Production of Nutrient-Enriched Vermicompost from Aquatic Macrophytes Supplemented with Kitchen Waste: Assessment of Nutrient Changes, Phytotoxicity, and Earthworm Biodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Feedstocks and Earthworms for Vermicomposting
2.2. Experimental Setup and Vermicomposting
- T1 = macrophyte mixture (M) only (azolla, lemna, and salvinia, total 500 gm).
- T2 = macrophyte mixture + bulking agent cow manure (M + CM, total 500 gm).
- T3 = macrophyte mixture + CM + nutrient supplements i.e., eggshell, bone meal, banana peel, and tea waste (M + CM + NS, total 500 gm, 250 gm M + CM, and other 250 gm nutrient supplements with each 62.5 gm).
2.3. Physico-Chemical Analysis of Initial Substrates and Final Vermicompost
2.4. Growth and Reproduction of Earthworms
2.5. Phytotoxicity Test Using Seed Bioassay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
3.1.1. pH
3.1.2. Electrical Conductivity (EC)
3.1.3. Total Nitrogen
3.1.4. Total Phosphorus
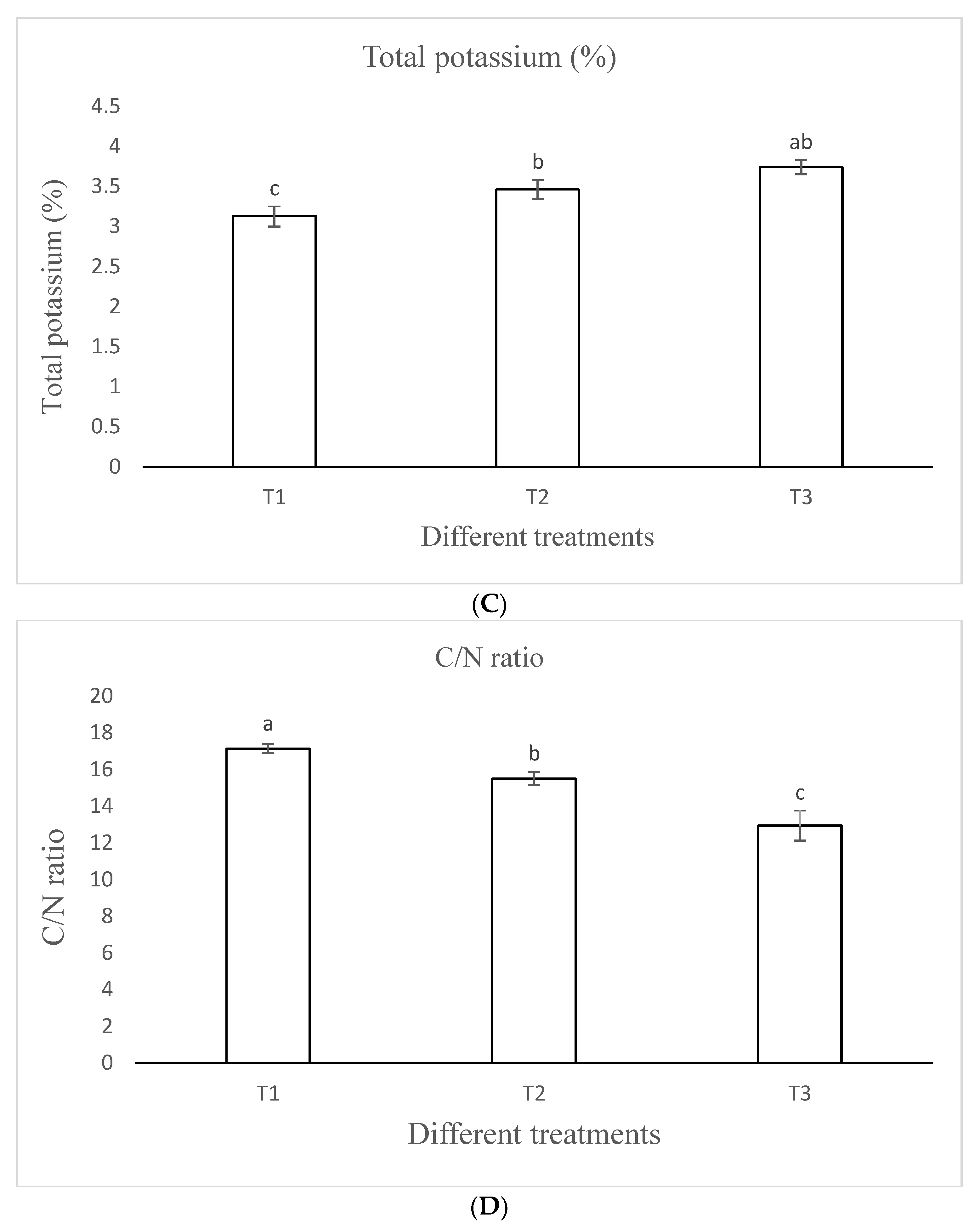
3.1.5. Total Potassium
3.1.6. Ash Content
3.1.7. Total Organic Carbon (TOC)
3.1.8. C/N Ratio
3.2. Mg, Na, and Micronutrients
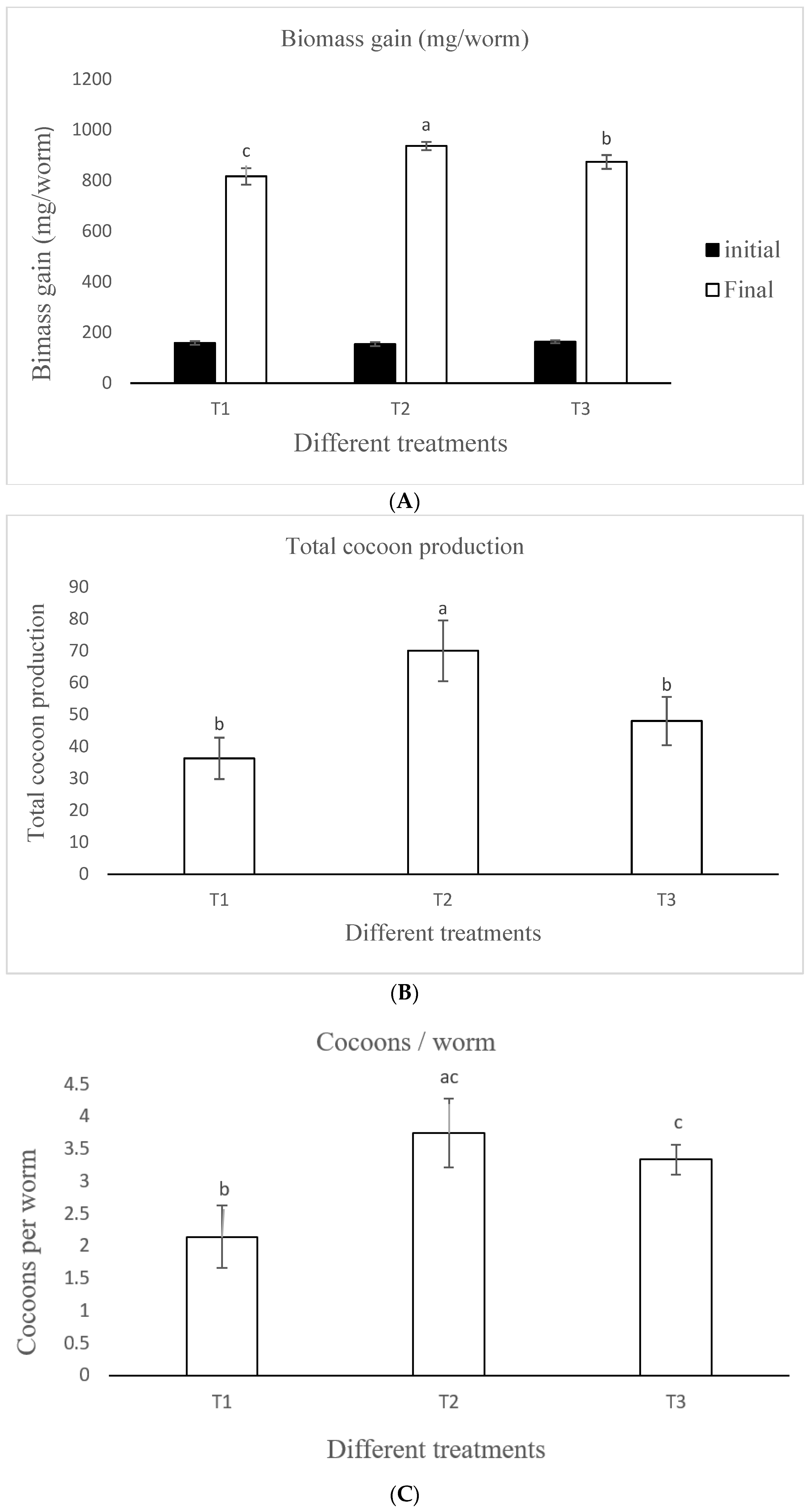
3.3. Earthworm Biomass Gain, Reproduction, and Mortality
3.3.1. Earthworm Biomass Gain
3.3.2. Growth Rate
3.3.3. Reproduction Rate
3.3.4. Population Buildup
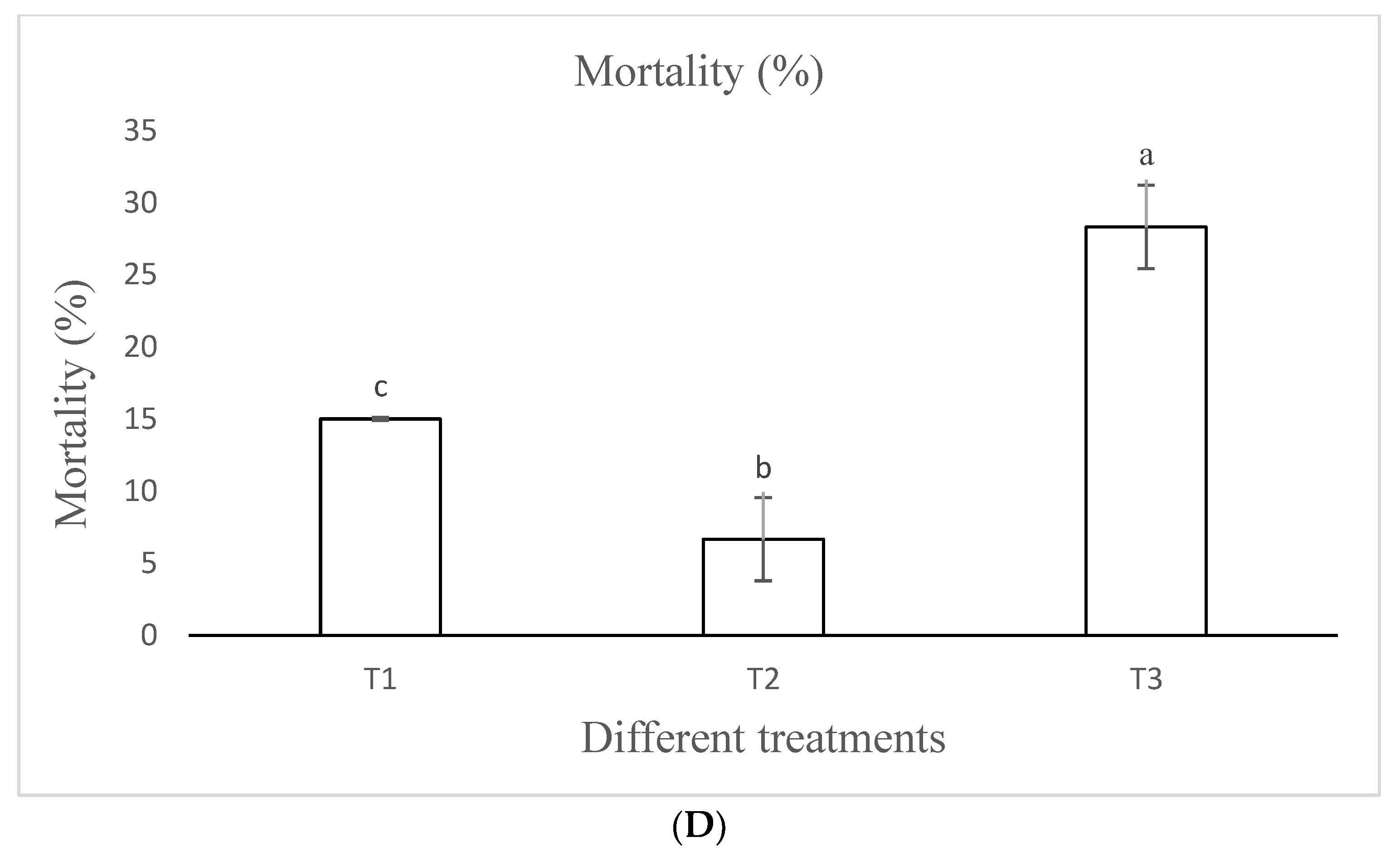
3.3.5. Mortality
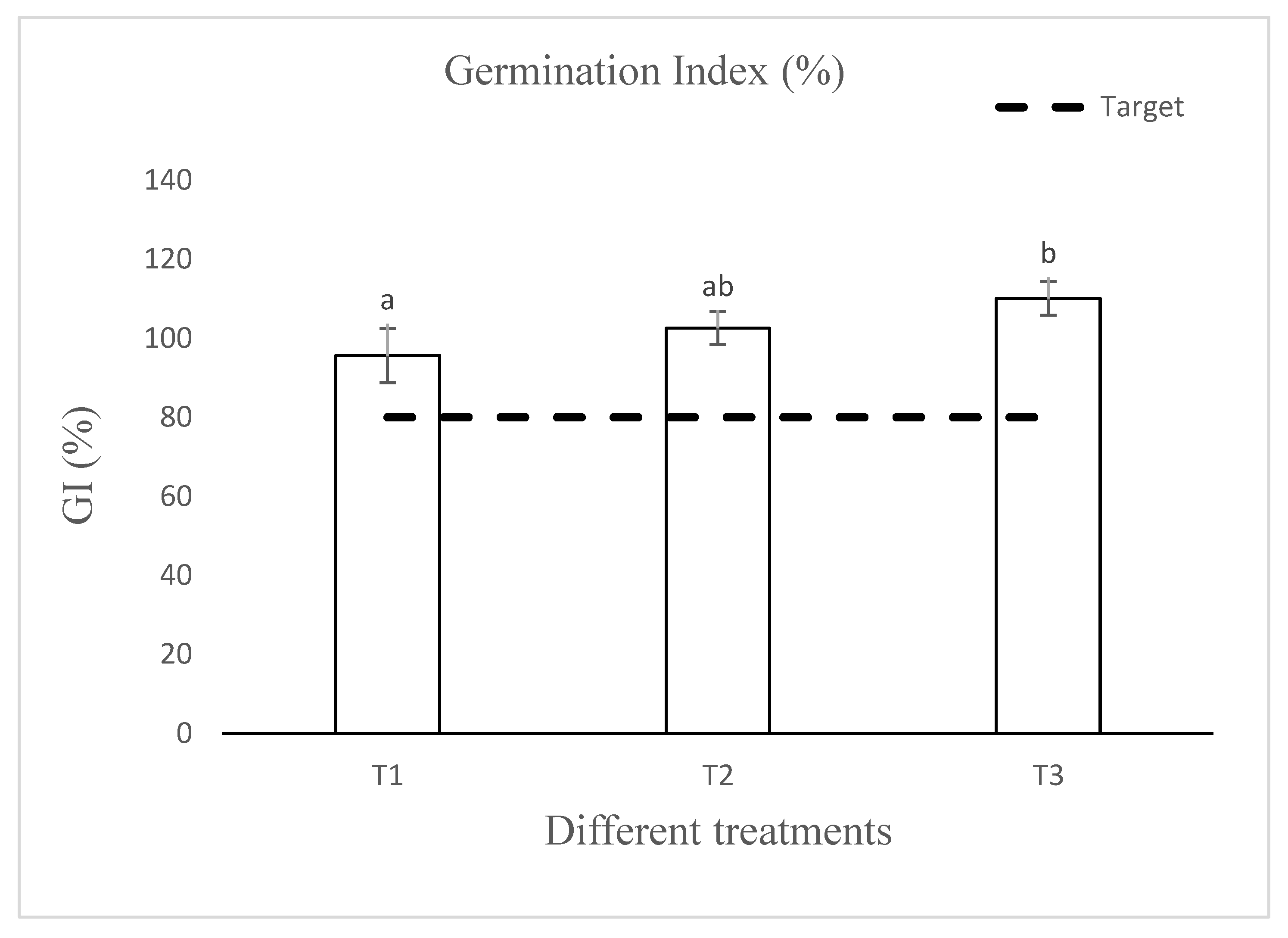
3.4. Seed Bioassay for Phytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, S.A.; Singh, J.; Vig, A.P. Earthworms as organic waste managers and biofertilizer producers. Waste Biomass Valor. 2018, 9, 1073–1086. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K.; Kannan, S.; Thangaraj, R. Recycling of leather industrial sludge through vermitechnology for a cleaner environment—A review. Ind. Crops Product. 