In Vitro Induction and Primary Evaluation of Octoploid Plants in Saskatoon Berry (Amelanchier alnifolia Nutt.)
Abstract
1. Introduction
2. Material and Methods
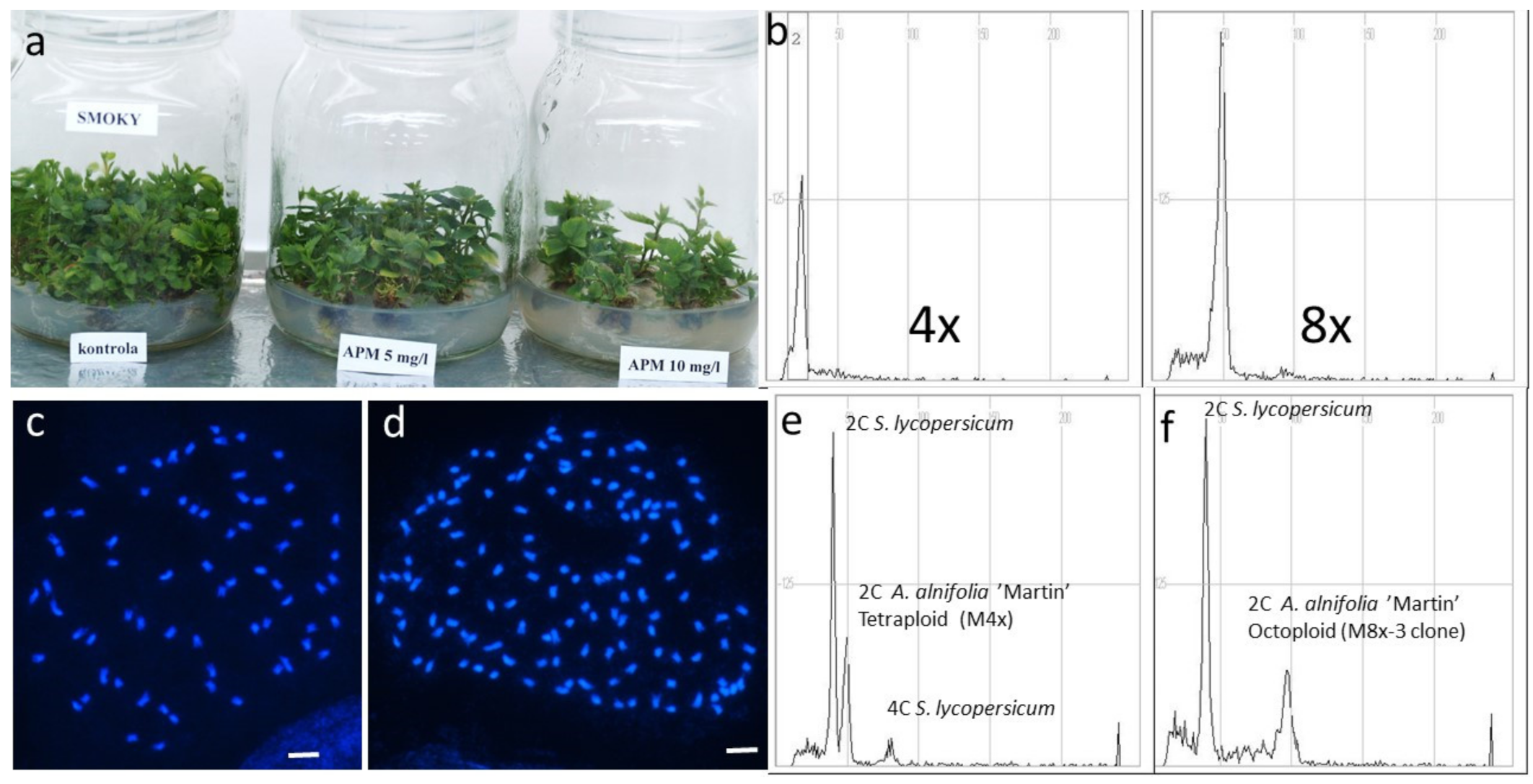
2.1. Induction of Octoploids
2.2. Detection of Octoploids
2.3. Confirmation of Octoploidy by Chromosome Counting
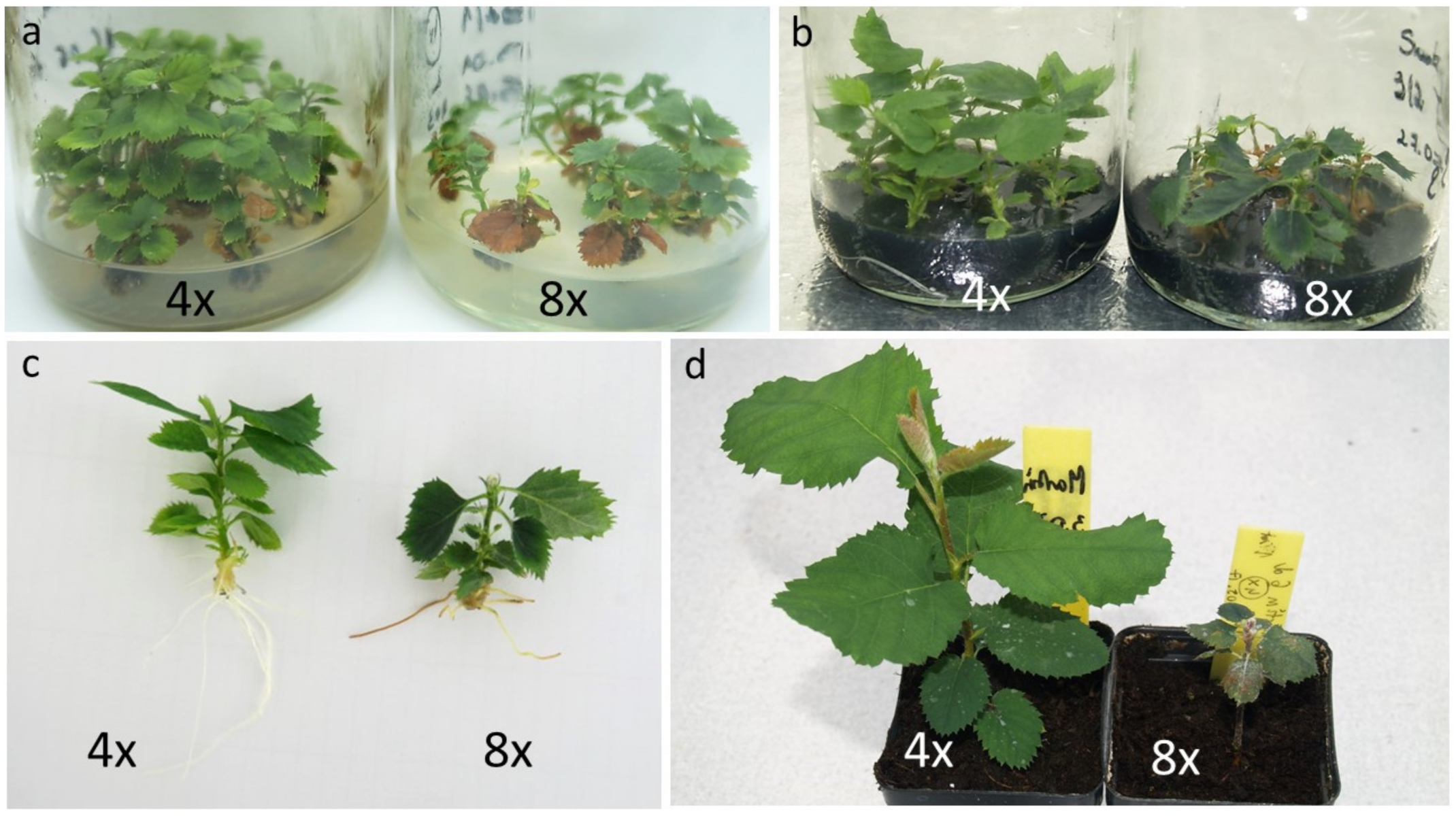
2.4. In Vitro Propagation and Rooting of Octoploids
2.5. Ethylene Measurements
2.6. Stomatal Size and Density
2.7. Statistical Analyses
3. Results and Discussion
4. Conclusions
- The described method of chromosome doubling was effective and allowed to obtain Saskatoon berry octoploids of tested cvs. ‘Smoky’ and ‘Martin’;
- Octoploids both cvs. ‘Smoky’ and ‘Martin’ were obtained after treatment with 250 mg L−1 colchicine and 5 mg L−1 APM;
- Trifluralin revealed the highest phytotoxic activity among the antimitotics used in Saskatoon berry mitotic polyploidization;
- Periodic cooling of in vitro shoot cultures increased the multiplication rate of octoploid plants of tested cultivars;
- In octoploid Saskatoon berry plants, the length of the stomata was significantly greater and their number was smaller compared with their tetraploid counterparts;
- The newly obtained octoploids Saskatoon berry showed slow growth and a tendency to premature dormancy;
- It is assumed that the phenomenon of slow growth and premature dormancy of newly formed octoploids Saskatoon berry may be associated with a change in the level and pattern of DNA methylation. Further research is required to confirm this hypothesis as well as to find factors that stimulate the growth of synthetic octoploids.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zatylny, A.M.; St-Pierre, R.G. Revised International Registry of cultivars and germplasm of the genus Amelanchier. Small Fruits Rev. 2003, 2, 51–80. [Google Scholar] [CrossRef]
- Bieniek, A.; Markuszewski, B.; Kopytowski, J.; Pluta, S.; Markowski, J. Yielding and fruit quality of several cultivars and breeding clones of Amelanchier alnifolia grown in north-eastern Poland. Zemdirb.-Agric. 2019, 106, 351–358. [Google Scholar] [CrossRef]
- Seliga, Ł.; Pluta, S. Growth and yielding of several Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes in central Poland. Zesz. Nauk. Inst. Ogrod. 2020, 28, 1–12, (In Polish with English abstract). [Google Scholar]
