Diversity in Root Architecture of Durum Wheat at Stem Elongation under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
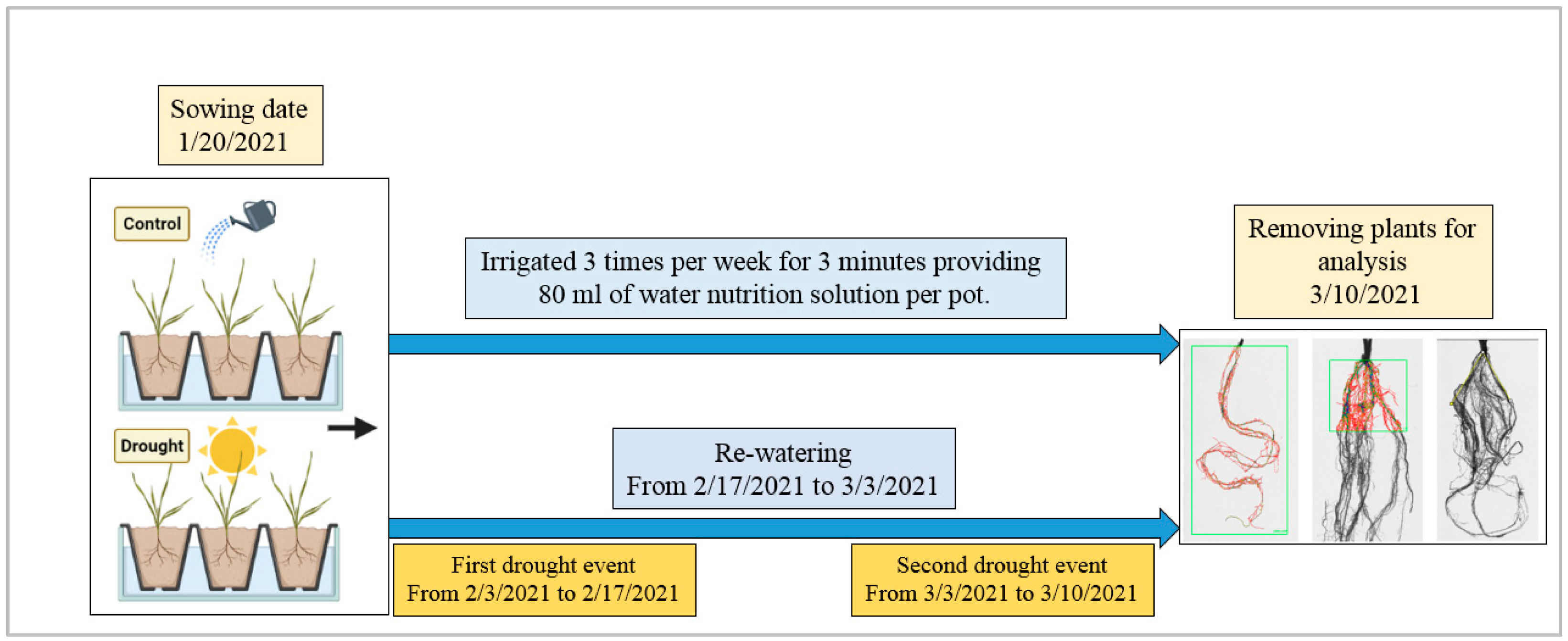
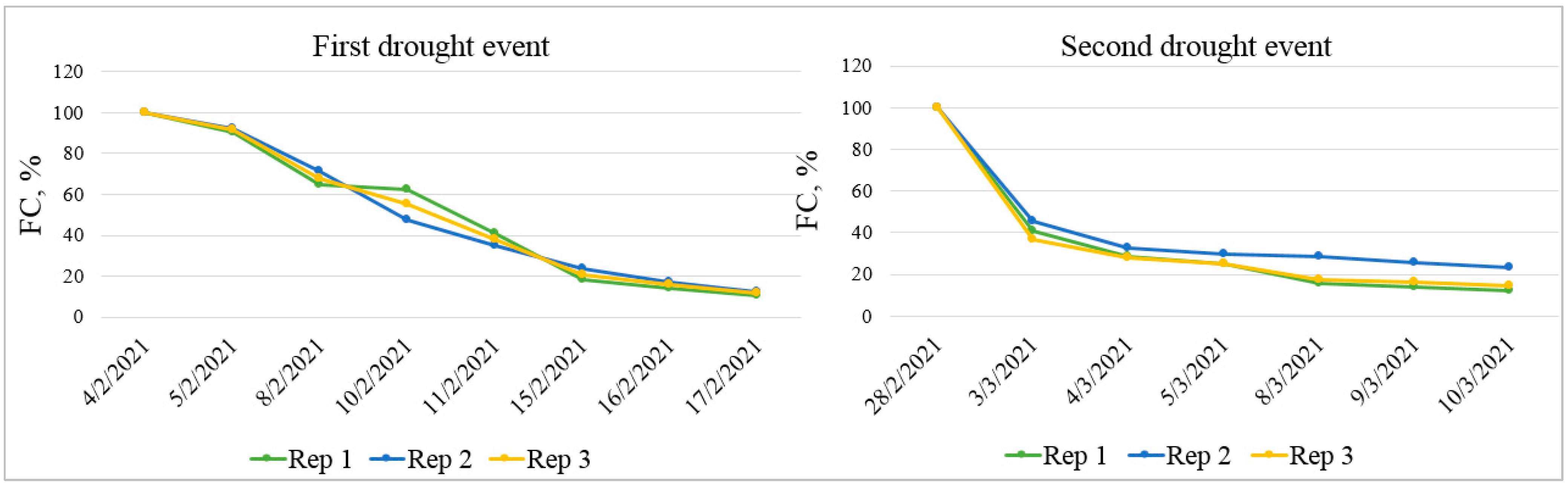
2.2. Greenhouse Experimental Design and Conditions
2.3. Phenotyping
2.4. Field Experiment
2.5. Statistical Analysis
3. Results
3.1. Drought Effect on Shoot Growth and Development in Greenhouse Experiment
3.2. Drought Effect on Whole Root System in Greenhouse Experiment
3.3. Drought Effect on Root Parameters at Topsoil in Greenhouse Experiment
3.4. The Comparison of Root System between Greenhouse and Field
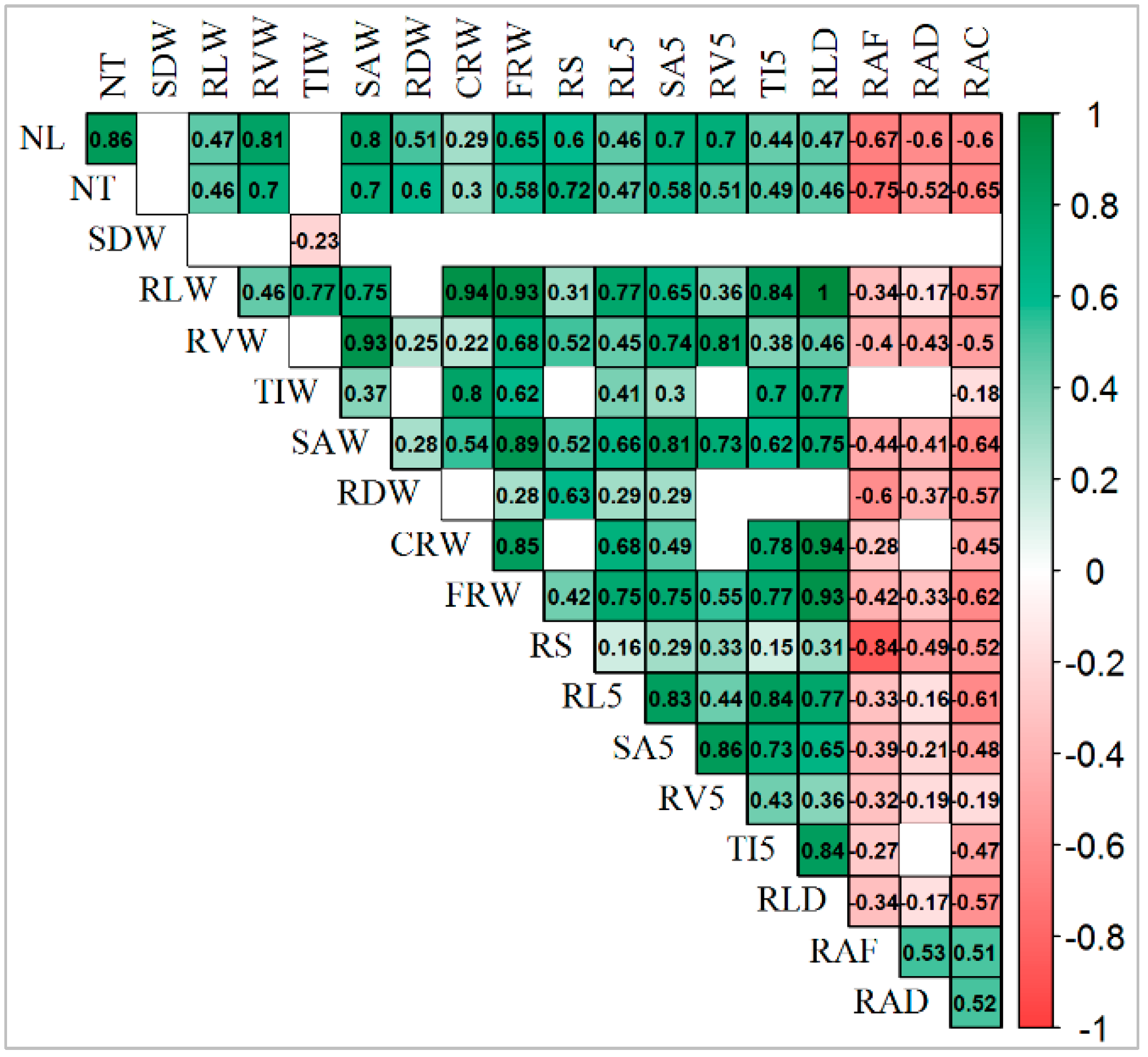
3.5. Correlation among Traits
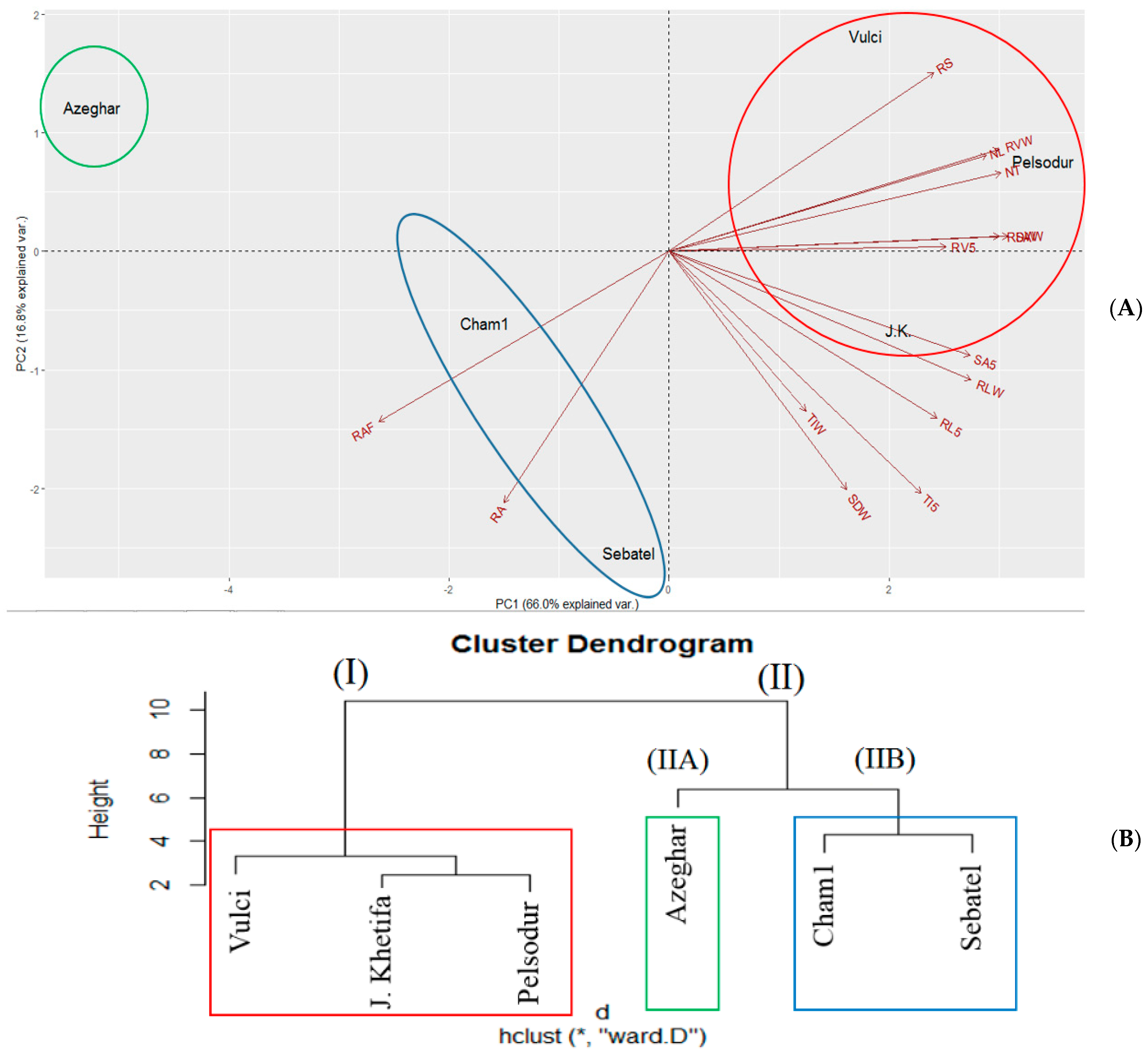
3.6. PCA in Response to Drought
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CR5 | number of crossings in the first 5 cm below the ground |
| CRF | number of crossings in the first 5 cm below the ground of plants from field |
| CRW | total number of crossings |
| FR5 | number of forks in the first 5 cm below the ground |
| FRF | number of forks in the first 5 cm below the ground of plants from field |
| FRW | total number of forks |
| NL | number of leave |
| NT | number of tillers |
| RAC | root angle of plants grown in greenhouse as control |
| RAD | root angle of plants grown in greenhouse under drought condition |
| RAF | root angle of plants grown in the field |
| RDW | root dry weight |
| RL5 | sum of roots lengths in the first 5 cm below the ground |
| RLD | root length density |
| RLF | sum of roots lengths in the first 5 cm below the ground of plants from field |
| RLW | sum of all root lengths |
| RS | root shoot ratio |
| RV5 | root volume in the first 5 cm below the ground |
| RVF | root volume in the first 5 cm below the ground of plants from field |
| RVW | total root volume |
| SA5 | root surface area in the first 5 cm below the ground |
| SAF | root surface area in the first 5 cm below the ground of plants from field |
| SAW | total root surface area |
| SDW | shoot dry weight |