2020, 155, 112791. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Rasool, S.; Ali, S.; Majid, S.; Rehman, M.U.; Ali, M.N.; Farooq, S. Vermicomposting: An eco-friendly approach for recycling/management of organic wastes. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; pp. 167–187. [Google Scholar] [CrossRef]
- Gusain, R.; Suthar, S. Vermicomposting of duckweed (Spirodela polyrhiza) by employing Eisenia fetida: Changes in nutrient contents, microbial enzyme activities and earthworm biodynamics. Bioresour. Technol. 2020, 311, 123585. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.; Pandey, B.; Gusain, R.; Gaur, R.Z.; Kumar, K. Nutrient changes and biodynamics of earthworm biological parameters during vermicomposting of water lettuce (Pistia sp.) biomass: A prominent weed of aquatic system. Environ. Sci. Poll. Res. 2018, 24, 199–207. [Google Scholar] [CrossRef]
- Singh, W.R.; Kalamdhad, A.S. Transformation of nutrients and heavy metals during vermicomposting of the invasive green weed Salvinia natans using Eisenia fetida. Inter. J. Recycl. Org. Waste Agric. 2016, 5, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Karmegam, N.; Jayakumar, M.; Govarthanan, M.; Kumar, P.; Ravindran, B.; Biruntha, M. Precomposting and green manure amendment for effective vermitransformation of hazardous coir industrial waste into enriched vermicompost. Bioresour. Technol. 2021, 319, 124136. [Google Scholar] [CrossRef]
- Sindhu, V.; Chatterjee, R.; Santhoshkumar, G.M.; Sinha, T. Enrichment of Organic Manures and Their Utilization in Vegetable Crops. Curr. J. App. Sci. Technol. 2020, 39, 10–24. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Ali, M.N.; Baba, Z.A.; Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron. Sustain. Develop. 2021, 41, 1–26. [Google Scholar] [CrossRef]