- Bakowska-Barczak, A.M.; Kołodziejczyk, P. Evaluation of Saskatoon berry (Amelanchier alnifolia Nutt.) cultivars for their polyphenol content, antioxidant properties, and storage stability. J. Agric. Food Chem. 2008, 56, 9933–9940. [Google Scholar] [CrossRef]
- Konopacka, D.; Piecko, J.; Mieszczakowska-Frac, M.; Markowski, J.; Rutkowski, K.; Kruczyńska, D.; Buczek, M. Saskatoon berry—A little known species of fruit, preliminary study on processing usefulness. Przemysł Ferment. I Owocowo-Warzywny 2017, 11, 8–11. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Pluta, S. The composition of bioactive compounds and antioxidant activity of Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in central Poland. Food Chem. 2017, 235, 234–243. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Seliga, Ł.; Pluta, S. Phytochemical composition and antioxidant capacity of seven Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in Poland. Molecules 2017, 22, 853. [Google Scholar] [CrossRef]
- Piecko, J.; Konopacka, D.; Mieszczakowska-Frąc, M.; Kruczyńska, D. The effectiveness of vacuum-microwave drying methods in the preservation of Amelanchier berries (Amelanchier canadensis L. Medik). Int. J. Food Eng. 2017, 13, 20160346. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Seliga, Ł.; Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem. 2020, 305, 125430. [Google Scholar] [CrossRef]
- Mazza, G. Compositional and functional properties of Saskatoon berry and blueberry. Int. J. Fruit Sci. 2005, 5, 101–120. [Google Scholar] [CrossRef]
- Żurawicz, E.; Pluta, S.; Kucharska, D. Amelanchier—A new berry crop in Poland with good potential for commercial cultivation. Acta Hortic. 2014, 1017, 251–255. [Google Scholar] [CrossRef]
- Aversano, R.; Ercolano, M.R.; Caruso, I.; Fasano, C.; Rosellini, D.; Carputo, D. Molecular tools for exploring polyploid genomes in plants. Int. J. Mol. Sci. 2012, 13, 10316–10335. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Liu, X.; Marchant, D.B.; Visger, C.J.; Soltis, D.E. Polyploidy and novelty: Gottlieb’s legacy. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130351. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Hieu, P.V. Polyploid gene expression and regulation in polysomic polyploids. Am. J. Plant Sci. 2019, 10, 1409–1443. [Google Scholar] [CrossRef]
- Parisod, C.; Holderegger, R.; Brochmann, C. Evolutionary consequences of autopolyploidy. New Phytol. 2010, 186, 5–17. [Google Scholar] [CrossRef]
