Sodium Selenate: An Environmental-Friendly Means to Control Tomato Bacterial Speck Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Pathogen, Inoculation and Disease Evaluation
2.3. Pathogen Growth in Planta
2.4. The Effect of Sodium Selenate on In Vitro Pst Growth
2.5. Determination of Selenium in Tomato Leaves
2.6. Plant Induced Resistance Markers
2.7. Statistical Analysis
- -
- Y is the bacterial growth reduction as a function of sodium selenate concentration,
- -
- D is the upper asymptote (positive control response),
- -
- C is the lower asymptote (response of the highest concentration tested),
- -
- a is the sodium selenate concentration that gives the intermediate response between the upper and the lower asymptotes,
- -
- b is the slope at the point of inflection.
3. Results
3.1. Bacterial Speck Disease Severity and Pathogen Growth in Leaves of Sodium Selenate Treated Tomato Plants
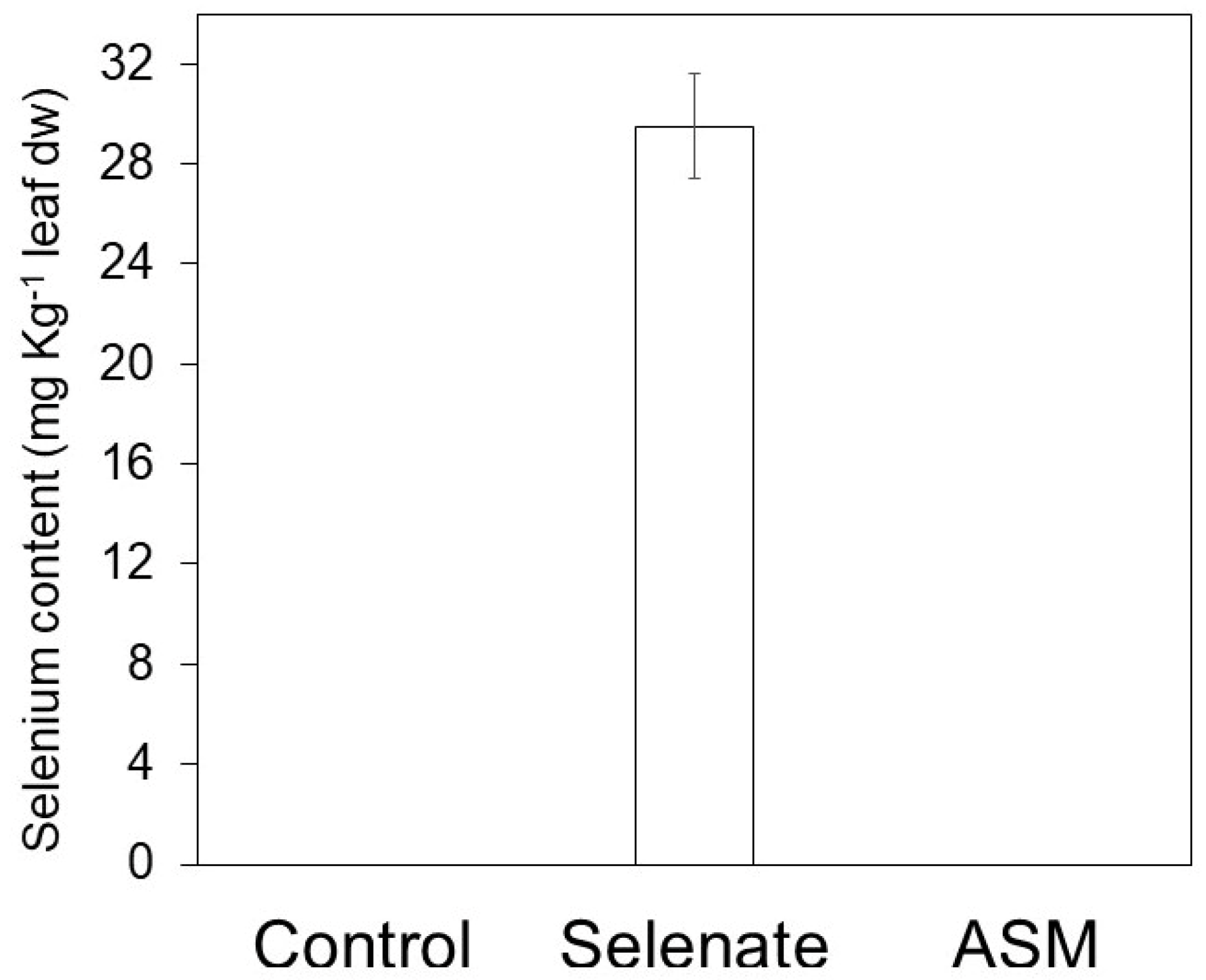
3.2. Level of Selenium in Leaves of Sodium Selenate Treated Tomato Plants
3.3. Callose Deposition in Leaves of Sodium Selenate Treated Tomato Plants
3.4. PR-1 and PIN2 Genes Expression in Leaves of Sodium Selenate Treated Tomato Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.; Dhankher, O.P.; Tripathi, R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yu, F.; Shao, W.; Ding, D.; Yu, Z.; Chen, F.; Geng, D.; Tan, X.; Lammi, M.J.; Guo, X. Associations between selenium content in hair and Kashin-Beck disease/Keshan Disease in children in northwestern China: A prospective cohort study. Biol. Trace Elem. Res. 2018, 184, 16–23. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Biosynthesis and structural characterization of selenium nanoparticles mediated by Zooglea ramigera. Powder Technol. 2013, 244, 26–29. [Google Scholar] [CrossRef]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotox. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: An in vitro and in vivo study. Front. Oncol. 2020, 9, 1541. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, D.L.; Carlson, B.A.; Tsuji, P.A.; Tobe, R.; Gladyshev, V.N. Selenium and cancer. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Elsevier: Amsterdam, The Netherlands, 2017; pp. 463–473. [Google Scholar]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Waraich, E.A.; Nawaz, F.; Ashraf, M.Y.; Khalid, M. Selenium (Se) improves drought tolerance in crop plants—A myth or fact? J. Sci. Food Agric. 2016, 96, 372–380. [Google Scholar] [CrossRef]