- Balachandar, R.; Baskaran, L.; Yuvaraj, A.; Thangaraj, R.; Subbaiya, R.; Ravindran, B.; Chang, S.W.; Karmegam, N. Enriched pressmud vermicompost production with green manure plants using Eudrilus eugeniae. Bioresour. Technol. 2020, 299, 122578. [Google Scholar] [CrossRef]
- Karmegam, N.; Rajasekar, K. Enrichment of biogas slurry vermicompost with Azotobacter chroococcum and Bacillus megaterium. J. Environ. Sci. Technol. 2012, 5, 91–108. [Google Scholar] [CrossRef] [Green Version]
- Unuofin, F.O.; Mnkeni, P.N.S. Optimization of Eisenia fetida stocking density for the bioconversion of rock phosphate enriched cow dung–waste paper mixtures. Waste Manage. 2014, 34, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Iftikar, W.; Sahariah, B.; Chattopadhyay, G.N. Vermicomposting converts fly ash to enrich soil fertility and sustain crop growth in red and lateritic soils. Resour. Conser. Recycl. 2012, 65, 100–106. [Google Scholar] [CrossRef]
- Mupambwa, H.A.; Dube, E.; Mnkeni, P.N. Fly ash composting to improve fertiliser value-a review. S. Afr. J. Sci. 2015, 111, 1–6. [Google Scholar] [CrossRef]
- Mupambwa, H.A.; Mnkeni, P.N.S. Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers: A review. Environ. Sci. Poll. Res. 2018, 25, 10577–10595. [Google Scholar] [CrossRef]
- Soobhany, N. Insight into the recovery of nutrients from organic solid waste through biochemical conversion processes for fertilizer production: A review. J. Clean. Prod. 2019, 241, 118413. [Google Scholar] [CrossRef]
- Gaonkar, M.; Chakraborty, A.P. Application of eggshell as fertilizer and calcium supplement tablet. Inter. J. Innovat. Res. Sci. Eng. Technol. 2016, 5, 3520–3525. [Google Scholar]
- Waheed, M.; Butt, M.S.; Shehzad, A.; Adzahan, N.M.; Shabbir, M.A.; Suleria, H.A.R.; Aadil, R.M. Eggshell calcium: A cheap alternative to expensive supplements. Trends Food Sci. Technol. 2019, 91, 219–230. [Google Scholar] [CrossRef]