- Yang, X.; Ye, C.Y.; Cheng, Z.M.; Tschaplinski, T.J.; Wullschleger, S.D.; Yin, W.; Xia, X.; Tuskan, G. Genomic aspects of research involving polyploid plants. Plant Cell Tissue Organ Cult. 2011, 104, 387–397. [Google Scholar] [CrossRef]
- Pruski, K.; Mohyuddin, M.; Grainger, G. Saskatoon (Amelanchier alnifolia Nutt.). In Biotechnology in Agriculture and Forestry Trees III; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 16. [Google Scholar]
- Burgess, M.B.; Cushman, K.R.; Doucette, E.T.; Frye, C.T.; Campbell, C.S. Understanding diploid diversity: A first step in unraveling polyploid, apomictic complexity in Amelanchier. Am. J. Bot. 2015, 102, 2041–2057. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Markiewicz, M.; Klamkowski, K.; Broniarek, A.; Marasek-Ciolakowska, A. The genetic background of the phenotypic variability observed in apple autotetraploids. Acta Hortic. 2021, 1307, 177–186. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Śliwińska, E. Estimation of DNA content in plants using flow cytometry. Post. Biol. Kom. 2008, 35 (Suppl. S24), 165–176, (In Polish with English abstract). [Google Scholar]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; He, H.; Bijman, P. Assessment of intergenomic recombination through GISH analysis of F1, BC1 and BC2 progenies of Tulipa gesneriana and T. fosteriana. Plant Sys. Evol. 2012, 298, 887–899. [Google Scholar] [CrossRef]
- Dyki, B.; Habdas, H. The method of isolation of epidermis of tomato and cucumber leaves for microscopic investigation of pathogenic fungus development. Acta Agrobot. 1996, 49, 123–129, (In Polish with English abstract). [Google Scholar] [CrossRef][Green Version]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Dhooghe, E.; Denis, S.; Eeckhaut, T.; Reheul, D.; Van Labeke, M.C. In vitro induction of tetraploids in Ranunculus. Euphytica 2009, 168, 33–40. [Google Scholar] [CrossRef]
- Dhooghe, E.; Grunewald, W.; Leus, L.; Van Labeke, M.C. In vitro polyploidisation of Helleborus species. Euphytica 2009, 165, 89–95. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Sowik, I.; Machlańska, A.; Kruczyńska, D.; Dyki, B. In vitro tetraploid induction of Malus × domestica Borkh. using leaf or shoot explants. Sci. Hort. 2017, 226, 379–388. [Google Scholar] [CrossRef]
- Niimi, H.; Watanabe, M.; Serizawa, H.; Koba, T.; Nakamura, I.; Mii, M. Amiprophosmethyl-induced efficient in vitro production of polyploids in raphanobrassica with the aid of aminoethoxyvinylglycine (AVG) in the culture medium. Breed. Sci. 2015, 65, 396–402. [Google Scholar] [CrossRef][Green Version]