- Banuelos, G. Phyto-products may be essential for sustainability and implementation of phytoremediation. Environ. Pollut. 2006, 144, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Doucha, J.; Lívanský, K.; Kotrbáček, V.; Zachleder, V. Production of Chlorella biomass enriched by selenium and its use in animal nutrition: A review. Appl. Microbiol. Biotechnol. 2009, 83, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.; Tew, K. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, Y.; Lin, Z.-Q.; Banuelos, G.; Li, W.; Yin, X. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS ONE 2013, 8, e65615. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The Role of selenium nanoparticles in agriculture and food technology. Biol. Trace Elem. Res. 2021, 200, 2528–2548. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Cheng, Q.; Hu, C.; Jia, W.; Cai, M.; Zhao, Y.; Tang, Y.; Yang, D.; Zhou, Y.; Sun, X.; Zhao, X. Selenium reduces the pathogenicity of Sclerotinia sclerotiorum by inhibiting sclerotial formation and germination. Ecotox. Environ. Saf. 2019, 183, 109503. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cai, M.; Hu, C.; Sun, X.; Cheng, Q.; Jia, W.; Yang, T.; Nie, M.; Zhao, X. Selenium (Se) reduces Sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ. Pollut. 2019, 254, 113051. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.-Q.; Zhu, Z.; Liu, Y.; Yuan, L.; Li, M. Effect of selenium on control of postharvest gray mold of tomato fruit and the possible mechanisms involved. Front. Microbiol. 2016, 6, 1441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-L.; Yin, X.-B.; Lin, Z.-Q.; Banuelos, G.S.; Yuan, L.-X.; Liu, Y.; Li, M. Inhibitory effect of selenium against Penicillium expansum and its possible mechanisms of action. Curr. Microbiol. 2014, 69, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jia, W.; Hu, C.; Nie, M.; Ming, J.; Cheng, Q.; Cai, M.; Sun, X.; Li, X.; Zheng, X. Selenium as a potential fungicide could protect oilseed rape leaves from Sclerotinia sclerotiorum infection. Environ. Pollut. 2020, 257, 113495. [Google Scholar] [CrossRef]
- WPTC. Tomato News. 2021. Available online: https://www.tomatonews.com/en (accessed on 28 January 2022).