- Budhalakoti, N. Formulation and standardisation of banana peel extracted insoluble dietary fibre-based buns. Curr. J. Appl. Sci. Technol. 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Aboul-Enein, A.M.; Salama, Z.A.; Gaafar, A.A.; Aly, H.F.; Abou-Elella, F.; Ahmed, H.A. Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. J. Chem. Pharm. Res. 2016, 8, 46–55. [Google Scholar]
- Kalemelawa, F.; Nishihara, E.; Endo, T.; Ahmad, Z.; Yeasmin, R.; Tenywa, M.M.; Yamamoto, S. An evaluation of aerobic and anaerobic composting of banana peels treated with different inoculums for soil nutrient replenishment. Bioresour. Technol. 2012, 126, 375–382. [Google Scholar] [CrossRef]
- Khayum, N.; Anbarasu, S.; Murugan, S. Biogas potential from spent tea waste: A laboratory scale investigation of co-digestion with cow manure. Energy 2018, 165, 760–768. [Google Scholar] [CrossRef]
- Cooper, J.M.; Butler, G.; Leifert, C. Life cycle analysis of greenhouse gas emissions from organic and conventional food production systems, with and without bio-energy options. NJAS-Wagening. J. Life Sci. 2011, 58, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Bhuvaneswari, A.; Kalaivanan, K.; Durairaj, S.; Selladurai, G. Effect of Bisphenol-A on the bioconversion of tea waste into vermicompost by Eudrilus eugeniae (Kinberg, 1867) at Different Intervals. Inter. J. Sci. Res. Biol. Sci. 2021, 8, 5–12. [Google Scholar] [CrossRef]
- Mäkelä, P.S.; Wasonga, D.O.; Solano Hernandez, A.; Santanen, A. seedling growth and phosphorus uptake in response to different phosphorus sources. Agronomy 2020, 10, 1089. [Google Scholar] [CrossRef]