- Podwyszyńska, M.; Gabryszewska, E.; Dyki, B.; Stępowska, A.; Kowalski, A.; Jasiński, A. Phenotypic and genome size changes (variation) in synthetic tetraploids of daylily (Hemerocallis) in relation to their diploid counterparts. Euphytica 2015, 203, 1–16. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Pluta, S. In vitro tetraploid induction of the blackcurrant (Ribes nigrum L.) and preliminary phenotypic observations. Zemdirb. Agric. 2019, 106, 151–158. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Soares, J.D.R.; Dos Santos, R.R.; Pasqual, M.; Silva, S.O. Colchicine and amiprophos-methyl (APM) in polyploidy induction in banana plant. Afr. J. Biotech. 2011, 10, 13476–13481. [Google Scholar]
- Talent, N.; Dickinson, T.A. Polyploidy in Crataegus and Mespilus (Rosaceae, Maloideae): Evolutionary inferences from flow cytometry of nuclear DNA amounts. Botany 2005, 83, 1268–1304. [Google Scholar]
- Burgess, M.B.; Cushman, K.R.; Doucette, E.T.; Talent, N.; Frye, C.T.; Campbell, C.S. Effects of apomixis and polyploidy on diversification and geographic distribution in Amelanchier (Rosaceae). Am. J. Bot. 2014, 101, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczyk, I.; Sliwinska, E. Leaves and seeds as materials for flow cytometric estimation of the genome size of 11 Rosaceae woody species containing DNA-staining inhibitors. J. Bot. 2010, 2010, 930895. [Google Scholar]
- Zonneveld, B.J. The DNA weights per nucleus (genome size) of more than 2350 species of the Flora of The Netherlands, of which 1370 are new to science, including the pattern of their DNA peaks. Forum Geobot. 2019, 8, 24–78. [Google Scholar]
- Pruski, K.; Nowak, J.; Grainger, G. Micropropagation of four cultivars of Saskatoon berry (Amelanchier alnifolia Nutt.). Plant Cell Tissue Organ Cult. 1990, 21, 103–109. [Google Scholar] [CrossRef]
- Kucharska, D. Sposób Rozmnażania Roślin Świdośliwy Amelanchier alnifolia Nutt. Polish Patent Office (UPRP) Patent No. 225459, 28 April 2017. [Google Scholar]
- Kucharska, D.; Orlikowska, T.; Maciorowski, R.; Kunka, M.; Niewiadomska-Wnuk, A. Storage of proliferating gooseberry cultures under slow growth conditions. Hort. Sci. 2021, 48, 134–140. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Cieślińska, M. Rooting shoots of apple varieties and their tetraploids obtained by the in vitro technique. Acta Sci. Pol. Hortorum Cultus 2018, 17, 49–64. [Google Scholar] [CrossRef]