| TI5 | numbers of tips in the first 5 cm below the ground |
| TIF | number of tips in the first 5 cm below the ground of plants from field |
| TIW | total number of tips |
References
- Nam, W.H.; Hayes, M.J.; Svoboda, M.D.; Tadesse, T.; Wilhite, D.A. Drought hazard assessment in the context of climate change for South Korea. Agric. Water Manag. 2015, 160, 106–117. [Google Scholar] [CrossRef]
- Mann, M.E.; Gleick, P.H. Climate change and California drought in the 21st century. Proc. Natl. Acad. Sci. USA 2015, 112, 3858–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, D.W. Stress metabolism: Its implication in breeding programmes. In Drought Tolerance in Winter Cereals; Srivastava, J.P., Porceddu, E., Acevedo, E., Varma, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987. [Google Scholar]
- Baird, J.; Gentry, J.; Lawrence, D.; Erbacher, A.; Aisthorpe, D.; Bell, L.; Anderson, B.; Brook, G.; Verrell, A.; Dunn, M.; et al. Nitrogen in the Farming System: Drought Implications on the Availability of Nitrogen. How Much Is There and Where Is It within the Profile? Does Cropping Sequence Influence Fallow Mineralisation Activity? Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2020/03/nitrogen-in-the-farming-system-drought-implications-on-the-availability-of-nitrogen.-how-much-is-there-and-where-is-it-within-the-profile-does-cropping-sequence-influence-fallow-mineralisation-activity (accessed on 21 April 2022).
- Sarto, M.V.M.; Sarto, J.R.W.; Rampim, L.; Rosset, J.S.; Bassegio, D.; da Costa, P.F.; Inagaki, A.M. Wheat phenology and yield under drought: A review. Aust. J. Crop Sci. 2017, 11, 941–946. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Carvalho, P.; Azam-Ali, S.; Foulkes, M.J. Quantifying relationships between rooting traits and water uptake under drought in Mediterranean barley and durum wheat. J. Integr. Plant Biol. 2014, 56, 455–469. [Google Scholar] [CrossRef]
- Rezzouk, F.Z.; Gracia-Romero, A.; Kefauver, S.C.; Nieto-Taladriz, M.T.; Serret, M.D.; Araus, J.L. Durum wheat ideotypes in Mediterranean environments differing in water and temperature conditions. Agric. Water Manag. 2022, 259, 107257. [Google Scholar] [CrossRef]