- Baker, A.M.; Trimm, J.R.; Sikora, F.J. Availability of phosphorus in bone meal. J. Assoc. Off. Anal. Chem. 1989, 72, 867–869. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Thangaraj, R.; Maheswaran, R. Decomposition of poultry litter through vermicomposting using earthworm Drawida sulcata and its effect on plant growth. Inter. J. Environ. Sci. Technol. 2019, 16, 7241–7254. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Recycling of lignocellulosic waste as vermicompost using earthworm Eisenia fetida. Environ. Sci. Poll. Res. 2019, 26, 14024–14035. [Google Scholar] [CrossRef]
- Tandon, H.L.S. Methods of Analysis of Soils, Plants, Waters, Fertilisers & Organic Manures; Fertiliser Development and Consultation Organisation: New Delhi, India, 2005. [Google Scholar]
- Zucconi, F. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Singh, C.K.; Kumar, A. Vermicomposting of terrestrial weeds Lantana camara L. and Parthenium hysterophorus L.: Agriculture solid waste. Ecol. Quest. 2017, 28, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Biruntha, M.; Karmegam, N.; Archana, J.; Selvi, B.K.; Paul, J.A.J.; Balamuralikrishnan, B.; Chang, S.W.; Ravindran, B. Vermiconversion of biowastes with low-to-high C/N ratio into value added vermicompost. Bioresour. Technol. 2020, 297, 122398. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Bioconversion of Lantana camara by vermicomposting with two different earthworm species in monoculture. Bioresour. Technol. 2020, 296, 122308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mueller, C.; Cai, Z. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol. Biochem. 2015, 84, 199–209. [Google Scholar] [CrossRef]
- Ramnarain, Y.I.; Ansari, A.A.; Ori, L. Vermicomposting of different organic materials using the epigeic earthworm Eisenia foetida. Inter. J. Recy. Org. Waste Agric. 2019, 8, 23–36. [Google Scholar] [CrossRef] [Green Version]
- CPHEEO. Municipal Solid Waste Management Manual. Part II: The Manual. Swach Bharath Mission; Ministry of Urban Development, Central Public Health and Environmental Engineering Organisation: New Delhi, India, 2016.
