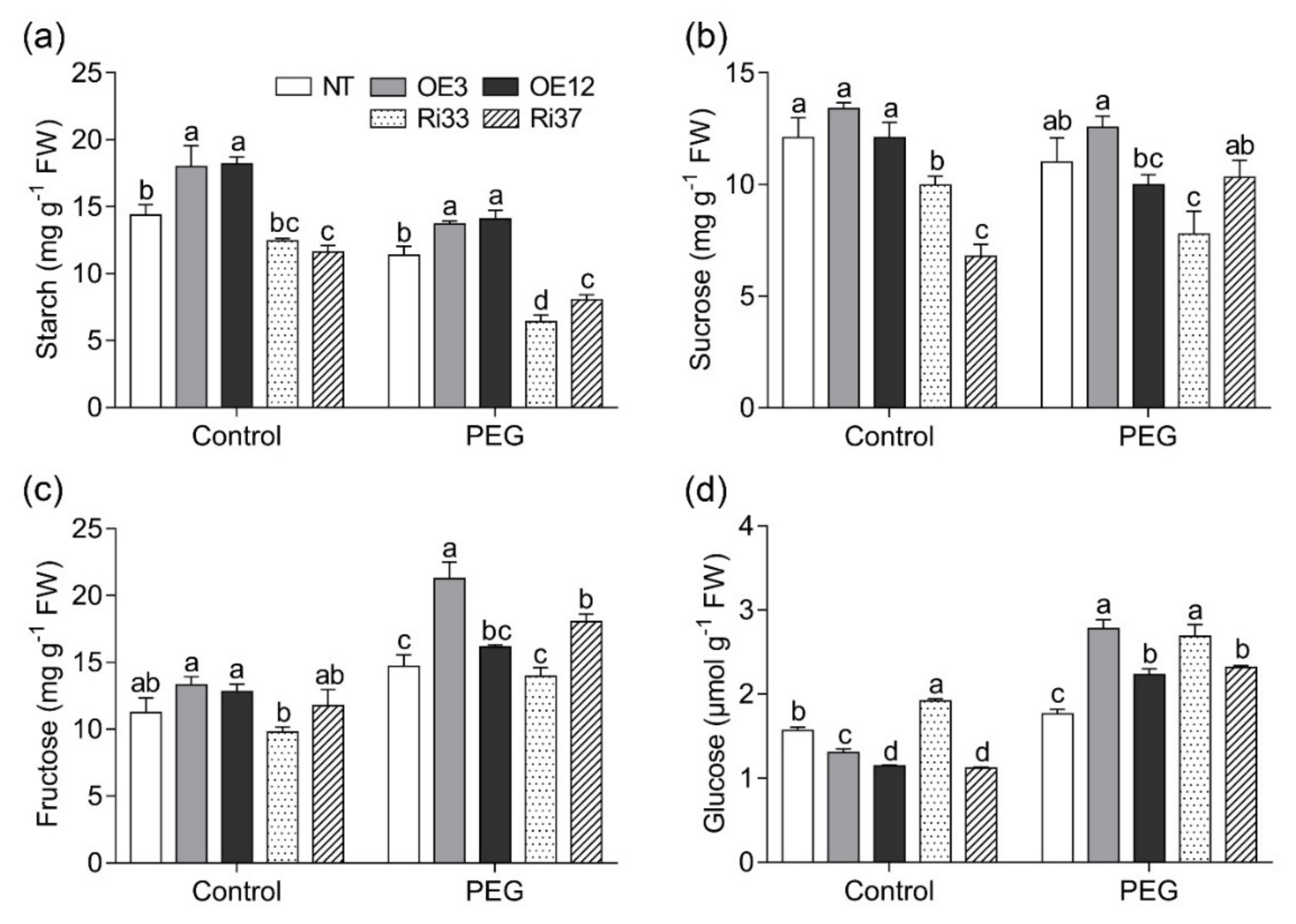
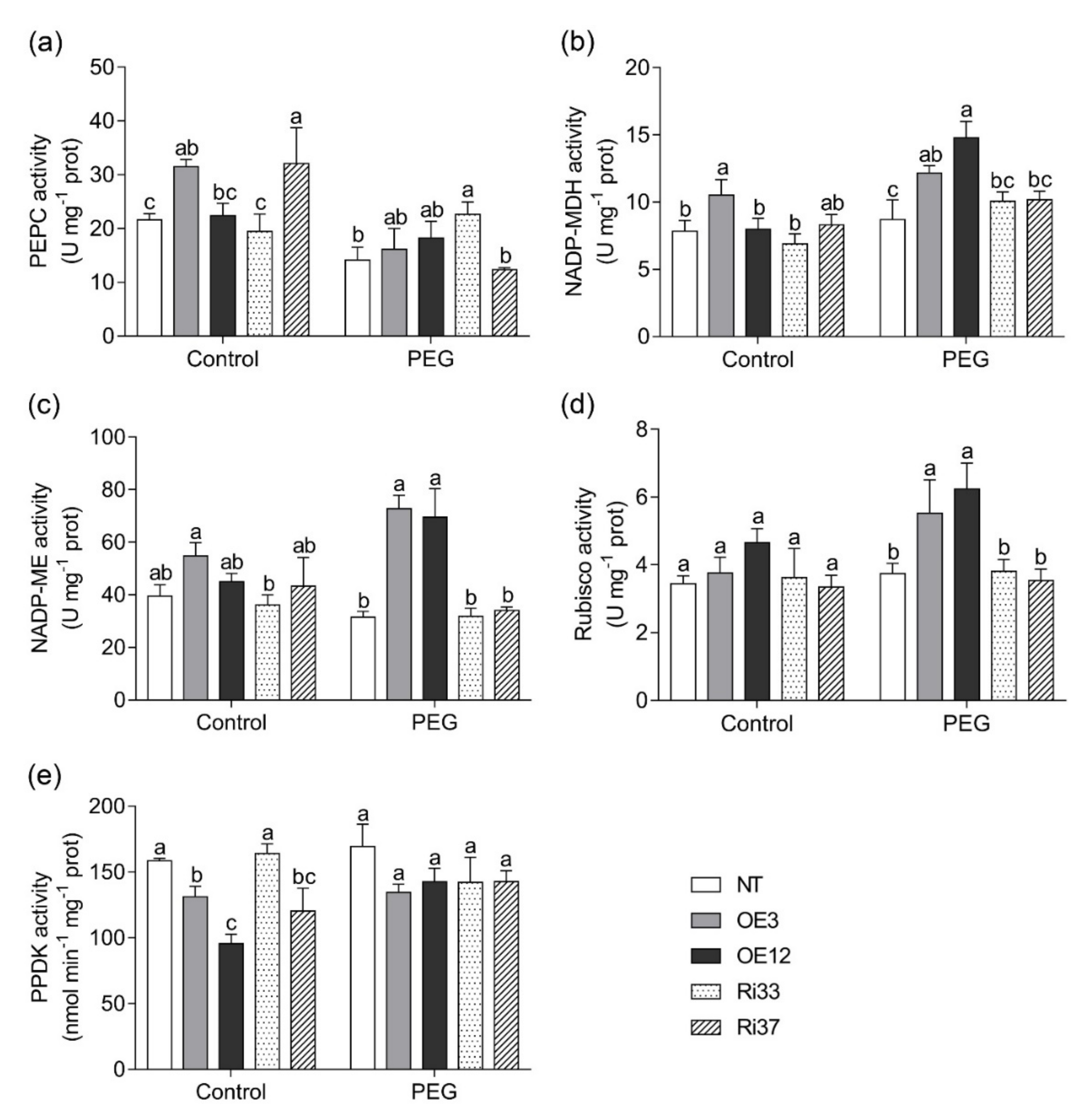

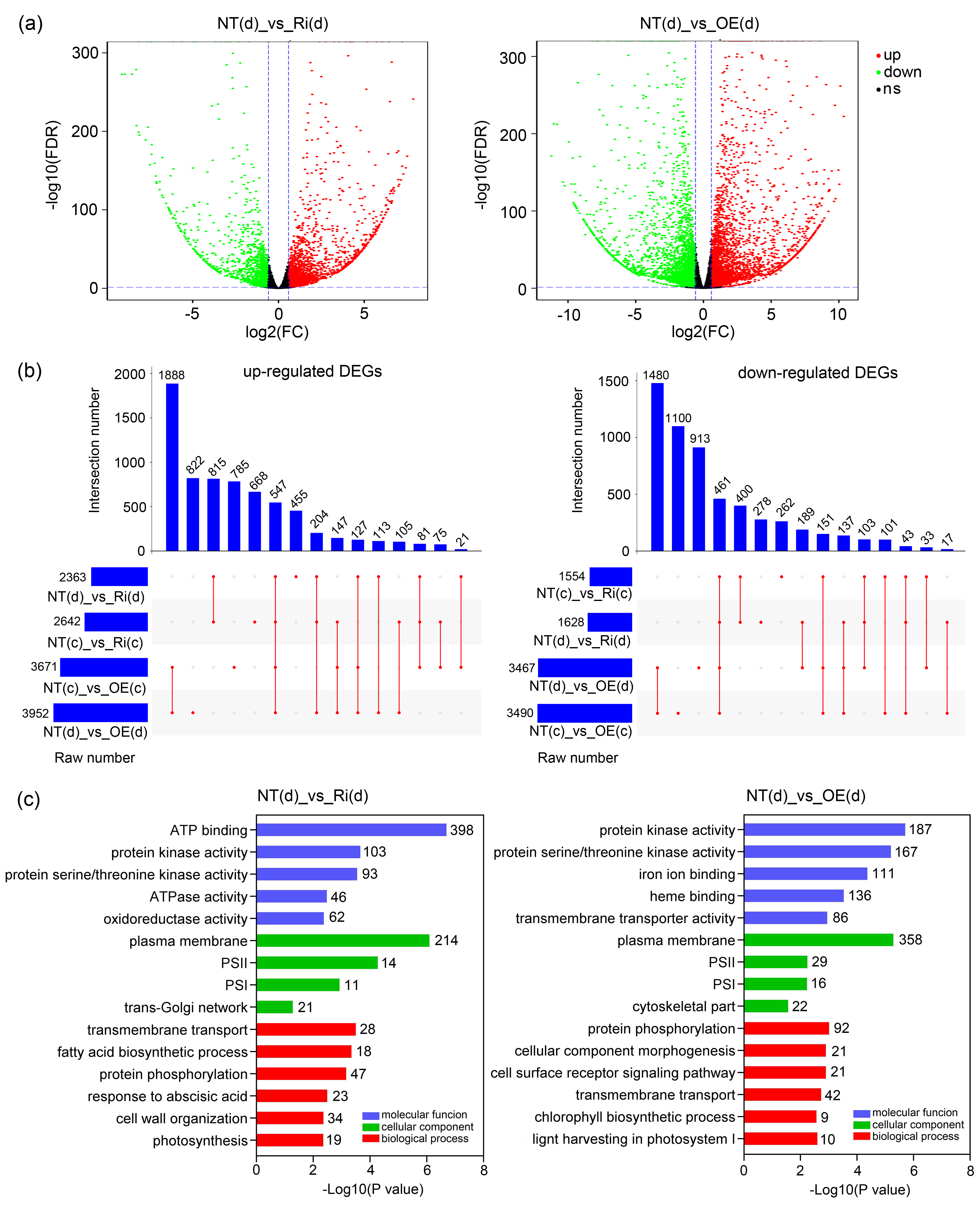
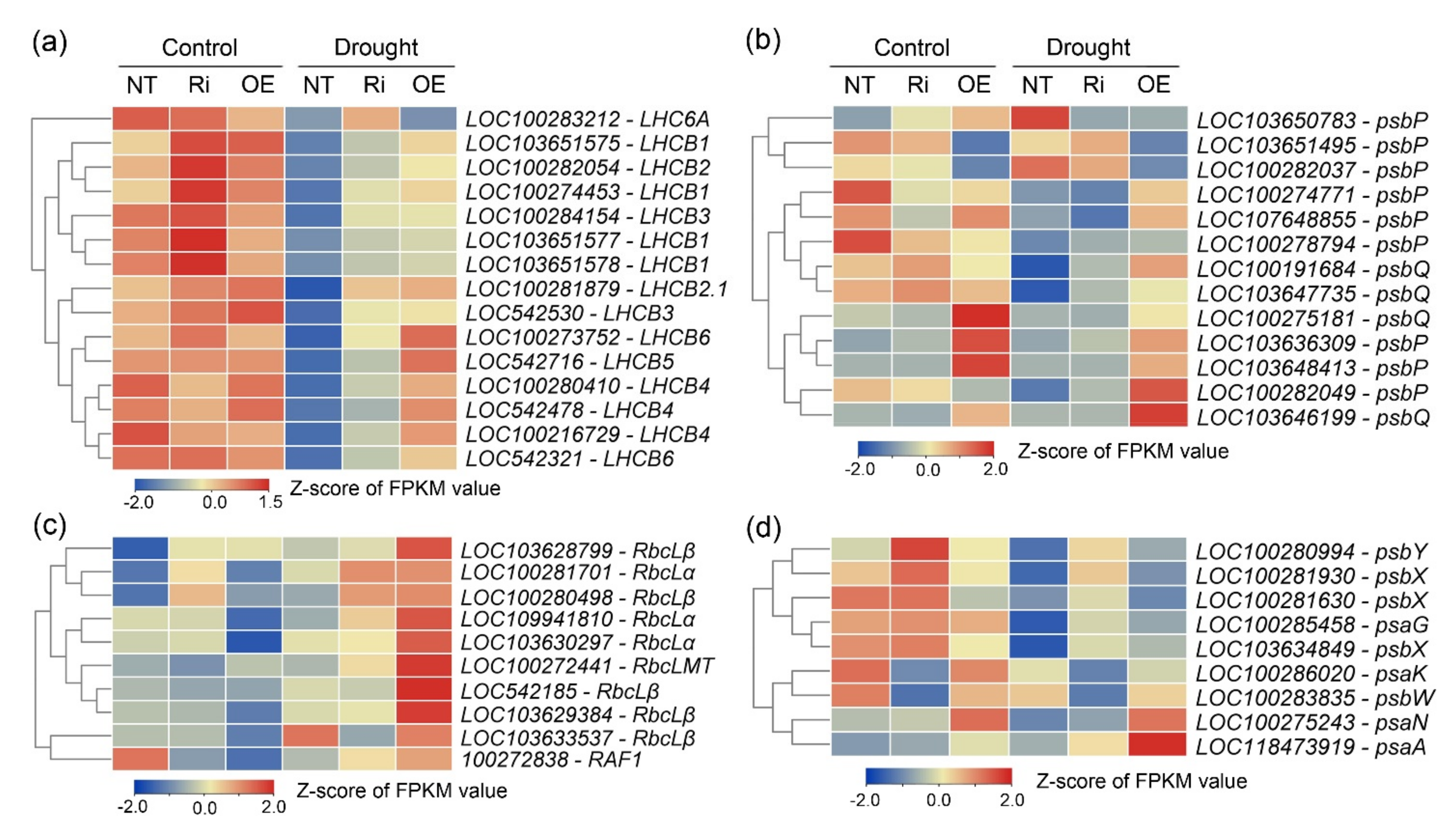
| Treatment | Line | Fresh Biomass (g Plant−1) | Dry Biomass (g Plant−1) | Root/Shoot Ratio | Area of the 4th Leaf (cm2) | Plant Height (cm) | ||
|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||||
| Control | NT | 6.62 ± 0.08 ab | 1.28 ± 0.02 bc | 0.44 ± 0.02 a | 0.09 ± 0.002 b | 0.19 ± 0.005 bc | 71.55 ± 2.32 a | 16.13 ± 0.18 b |