- Saeidi, M.; Ardalani, S.; Jalali-Honarmand, S.; Ghobadi, M.E.; Abdoli, M. Evaluation of drought stress at vegetative growth stage on the grain yield formation and some physiological traits as well as fluorescence parameters of different bread wheat cultivars. Acta Biol. Szeged. 2015, 59, 35–44. [Google Scholar]
- Blum, A.; Ramaiah, S.; Kanemasu, E.T.; Paulsen, G.M. Wheat recovery from drought stress at the tillering stage of development. F. Crop. Res. 1990, 24, 67–85. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Z.; Zhu, M.; Li, C.; Zhu, X.; Guo, W. Does cyclic water stress damage wheat yield more than a single stress? PLoS ONE 2018, 13, e0195535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Water Transport in and to Roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 245–265. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Christopher, J.; deVoil, P.; Hammer, G.L.; Manschadi, A.M.; Christopher, J.; deVoil, P.; Hammer, G.L. The role of root architectural traits in adaptation of wheat to water-limited environments. Funct. Plant Biol. 2006, 33, 823–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Castaneda, C.; Richards, R.A.; Farquhar, G.D. Variation in Early Vigor between Wheat and Barley. Crop Sci. 1995, 35, 472–479. [Google Scholar] [CrossRef]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef]
- Sanguineti, M.C.; Li, S.; MacCaferri, M.; Corneti, S.; Rotondo, F.; Chiari, T.; Tuberosa, R. Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann. Appl. Biol. 2007, 151, 291–305. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; DeVoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Tuberosa, R. Genetic and genomic dissection of maize root development and architecture. Curr. Opin. Plant Biol. 2009, 12, 172–177. [Google Scholar] [CrossRef]
- Maccaferri, M.; El-Feki, W.; Nazemi, G.; Salvi, S.; Canè, M.A.; Colalongo, M.C.; Stefanelli, S.; Tuberosa, R. Prioritizing quantitative trait loci for root system architecture in tetraploid wheat. J. Exp. Bot. 2016, 67, 1161–1178. [Google Scholar] [CrossRef]