- He, P.; Cheng, L.; Li, H.; Wang, H.; Li, L. A Comparative Analysis of DNA Methylation in Diploid and Tetraploid Apple (Malus × domestica Borkh.). Czech J. Genet. Plant Breed. 2017, 53, 63–68. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Chen, G.; Wang, J. DNA methylation alteration is a major consequence of genome doubling in autotetraploid Brassica Rapa. Arch. Biol. Sci. 2017, 69, 689–697. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Markiewicz, M.; Broniarek-Niemiec, A.; Matysiak, B.; Marasek-Ciolakowska, A. Apple autotetraploids with enhanced resistance to apple scab (Venturia inaequalis) due to genome duplication-phenotypic and genetic evaluation. Int. J. Mol. Sci. 2021, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’ apple trees. Sci. Hort. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Korban, S.S.; Wannarat, W.; Rayburn, C.M.; Tatum, T.C.; Raybur, A.L. Genome size and nucleotypic variation in Malus germplasm. Genome 2009, 52, 149–155. [Google Scholar] [CrossRef]
| Antimitotics (mg L−1) | 1st Subculture | 2nd Subculture | ||
|---|---|---|---|---|
| Number of Shoots/Explant | Explant Survival (%) | Number of Shoots/Explant | Explant Survival (%) | |
| ‘SMOKY’ | ||||
| Control | 10.0 a * | 100 | 8.5 a | 100 |
| Oryzalin 5 | 7.1 ab | 96.4 | 4.0 ab | 93.7 |
| Oryzalin 10 | 4.9 b | 87.5 | 3.1 b | 87.5 |
| Colchicine 125 | 7.1 ab | 100 | 5.3 ab | 87.5 |
| Colchicine 250 | 5.8 b | 93.7 | 4.6 ab | 93.7 |
| Trifluralin 50 | - | 0 | - | 0 |
| Trifluralin 100 | - | 0 | - | 0 |
| APM 5 | 7.6 ab | 93.7 | 5.9 ab | 93.7 |
| APM 10 | 3.7 b | 81.3 | 3.7 ab | 56.2 |
| p ** | 0.000 | 0.044 | ||
| ‘MARTIN’ | ||||
| Control | 11.8 a | 100 | 6.6 ab | 100 |
| Oryzalin 5 | 5.1 b | 100 | 10.1 a | 100 |
| Oryzalin 10 | 3.0 b | 100 | 4.6 b | 87.5 |
| Colchicine 125 | 7.4 ab | 93.7 | 7.8 ab | 87.5 |
| Colchicine 250 | 3.6 b | 93.7 | 5.6 ab | 87.5 |
| Trifluralin 50 | - | 0 | - | 0 |
| Trifluralin 100 | - | 0 | - | 0 |
| APM 5 | 7.6 ab | 93.7 | 5.4 ab | 100 |
| APM 10 | 5.4 b | 100 | 3.5 b | 100 |
| p | 0.000 | 0.003 | ||
| Genotype | Nuclear 2C DNA Content (pg) | Ploidy Level | |
|---|---|---|---|
| ‘Smoky’ | Control (4x) | 2.28 ± 0.014 c * | 4x |
| (S8x-1) | 4.60 ± 0.014 b | 8x | |
| (S8x-2) | 4.65 ± 0.026 a | 8x | |
| p ** | 0.000 | ||
| ‘Martin’ | Control (4x) | 2.29 ± 0.026 b | 4x |
| (M8x-1) | 4.55 ± 0.026 a | 8x | |
| (M8x-2) | 4.58 ± 0.052 a | 8x | |
| (M8x-3) | 4.59 ± 0.016 a | 8x | |
| (M48-4) | 4.57 ± 0.015 a | 8x | |
| p | 0.000 | ||
| Genotype | Shoot Multiplication Rate (Number of Shoots/Primary Shoot) | Rooting | |||