- Gong, X.; Li, S.; Carson, M.A.; Chang, S.X.; Wu, Q.; Wang, L.; An, Z.; Sun, X. Spent mushroom substrate and cattle manure amendments enhance the transformation of garden waste into vermicomposts using the earthworm Eisenia fetida. J. Environ. Manag. 2019, 248, 109263. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kauser, H.; Jain, M.S.; Khwairakpam, M.; Kalamdhad, A.S. Biogenic stabilization and heavy metal immobilization during vermicomposting of vegetable waste with biochar amendment. J. Hazard. Mater. 2020, 390, 121366. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Suthar, S. Vermicomposting of invasive weed Ageratum conyzoids: Assessment of nutrient mineralization, enzymatic activities, and microbial properties. Bioresour. Technol. 2020, 312, 123537. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, R.; Biruntha, M.; Yuvaraj, A.; Thangaraj, R.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Earthworm intervened nutrient recovery and greener production of vermicompost from Ipomoea staphylina–An invasive weed with emerging environmental challenges. Chemosphere 2021, 263, 128080. [Google Scholar] [CrossRef]
- Karmegam, N.; Vijayan, P.; Prakash, M.; Paul, J.A. Vermicomposting of paper industry sludge with cow dung and green manure plants using Eisenia fetida: A viable option for cleaner and enriched vermicompost production. J. Clean. Product. 2019, 228, 718–728. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Thangaraj, R.; Ravindran, B.; Chang, S.W.; Karmegam, N. Centrality of cattle solid wastes in vermicomposting technology–A cleaner resource recovery and biowaste recycling option for agricultural and environmental sustainability. Environ. Poll. 2021, 268, 115688. [Google Scholar] [CrossRef]
- Meng, L.; Li, W.; Zhang, S.; Wu, C.; Lv, L. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Bioresour. Technol. 2017, 226, 39–45. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V.K. Biotransformation of bakery industry sludge into valuable product using vermicomposting. Bioresour. Technol. 2019, 274, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.; Khwairakpam, M. Management of invasive weed Parthenium hysterophorus through vermicomposting using a polyculture of Eisenia fetida and Eudrilus eugeniae. Environ. Sci. Pollut. Res. 2021, 28, 29710–29719. [Google Scholar] [CrossRef] [PubMed]
- Ananthavalli, R.; Ramadas, V.; Paul, J.A.J.; Selvi, B.K.; Karmegam, N. Vermistabilization of seaweeds using an indigenous earthworm species, Perionyx excavatus (Perrier). Ecol. Eng. 2019, 130, 23–31. [Google Scholar] [CrossRef]
- Rai, R.; Singh, R.K.; Suthar, S. Production of compost with biopesticide property from toxic weed Lantana: Quantification of alkaloids in compost and bacterial pathogen suppression. J. Hazard. Mater. 2021, 401, 123332. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Suthar, S. Vermistabilization of paper mill wastewater sludge using Eisenia fetida. Bioresour. Technol. 2013, 128, 193–198. [Google Scholar] [CrossRef]
- Song, X.; Liu, M.; Wu, D.; Qi, L.; Ye, C.; Jiao, J.; Hu, F. Heavy metal and nutrient changes during vermicomposting animal manure spiked with mushroom residues. Waste Manag. 2014, 34, 1977–1983. [Google Scholar] [CrossRef]
- Suthar, S. Vermicomposting of vegetable-market solid waste using Eisenia fetida: Impact of bulking material on earthworm growth and decomposition rate. Ecol. Eng. 2009, 35, 914–920. [Google Scholar] [CrossRef]