- Richard, C.A.I.; Hickey, L.T.; Fletcher, S.; Jennings, R.; Chenu, K.; Christopher, J.T. High-throughput phenotyping of seminal root traits in wheat. Plant Methods 2015, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.T.; Famoso, A.N.; Zhao, K.; Shaff, J.E.; Craft, E.J.; Bustamante, C.D.; Mccouch, S.R.; Aneshansley, D.J.; Kochian, L.V. High-throughput two-dimensional root system phenotyping platform facilitates genetic analysis of root growth and development. Plant Cell Environ. 2013, 36, 454–466. [Google Scholar] [CrossRef]
- El Hassouni, K.; Alahmad, S.; Belkadi, B.; Filali-Maltouf, A.; Hickey, L.T.; Bassi, F.M. Root system architecture and its association with yield under different water regimes in Durum wheat. Crop Sci. 2018, 58, 2331–2346. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, Y.; He, R.; Ding, Q. Phenotyping field-state wheat root system architecture for root foraging traits in response to environment × management interactions. Sci. Rep. 2018, 8, 2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gesimba, R.M.; Njoka, E.; Kinyua, M. Root Characteristics of Drought Tolerant Bread Wheat (Triticum aestivum) Genotypes at Seedling Stage. Asian J. Plant Sci. 2004, 4, 512–515. [Google Scholar] [CrossRef]
- Mondini, L.; Nachit, M.; Porceddu, E.; Pagnotta, M.A. Identification of SNP mutations in DREB1, HKT1, and WRKY1 genes involved in drought and salt stress tolerance in durum wheat (Triticum turgidum L. var durum). Omics A J. Integr. Biol. 2012, 16, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Nachit, M.M.; Elouafi, I.; Pagnotta, M.A.; El Saleh, A.; Iacono, E.; Labhilili, M.; Asbati, A.; Azrak, M.; Hazzam, H.; Benscher, D.; et al. Molecular linkage map for an intraspecific recombinant inbred population of durum wheat (Triticum turgidum L. var. durum). Theor. Appl. Genet. 2001, 102, 177–186. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Pecetti, L. Developing a tall durum wheat plant type for semi-arid, Mediterranean cereal–livestock farming systems. F. Crop. Res. 2003, 80, 157–164. [Google Scholar] [CrossRef]