- Alexander, S.; Kim, S.; Waldenmaier, C. First report of copper-tolerant Pseudomonas syringae pv. tomato in Virginia. Plant Dis. 1999, 83, 964. [Google Scholar] [CrossRef]
- Molinari, S. Systemic acquired resistance activation in Solanaceous crops as a management strategy against root-knot nematodes. Pest Manag. Sci. 2016, 72, 888–896. [Google Scholar] [CrossRef]
- Quaglia, M.; Bocchini, M.; Orfei, B.; D’Amato, R.; Famiani, F.; Moretti, C.; Buonaurio, R. Zinc phosphate protects tomato plants against Pseudomonas syringae pv. tomato. J. Plant Dis. Prot. 2021, 128, 989–998. [Google Scholar] [CrossRef]
- Scarponi, L.; Buonaurio, R.; Martinetti, L. Persistence and translocation of a benzothiadiazole derivative in tomato plants in relation to systemic acquired resistance against Pseudomonas syringae pv tomato. Pest Manag. Sci. 2001, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Buonaurio, R.; Stravato, V.; Cappelli, C. Occurrence of Pseudomonas syringae pv tomato race 1 in Italy on Pto gene-bearing tomato plants. J. Phytopathol. 1996, 144, 437–440. [Google Scholar] [CrossRef]
- Lamari, L. Assess: Image Analysis Software for Plant Disease Quantification; APS Press: St. Paul, MN, USA, 2002. [Google Scholar]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose deposition: A multifaceted plant defense response. MPMI 2011, 24, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaglia, M.; Baglivo, F.; Moretti, C. Postharvest β-aminobutyric-acid–primed resistance is not effective in the control of Penicillium expansum Link. on ‘Golden delicious’ apple fruit. J. Crop Prot. 2017, 102, 43–48. [Google Scholar] [CrossRef]
- Goyal, R.K.; Fatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-related protein 1b1 (PR1b1) is a major tomato fruit protein responsive to chilling temperature and upregulated in high polyamine transgenic genotypes. Front. Plant Sci. 2016, 7, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagna, A.; Ederli, L.; Pasqualini, S.; Mensuali-Sodi, A.; Baldan, B.; Donnini, S.; Ranieri, A. The tomato ethylene receptor LE-ETR3 (NR) is not involved in mediating ozone sensitivity: Causal relationships among ethylene emission, oxidative burst and tissue damage. New Phytol. 2007, 174, 342–356. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Onofri, A.; Pannacci, E. Spreadsheet tools for biometry classes in crop science programmes. Commun. Biometry Crop. Sci. 2014, 9, 43–53. [Google Scholar]
- Streibig, J.C.; Kudsk, P. Dose-response curves and statistical models. In Herbicide Bioassay; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Pestemer, W.; Günther, P. Growth inhibition of plants as a bioassay for herbicide analysis. In Analysis of Pesticides in Ground and Surface Water I; Springer: Berlin/Heidelberg, Germany, 1995; pp. 219–231. [Google Scholar]
- Companioni, B.; Medrano, J.; Torres, J.; Flores, A.; Rodríguez, E.; Benavides, A. Protective action of sodium selenite against Fusarium wilt in tomato: Total protein contents, levels of phenolic compounds and changes in antioxidant potential. In Proceedings of the II International Symposium on Soilless Culture and Hydroponics 947, Puebla, Mexico, 15–19 May 2011; pp. 321–327. [Google Scholar]