- Aira, M.; Monroy, F.; Domínguez, J. Earthworms strongly modify microbial biomass and activity triggering enzymatic activities during vermicomposting independently of the application rates of pig slurry. Sci. Total Environ. 2007, 385, 252–261. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Suthar, S. Nutrient scaling of duckweed (Spirodela polyrhiza) biomass in urban wastewater and its utility in anaerobic co-digestion. Process Saf. Environ. Prot. 2017, 107, 138–146. [Google Scholar] [CrossRef]
- Gusain, R.; Suthar, S. Biofuel production efficiency of biomass of some aquatic weeds (Lemna gibba, Lemna minor, Pistia stratiotes and Eichhornia sp.). Process Saf. Environ. Prot. 2017, 109, 233–241. [Google Scholar] [CrossRef]
- Attia-Ismail, S.A. Factors limiting and methods of improving nutritive and feeding values of halophytes in arid, semi-arid and coastal areas. In Proceedings of the International Conference on Biosaline Agriculture and High Salinity Tolerance, Mugla, Turkey, 9–14 January 2005; pp. 9–14. [Google Scholar]
- Attia-Ismail, S.A. Plant secondary metabolites: Deleterious effects, remediation. In Plants, Pollutants and Remediation; Springer: Dordrecht, The Netherlands, 2015; pp. 157–178. [Google Scholar] [CrossRef]
- Hendriksen, N.B. Leaf litter selection by detritivore and geophagous earthworms. Biol. Fertil. Soils 1990, 10, 17–21. [Google Scholar]
- Suthar, S.; Gairola, S. Nutrient recovery from urban forest leaf litter waste solids using Eisenia fetida. Ecol. Eng. 2014, 71, 660–666. [Google Scholar] [CrossRef]
- Sáez, J.A.; Pérez-Murcia, M.D.; Vico, A.; Martínez-Gallardo, M.R.; Andreu-Rodriguez, F.J.; López, M.J.; Moral, R. Olive mill wastewater-evaporation ponds long term stored: Integrated assessment of in situ bioremediation strategies based on composting and vermicomposting. J. Hazard. Mater. 2021, 402, 123481. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Using cow dung and spent coffee grounds to enhance the two-stage co-composting of green waste. Bioresour. Technol. 2017, 245, 152–161. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Tea Waste | Eggshell | Bone Meal | Banana Peel | Cow Manure |
|---|---|---|---|---|---|
| Ash (%) | 5.67 ± 0.78 | 95.67 ± 1.06 | 49.47 ± 1.90 | 13.34 ± 0.95 | 28.50 ± 2.64 |
| O.M. (%) | 94.33 ± 0.79 | 4.33 ± 1.06 | 50.53 ±1.89 | 86.66 ± 0.95 | 71.50 ± 3.36 |
| O.C (%) | 54.72 ± 0.45 | 2.51 ± 0.61 | 29.31 ± 1.10 | 50.27 ± 0.55 | 41.46 ± 1.53 |
| pH | 6.42 ± 0.04 | 7.86 ± 0.17 | 6.75 ± 0.07 | 5.51 ± 0.03 | 8.01 ± 0.05 |
| EC (mS cm−1) | 0.96 ± 0.07 | 0.21 ± 0.01 | 2.97 ± 0.16 | 9.28 ± 1.68 | 1.48 ± 0.15 |
| TKN (%) | 4.32 ± 0.54 | 0.47 ± 0.09 | 3.69 ± 0.06 | 1.28 ± 0.05 | 1.35 ± 0.26 |
| TP (%) | 0.12 ± 0.06 | 0.009 ± 0.01 | 4.01 ± 0.18 | 0.03 ± 0.01 | 0.31 ± 0.01 |
| TK (%) | 0.61 ±0.23 | 0.08 ±0.21 | 0.56 ±0.19 | 8.6 ±0.71 | 0.72 ± 0.01 |
| Mg (%) | 0.09 ±0.007 | 0.15 ±0.03 | 0.43 ±0.04 | 0.08 ±0.01 | 0.32 ± 0.01 |
| Na (mg kg−1) | 146.67 ± 7.92 | 137.76 ± 9.31 | 599.82 ± 12.40 | 59.84 ± 5.26 | 229.46 ± 13.90 |
| Cu (mg kg−1) | 7.16 ±0.74 | 3.07 ± 0.35 | 8.91 ±1.18 | 5.24 ± 0.43 | 7.53 ± 0.89 |
| Zn (mg kg−1) | 21.75 ± 2.67 | 6.81 ± 2.01 | 72.53 ± 6.80 | 26.42 ± 1.16 | 68.94 ± 4.76 |
| Fe (mg kg−1) | 81.89 ± 9.59 | 4.37 ± 1.34 | 729.56 ± 44.50 | 12.26 ± 1.87 | 793.46 ± 9.85 |
| Mn (mg kg−1) | 137.52 ± 22.16 | BDL | 84.87 ± 9.89 | 9.25 ± 2.17 | 222.73 ± 8.71 |