- Katerji, N.; Van Hoorn, J.W.; Fares, C.; Hamdy, A.; Mastrorilli, M.; Oweis, T. Salinity effect on grain quality of two durum wheat varieties differing in salt tolerance. Agric. Water Manag. 2005, 75, 85–91. [Google Scholar] [CrossRef]
- Katerji, N.; Van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M.; Oweis, T. Salt tolerance analysis of chickpea, faba bean and durum wheat varieties: I. Chickpea and faba bean. Agric. Water Manag. 2005, 72, 177–194. [Google Scholar] [CrossRef]
- Mondini, L.; Nachit, M.M.; Porceddu, E.; Pagnotta, M.A. HRM technology for the identification and characterization of INDEL and SNPs mutations in genes involved in drought and salt tolerance of durum wheat. Plant Genet. Resour. 2011, 9, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Mondini, L.; Nachit, M.M.; Pagnotta, M.A. Allelic variants in durum wheat (Triticum turgidum L. var. durum) DREB genes conferring tolerance to abiotic stresses. Mol. Genet. Genom. 2015, 290, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Monneveux, P.; Rekika, D.; Acevedo, E.; Merah, O. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Sci. 2006, 170, 867–872. [Google Scholar] [CrossRef]
- Pagnotta, M.A.; Luca, B.; Poala, F. Selection of Durum Wheat Lines under Organic Management—Preliminary Results/Zenodo. Available online: https://zenodo.org/record/4675513#.YkGRWFXP3IU (accessed on 28 March 2022).
- Grewal, K.S.; Buchan, G.D.; Tonkin, P.J. Estimation of field capacity and wilting point of some new zealand soils from their saturation percentages. N. Z. J. Crop Hortic. Sci. 1990, 18, 241–246. [Google Scholar] [CrossRef]
- GitHub—Taiyun/Corrplot: A Visual Exploratory Tool on Correlation Matrix. Available online: https://github.com/taiyun/corrplot (accessed on 23 December 2021).
- Vincent, Q.V. GitHub—Vqv/Ggbiplot: A Biplot Based on Ggplot2. Available online: https://github.com/vqv/ggbiplot (accessed on 23 December 2021).