| OE3 | 7.44 ± 0.21 a | 1.65 ± 0.10 a | 0.47 ± 0.02 a | 0.12 ± 0.011 a | 0.23 ± 0.005 a | 59.65 ± 1.30 c | 14.50 ± 0.20 c | |
| OE12 | 6.44 ± 0.19 b | 1.39 ± 0.07 b | 0.42 ± 0.02 a | 0.09 ± 0.004 b | 0.22 ± 0.008 ab | 61.71 ± 1.25 bc | 13.98 ± 0.31 c | |
| Ri33 | 7.04 ± 0.39 ab | 1.23 ± 0.11 bc | 0.45 ± 0.02 a | 0.09 ± 0.004 b | 0.18 ± 0.004 c | 69.66 ± 0.68 ab | 16.98 ± 0.61 ab | |
| Ri37 | 6.36 ± 0.15 b | 1.13 ± 0.06 c | 0.42 ± 0.01 a | 0.07 ± 0.003 b | 0.18 ± 0.007 c | 57.42 ± 3.55 c | 17.63 ± 0.17 a | |
| Drought | NT | 2.21 ± 0.04 a | 0.32 ± 0.01 b | 0.33 ± 0.02 a | 0.12 ± 0.006 c | 0.14 ± 0.003 b | 58.09 ± 0.66 a | 13.93 ± 0.29 a |