|---|---|---|---|---|---|
| Standard Conditions | After Cooling at 4 °C | Number of Roots/Shoot | % of Rooted Plants | ||
| ‘Smoky’ | Control (4x) | 4.2 b * | 4.0 b | 3.6 a | 100 |
| (S8x-1) | 3.8 b | 7.3 a | 2.8 b | 84.0 | |
| (S8x-2) | 5.9 a | 8.1 a | 2.7 b | 82.0 | |
| p ** | 0.004 | 0.000 | 0.010 | ||
| ‘Martin’ | Control (4x) | 3.3 a | 5.5 b | 3.6 a | 100 |
| (M8x-1) | 2.1 ab | 5.5 b | 1.9 b | 84.4 | |
| (M8x-2) | 2.9 ab | 7.5 a | 2.5 b | 72.2 | |
| (M8x-3) | 2.4 ab | 7.7 a | 1.9 b | 32.7 | |
| (M48-4) | 1.9 b | 7.2 a | 2.4 b | 75.0 | |
| p | 0.001 | 0.000 | 0.000 | ||
| Genotype | Ethylene Concentration (nl gFW−1 h−1) | |
|---|---|---|
| ‘Smoky’ | Control (4x) | 8.4 a * |
| (S8x-1) | 1.7 b | |
| (S8x-2) | 2.6 b | |
| p ** | 0.000 | |
| ‘Martin’ | Reference (4x) | 2.7 a |
| (M8x-1) | 2.2 ab | |
| (M8x-2) | 1.1 ab | |
| (M8x-3) | 0.9 b | |
| (M8x-4) | 1.8 ab | |
| p | 0.007 | |
| Genotype | Number of Stomata (items/1 mm2) | Length of Stomata (μm) | |
|---|---|---|---|
| ‘Smoky’ | Contro (4x) | 116.0 a* | 26.4 b |
| (S8x-1) | 79.0 b | 29.6 ab | |
| (S8x-2) | 50.0 c | 38.9a | |
| p ** | 0.000 | 0.000 | |
| ‘Martin’ | Contro (4x) | 93.0 a | 28.4 b |
| (M8x-2) | 65.3 b | 32.9 a | |
| p | 0.000 | 0.000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska, D.; Podwyszyńska, M.; Trzewik, A.; Marasek-Ciołakowska, A.; Pluta, S.; Seliga, Ł. In Vitro Induction and Primary Evaluation of Octoploid Plants in Saskatoon Berry (Amelanchier alnifolia Nutt.). Agronomy 2022, 12, 1215. https://doi.org/10.3390/agronomy12051215
Kucharska D, Podwyszyńska M, Trzewik A, Marasek-Ciołakowska A, Pluta S, Seliga Ł. In Vitro Induction and Primary Evaluation of Octoploid Plants in Saskatoon Berry (Amelanchier alnifolia Nutt.). Agronomy. 2022; 12(5):1215. https://doi.org/10.3390/agronomy12051215
Chicago/Turabian StyleKucharska, Danuta, Małgorzata Podwyszyńska, Aleksandra Trzewik, Agnieszka Marasek-Ciołakowska, Stanisław Pluta, and Łukasz Seliga. 2022. "In Vitro Induction and Primary Evaluation of Octoploid Plants in Saskatoon Berry (Amelanchier alnifolia Nutt.)" Agronomy 12, no. 5: 1215. https://doi.org/10.3390/agronomy12051215
APA StyleKucharska, D., Podwyszyńska, M., Trzewik, A., Marasek-Ciołakowska, A., Pluta, S., & Seliga, Ł. (2022). In Vitro Induction and Primary Evaluation of Octoploid Plants in Saskatoon Berry (Amelanchier alnifolia Nutt.). Agronomy, 12(5), 1215. https://doi.org/10.3390/agronomy12051215