- Reynolds, M.; Langridge, P. Physiological Breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alahmad, S.; El Hassouni, K.; Bassi, F.M.; Dinglasan, E.; Youssef, C.; Quarry, G.; Aksoy, A.; Mazzucotelli, E.; Juhász, A.; Able, J.A.; et al. A major root architecture QTL responding to water limitation in durum wheat. Front. Plant Sci. 2019, 10, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, K.A.; Putz, A.; Gilmer, F.; Heinz, K.; Fischbach, A.; Pfeifer, J.; Faget, M.; Blossfeld, S.; Ernst, M.; Dimaki, C.; et al. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct. Plant Biol. 2012, 39, 891–904. [Google Scholar] [CrossRef] [Green Version]
- Gregory, P.J.; Bengough, A.G.; Grinev, D.; Schmidt, S.; Thomas, W.T.B.; Wojciechowski, T.; Young, I.M. Root phenomics of crops: Opportunities and challenges. Funct. Plant Biol. 2009, 36, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Nakhforoosh, A.; Nagel, K.A.; Fiorani, F.; Bodner, G. Deep soil exploration vs. topsoil exploitation: Distinctive rooting strategies between wheat landraces and wild relatives. Plant Soil 2021, 459, 397–421. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Bengough, A.G.; Gordon, D.C.; Al-Menaie, H.; Ellis, R.P.; Allan, D.; Keith, R.; Thomas, W.T.B.; Forster, B.P. Gel observation chamber for rapid screening of root traits in cereal seedlings. Plant Soil 2004, 262, 63–70. [Google Scholar] [CrossRef]
- York, L.; Slack, S.; Bennett, M.; Foulkes, M.J. Wheat shovelomics I: A field phenotyping approach for characterising the structure and function of root systems in tillering species. bioRxiv 2018, 280875. [Google Scholar] [CrossRef]
- Tracy, S.R.; Nagel, K.A.; Postma, J.A.; Fassbender, H.; Wasson, A.; Watt, M. Crop Improvement from Phenotyping Roots: Highlights Reveal Expanding Opportunities. Trends Plant Sci. 2020, 25, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Roselló, M.; Royo, C.; Sanchez-Garcia, M.; Soriano, J.M. Genetic Dissection of the Seminal Root System Architecture in Mediterranean Durum Wheat Landraces by Genome-Wide Association Study. Agronomy 2019, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Mengistu, D.K.; Kiros, A.Y.; Pè, M.E. Phenotypic diversity in Ethiopian durum wheat (Triticum turgidum var. durum) landraces. Crop J. 2015, 3, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Alemu, A.; Feyissa, T.; Maccaferri, M.; Sciara, G.; Tuberosa, R.; Ammar, K.; Badebo, A.; Acevedo, M.; Letta, T.; Abeyo, B. Genome-wide association analysis unveils novel QTLs for seminal root system architecture traits in Ethiopian durum wheat. BMC Genom. 2021, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Ober, E.S.; Alahmad, S.; Cockram, J.; Forestan, C.; Hickey, L.T.; Kant, J.; Maccaferri, M.; Marr, E.; Milner, M.; Pinto, F.; et al. Wheat root systems as a breeding target for climate resilience. Theor. Appl. Genet. 2021, 134, 1645–1662. [Google Scholar] [CrossRef]
- Puccio, G.; Ingraffia, R.; Giambalvo, D.; Amato, G.; Frenda, A.S. Morphological and Physiological Root Traits and Their Relationship with Nitrogen Uptake in Wheat Varieties Released from 1915 to 2013. Agronomy 2021, 11, 1149. [Google Scholar] [CrossRef]