| OE3 | 2.86 ± 0.05 a | 0.47 ± 0.02 a | 0.36 ± 0.01 a | 0.15 ± 0.008 ab | 0.17 ± 0.002 ab | 56.10 ± 0.39 a | 12.05 ± 0.22 b | |
| OE12 | 2.27 ± 0.10 a | 0.45 ± 0.02 a | 0.33 ± 0.01 a | 0.17 ± 0.008 a | 0.20 ± 0.010 a | 56.70 ± 2.02 a | 11.83 ± 0.13 b | |
| Ri33 | 2.50 ± 0.24 a | 0.37 ± 0.02 b | 0.34 ± 0.02 a | 0.13 ± 0.006 bc | 0.15 ± 0.005 b | 57.94 ± 1.50 a | 14.23 ± 0.42 a | |
| Ri37 | 2.25 ± 0.26 a | 0.33 ± 0.01 b | 0.33 ± 0.01 a | 0.14 ± 0.006 c | 0.15 ± 0.005 b | 48.93 ± 1.20 b | 15.10 ± 0.46 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Abulaizi, A.; Wang, C.; Lan, H. Overexpression of CgbHLH001, a Positive Regulator to Adversity, Enhances the Photosynthetic Capacity of Maize Seedlings under Drought Stress. Agronomy 2022, 12, 1149. https://doi.org/10.3390/agronomy12051149
Zhao H, Abulaizi A, Wang C, Lan H. Overexpression of CgbHLH001, a Positive Regulator to Adversity, Enhances the Photosynthetic Capacity of Maize Seedlings under Drought Stress. Agronomy. 2022; 12(5):1149. https://doi.org/10.3390/agronomy12051149
Chicago/Turabian StyleZhao, Haiju, Ailiman Abulaizi, Changhai Wang, and Haiyan Lan. 2022. "Overexpression of CgbHLH001, a Positive Regulator to Adversity, Enhances the Photosynthetic Capacity of Maize Seedlings under Drought Stress" Agronomy 12, no. 5: 1149. https://doi.org/10.3390/agronomy12051149
APA StyleZhao, H., Abulaizi, A., Wang, C., & Lan, H. (2022). Overexpression of CgbHLH001, a Positive Regulator to Adversity, Enhances the Photosynthetic Capacity of Maize Seedlings under Drought Stress. Agronomy, 12(5), 1149. https://doi.org/10.3390/agronomy12051149