- Hanson, B.; Garifullina, G.F.; Lindblom, S.D.; Wangeline, A.; Ackley, A.; Kramer, K.; Norton, A.P.; Lawrence, C.B.; Pilon-Smits, E.A. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 2003, 159, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Ortiz, E.J.; Gonzalez-Gil, G.; Saikaly, P.E.; van Hullebusch, E.D.; Lens, P.N. Effects of selenium oxyanions on the white-rot fungus Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2015, 99, 2405–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Hu, C.; Ming, J.; Zhao, Y.; Xin, J.; Sun, X.; Zhao, X. Action of selenium against Sclerotinia sclerotiorum: Damaging membrane system and interfering with metabolism. Pestic. Biochem. Phys. 2018, 150, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Hu, C.; Xu, J.; Ming, J.; Zhao, Y.; Cai, M.; Sun, X.; Liu, X.; Zhao, X. Dissolved organic matter derived from rape straw pretreated with selenium in soil improves the inhibition of Sclerotinia sclerotiorum growth. J. Hazard. Mater. 2019, 369, 601–610. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Y.; Zhang, F.; Hu, J.T.; Zhao, Y.M.; Cheng, B.L. Effects of selenium on Fusarium growth and associated fermentation products and the relationship with chondrocyte viability. Biomed. Environ. Sci. 2017, 30, 134–138. [Google Scholar]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Kolozsváriné Nagy, J.; Király, L. The versatile roles of sulfur-containing biomolecules in plant defense—A road to disease resistance. Plants 2020, 9, 1705. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Slusarenko, A.J.; Gruhlke, M.C.H. Sulfur and sulfur compounds in plant defence. Nat. Prod. Commun. 2012, 7, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Sekowska, A.; Kung, H.F.; Danchin, A. Sulfur metabolism in Escherichia coli and related bacteria: Facts and fiction. J. Mol. Microbiol. Biotechnol. 2000, 2, 145–177. [Google Scholar]
- Yunis, H.; Bashan, Y.; Okon, Y.; Henis, Y. Weather dependence, yield losses, and control of bacterial speck of tomato caused by Pseudomonas tomato. Plant Dis. 1980, 64, 937–939. [Google Scholar] [CrossRef]
- Bender, C.L.; Cooksey, D.A. Indigenous plasmids in Pseudomonas syringae pv. tomato: Conjugative transfer and role on copper resistance. J. Bacteriol. 1986, 165, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.S.; Cooksey, D. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoewyk, D.; Takahashi, H.; Inoue, E.; Hess, A.; Tamaoki, M.; Pilon-Smits, E.A. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol. Plant. 2008, 132, 236–253. [Google Scholar] [CrossRef]
- Tamaoki, M.; Maruyama-Nakashita, A. Molecular mechanisms of selenium responses and resistance in plants. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 35–51. [Google Scholar]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.M.; De Britto, S.; Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J. Biotech. 2021, 325, 196–206. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Faizy, S.E.-D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.-D.; Shalaby, T.; Bayoumi, Y. Selenium and nano-selenium biofortification for human health: Opportunities and challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef]
- Fowler, J.H.; Narváez-Vásquez, J.; Aromdee, D.N.; Pautot, V.; Holzer, F.M.; Walling, L.L. Leucine aminopeptidase regulates defense and wound signalling in tomato downstream of jasmonic acid. Plant Cell 2009, 21, 1239–1251. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, C.; Bocchini, M.; Quaglia, M.; Businelli, D.; Orfei, B.; Buonaurio, R. Sodium Selenate: An Environmental-Friendly Means to Control Tomato Bacterial Speck Disease. Agronomy 2022, 12, 1351. https://doi.org/10.3390/agronomy12061351
Moretti C, Bocchini M, Quaglia M, Businelli D, Orfei B, Buonaurio R. Sodium Selenate: An Environmental-Friendly Means to Control Tomato Bacterial Speck Disease. Agronomy. 2022; 12(6):1351. https://doi.org/10.3390/agronomy12061351
Chicago/Turabian StyleMoretti, Chiaraluce, Marika Bocchini, Mara Quaglia, Daniela Businelli, Benedetta Orfei, and Roberto Buonaurio. 2022. "Sodium Selenate: An Environmental-Friendly Means to Control Tomato Bacterial Speck Disease" Agronomy 12, no. 6: 1351. https://doi.org/10.3390/agronomy12061351