- Gowda, V.R.P.; Henry, A.; Yamauchi, A.; Shashidhar, H.E.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crops Res. 2011, 122, 1–13. [Google Scholar] [CrossRef]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Genotype | Shoot Dry Weight (g) | Number of Leaves | Number of Tillers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Drought | D/C, % | Control | Drought | D/C, % | Control | Drought | D/C, % | |
| Azeghar | 2.1 ± 0.1a | 0.51 ± 0.1c | −75.7 *** | 16 ± 3d | 7b | −55.3 * | 3d | 1c | −66.7 * |
| Cham1 | 2.1 ± 0.1a | 0.50 ± 0.1bc | −76.3 *** | 23 ± 2c | 99 ± 1b | −62.3 *** | 5 ± 1bc | 3 ± 1b | −42.9 * |
| J. Khetifa | 2.6 ± 0.2a | 0.63 ± 0.2a | −75.9 *** | 37 ± 1a | 16 ± 1a | −56.4 *** | 9a | 4 ± 1a | −51.9 *** |
| Pelsodur | 1.6 ± 0.1b | 0.58 ± 0.1a | −63.7 * | 31 ± 1ab | 15 ± 2a | −51.1 *** | 10 ± 1a | 5 ± 1a | −50.0 *** |
| Sebatel | 2.1 ± 0.1a | 0.66 ± 0.1a | −68.4 ** | 17 ± 4d | 10 ± 1b | −43.1ns | 4 ± 1cd | 2 ± 1b | −36.4ns |
| Vulci | 2.0 ± 0.1a | 0.55 ± 0.1ab | −72.1 ** | 30 ± 6b | 16 ± 3a | −47.2 *** | 6 ± 1b | 4 ± 1a | −23.5ns |
| Genotype | ns | *** | *** | ||||||
| Treatments | *** | *** | *** | ||||||
| G × T | ns | ** | *** | ||||||
| Genotype | RVW (cm3) | RLW (cm) | TIW | RDW (g) | ||||
| Control | Drought | Control | Drought | Control | Drought | Control | Drought | |
| Azeghar | 5.3 ± 1.1b | 0.97 ± 0.3c | 989 ± 84 | 569 ± 186b | 1989 ± 217 | 1314 ± 329b | 0.53 ± 0.1c | 0.17 ± 0.1c |
| Cham1 | 7.0 ± 0.3b | 1.5 ± 0.5bc | 1056 ± 182 | 816 ± 125ab | 2009 ± 312 | 2299 ± 524a | 0.83 ± 0.1a | 0.26 ± 0.1ab |
| J. Khetifa | 9.7 ± 1.9a | 2.2 ± 0.2ab | 973 ± 124 | 887 ± 107a | 2218 ± 309 | 1970 ± 168ab | 0.86 ± 0.1a | 0.30 ± 0.1a |
| Pelsodur | 6.4 ± 0.9b | 2.7 ± 0.4a | 835 ± 187 | 912 ± 190a | 1775 ± 556 | 1812 ± 438ab | 0.68 ± 0.1b | 0.36 ± 0.1a |
| Sebatel | 6.1 ± 1.6b | 1.5 ± 0.1abc | 921 ± 231 | 806 ± 168ab | 1704 ± 385 | 1799 ± 489ab | 0.62 ± 0.1b | 0.25 ± 0.1ab |
| Vulci | 9.4 ± 0.5a | 2.4 ± 1.5ab | 992 ± 93 | 768 ± 36ab | 2024 ± 68 | 1742 ± 316ab | 0.72 ± 0.1b | 0.29 ± 0.1a |
| Genotype | *** | ns | ns | * | ||||
| Treatments | *** | ** | ns | *** | ||||
| G × T | ** | ns | ns | ns | ||||
| Genotype | Root/Shoot Ratio | SAW (cm2) | Root Angle (°) | RLD (cm cm−3) | ||||
| Control | Drought | Control | Drought | Control | Drought | Control | Drought | |
| Azeghar | 0.25 ± 0.1c | 0.33 ± 0.1c | 256 ± 33 | 83 ± 26c | 125 ± 4a | 108 ± 5b | 0.39 ± 0.03 | 0.23 ± 0.07b |
| Cham1 | 0.42 ± 0.2ab | 0.51 ± 0.0b | 304 ± 28 | 123 ± 24bc | 115 ± 8ab | 121 ± 1a | 0.42 ± 0.07 | 0.33 ± 0.05ab |
| J. Khetifa | 0.33 ± 0.1abc | 0.48 ± 0.1b | 345 ± 56 | 156 ± 15ab | 102 ± 2c | 105 ± 4b | 0.39 ± 0.05 | 0.35 ± 0.04a |
| Pelsodur | 0.46 ± 0.1a | 0.63 ± 0.1a | 281 ± 72 | 175 ± 15a | 94 ± 2c | 98 ± 3c | 0.33 ± 0.07 | 0.36 ± 0.08a |
| Sebatel | 0.3 ± 0.02bc | 0.38 ± 0.0c | 265 ± 68 | 124 ± 14bc | 114 ± 9b | 121 ± 1a | 0.37 ± 0.09 | 0.32 ± 0.07ab |
| Vulci | 0.43 ± 0.02ab | 0.67 ± 0.1a | 315 ± 36 | 147 ± 50ab | 114 ± 6b | 106 ± 1b | 0.31 ± 0.04 | 0.31 ± 0.01ab |
| Genotype | *** | * | *** | ns | ||||
| Treatments | *** | *** | ns | ** | ||||
| G × T | ns | ns | *** | ns | ||||
| Genotype | RL5 Root Length (cm) | SA5 Root Surface Area (cm2) | TI5 Tips | RV5 Root Volume (cm3) | ||||