| C/N | 12.67 ± 0.34 | 5.34 ± 0.18 | 7.94 ± 0.49 | 39.27 ± 1.76 | 30.71 ± 1.05 |
| Parameters | T1 | T2 | T3 | F-Value | p-Value |
|---|---|---|---|---|---|
| Ash (%) | 31.76 ± 0.89 b | 32.36 ± 1.07 ab | 35.92 ± 2.41 a | 5.8568 | 0.0389 |
| O.C (%) | 39.58 ± 0.52 a | 39.23 ± 0.62 ab | 37.16 ± 1.39 b | 5.8731 | 0.0387 |
| pH | 7.65 ± 0.14 a | 7.81 ± 0.10 a | 7.98 ± 0.17 a | 3.9588 | 0.0801 |
| EC (mS cm−1) | 3.37 ± 0.19 b | 3.45 ± 0.25 ab | 3.94 ± 0.18 a | 6.3857 | 0.0326 |
| TKN (%) | 2.31 ± 0.04 c | 2.53 ± 0.02 b | 2.87 ± 0.10 a | 57.010 | 0.0001 |
| TP (%) | 0.73 ± 0.04 b | 0.78 ± 0.05 ab | 0.86 ± 0.04 a | 5.2143 | 0.0487 |
| TK (%) | 3.13 ± 0.13 c | 3.46 ± 0.12 b | 3.74 ± 0.09 ab | 19.7481 | 0.0023 |
| Mg (%) | 0.48 ± 0.02 b | 0.53 ± 0.03 ab | 0.55 ± 0.01 a | 6.5000 | 0.0315 |
| Na (%) | 0.29 ± 0.01 c | 0.37 ± 0.01 ab | 0.40 ± 0.02 a | 28.6774 | 0.0008 |
| Cu (mg kg−1) | 10.67 ± 0.61 b | 12.34 ± 0.74 b | 15.29 ± 0.68 a | 35.3508 | 0.0005 |
| Zn (mg kg−1) | 64.74 ± 3.87 b | 72.36 ± 4.32 b | 81.55 ± 2.40 a | 16.1641 | 0.0038 |
| Fe (mg kg−1) | 1276.87 ± 17.26 b | 1320.12 ± 15.11 b | 1397.12 ± 21.44 a | 29.1343 | 0.0008 |
| Mn (mg kg−1) | 634.92 ± 11.51 c | 697.31 ± 13.89 b | 721.69 ± 11.18 ab | 39.946 | 0.0003 |
| C/N | 17.13 ± 0.24 a | 15.50 ± 0.35 b | 12.94 ± 0.83 c | 44.6269 | 0.0002 |
| Parameters | T1 | T2 | T3 | F-Value | p-Value |
|---|---|---|---|---|---|
| Initial mean individual weight (mg worm−1) | 158.71 ± 6.98 a | 154.02 ± 7.74 a | 163.24 ± 5.62 a | 1.3359 | 0.3312 |
| Final mean individual weight (mg worm−1) | 816.33 ± 32.48 c | 936.30 ± 16.28 a | 873.47 ± 27.21 b | 20.723 | 0.002 |
| Net weight gain (mg worm−1) | 657.61 ± 16.72 b | 782.27 ± 13.34 a | 710.23 ±32.79 b | 22.985 | 0.001 |
| Maximum growth rate (mg worm−1 day−1) | 10.96 ± 0.28 b | 13.04 ± 0.22 a | 11.83 ± 0.54 b | 23.039 | 0.001 |
| Mortality (%) | 15.00 ± 0.00 b | 6.67 ± 2.89 c | 28.33 ± 2.89 a | 64.5000 | <0.001 |
| Total cocoons produced | 36.33 ± 6.51 b | 70.00 ± 9.54 a | 48.0 ± 7.55 b | 13.819 | 0.005 |
| Cocoons per worm | 2.15 ± 0.48 b | 3.75 ± 0.52 ac | 3.34 ± 0.23 c | 11.119 | 0.009 |
| Total juveniles | 13.67 ± 3.51 b | 26.67 ± 6.42 a | 17.00 ± 4.58 b | 5.495 | 0.044 |
| Juveniles per worm | 0.81 ± 0.24 b | 1.57 ± 0.23 a | 1.19 ± 0.32 b | 5.930 | 0.037 |
| Total population at the end | 30.67 ± 3.21 b | 45.33 ± 6.67 a | 31.33 ± 5.03 b | 7.716 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatoo, A.M.; Bhat, S.A.; Ali, M.N.; Baba, Z.A.; Zaheen, Z. Production of Nutrient-Enriched Vermicompost from Aquatic Macrophytes Supplemented with Kitchen Waste: Assessment of Nutrient Changes, Phytotoxicity, and Earthworm Biodynamics. Agronomy 2022, 12, 1303. https://doi.org/10.3390/agronomy12061303
Yatoo AM, Bhat SA, Ali MN, Baba ZA, Zaheen Z. Production of Nutrient-Enriched Vermicompost from Aquatic Macrophytes Supplemented with Kitchen Waste: Assessment of Nutrient Changes, Phytotoxicity, and Earthworm Biodynamics. Agronomy. 2022; 12(6):1303. https://doi.org/10.3390/agronomy12061303
Chicago/Turabian StyleYatoo, Ali Mohd, Sartaj Ahmad Bhat, Md. Niamat Ali, Zahoor Ahmad Baba, and Zarka Zaheen. 2022. "Production of Nutrient-Enriched Vermicompost from Aquatic Macrophytes Supplemented with Kitchen Waste: Assessment of Nutrient Changes, Phytotoxicity, and Earthworm Biodynamics" Agronomy 12, no. 6: 1303. https://doi.org/10.3390/agronomy12061303