| Control | Drought | Control | Drought | Control | Drought | Control | Drought | |
| Azeghar | 144 ± 35 | 96 ± 12c | 40 ± 12b | 17. ± 1.5b | 341 ± 60 | 181 ± 43b | 0.9 ± 0.3 | 0.24 ± 0.03b |
| Cham1 | 149 ± 33 | 124 ± 7bc | 46 ± 9ab | 21 ± 3b | 311 ± 82 | 375 ± 38a | 1.14 ± 0.2 | 0.28 ± 0.07b |
| J. Khetifa. | 102 ± 27 | 181 ± 28ab | 57 ± 15ab | 34 ± 3a | 231 ± 79 | 451 ± 60a | 2.88 ± 0.9 | 0.52 ± 0.07ab |
| Pelsodur | 133 ± 4 | 217 ± 49a | 51 ± 3ab | 36 ± 4a | 283 ± 63 | 396 ± 89a | 1.58 ± 0.2 | 0.49 ± 0.01ab |
| Sebatel | 120 ± 42 | 202 ± 58a | 43 ± 15b | 36 ± 7a | 246 ± 97 | 435 ± 137a | 1.26 ± 0.4 | 0.52 ± 0.05ab |
| Vulci | 138 ± 71 | 140 ± 16bc | 63 ± 3a | 33 ± 13a | 261 ± 97 | 326 ± 57a | 3.04 ± 2.3 | 0.65 ± 0.44a |
| Genotype | ns | ** | ns | ns | ||||
| Treatments | *** | *** | ** | *** | ||||
| G × T | ns | ns | ns | ns | ||||
| Genotype | (RL5/RLW) * 100 | (SA5/SAW) * 100 | RL5/TI5 | (RV5/RVW) * 100 | ||||
| Control | Drought | Control | Drought | Control | Drought | Control | Drought | |
| Azeghar | 14.7 | 17.8bc | 15.6ab | 21.4bc | 0.4 | 0.5 | 16.6 | 26.5 |
| Cham1 | 14.2 | 15.4c | 15.1b | 17.2c | 0.5 | 0.3 | 16.3 | 19.4 |
| J. Khetifa | 10.6 | 20.5ab | 16.5ab | 22.0bc | 0.4 | 0.4 | 28.3 | 23.7 |
| Pelsodur | 16.5 | 23.7a | 18.9ab | 20.7bc | 0.5 | 0.6 | 21.7 | 18.1 |
| Sebatel | 12.8 | 24.8a | 16.2ab | 29.1a | 0.5 | 0.5 | 20.6 | 34.3 |
| Vulci | 13.6 | 18.2bc | 20.1a | 22.5b | 0.5 | 0.4 | 37.2 | 28.3 |
| Genotype | ns | ** | ns | ns | ||||
| Treatments | *** | *** | ns | ns | ||||
| G × T | ns | * | ns | ns | ||||
| Azeghar | Cham1 | J. Khetifa | Pelsodur | Sebatel | Vulci | ANOVA | |
|---|---|---|---|---|---|---|---|
| RVF, cm3 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.0 | ns |
| RLF, cm | 59 ± 4cd | 89 ± 6a | 84 ± 15ab | 49 ± 6de | 38 ± 3e | 69 ± 11bc | *** |
| SAF, cm2 | 11.8 ± 2.1bc | 19.1 ± 3.4a | 14.5 ± 1.3b | 11.3 ± 2.7bc | 8.2 ± 2.9c | 15.0 ± 2.2ab | ** |
| TIF | 224 ± 48bc | 266 ± 44b | 390 ± 65a | 218 ± 83bc | 142 ± 30c | 261 ± 20b | ** |
| FRF | 223 ± 5bc | 345 ± 34ab | 449 ± 88a | 248 ± 84bc | 114 ± 50c | 446 ± 155a | ** |
| CRF | 22 ± 8 | 28 ± 7 | 50 ± 29 | 15 ± 3 | 8 ± 2 | 44 ± 29 | ns |
| RAF, ° | 112 ± 6a | 102 ± 5ab | 93 ± 6bc | 84 ± 7cd | 105 ± 5ab | 76 ± 13d | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanavičiūtė, I.; Bonfiglioli, L.; Pagnotta, M.A. Diversity in Root Architecture of Durum Wheat at Stem Elongation under Drought Stress. Agronomy 2022, 12, 1329. https://doi.org/10.3390/agronomy12061329
Urbanavičiūtė I, Bonfiglioli L, Pagnotta MA. Diversity in Root Architecture of Durum Wheat at Stem Elongation under Drought Stress. Agronomy. 2022; 12(6):1329. https://doi.org/10.3390/agronomy12061329
Chicago/Turabian StyleUrbanavičiūtė, Ieva, Luca Bonfiglioli, and Mario A. Pagnotta. 2022. "Diversity in Root Architecture of Durum Wheat at Stem Elongation under Drought Stress" Agronomy 12, no. 6: 1329. https://doi.org/10.3390/agronomy12061329
APA StyleUrbanavičiūtė, I., Bonfiglioli, L., & Pagnotta, M. A. (2022). Diversity in Root Architecture of Durum Wheat at Stem Elongation under Drought Stress. Agronomy, 12(6), 1329. https://doi.org/10.3390/agronomy12061329








