Effect of Artificial Light Treatment on the Physiological Property and Biological Activity of the Aerial and Underground Parts of Atractylodes macrocephala
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Artificial Light Treatment
2.2. Measurement of Plant Growth Characteristics and Chlorophyll Content
2.3. Extraction and Concentration
2.4. DPPH Readical Scavenging Assay
2.5. Total Phenol Content
2.6. Total Flavonoid Content
2.7. Antimicrobial Activity Assay
2.8. Tyrosinase Inhibitory Activity Assay
2.9. MTT Analysis
2.10. Nitric Oxide (NO) Production Rate and Expression of Inflammatory Genes in LPS-Treated Raw 264.7 Cells
2.11. Statistical Processing Analysis
3. Results and Discussion
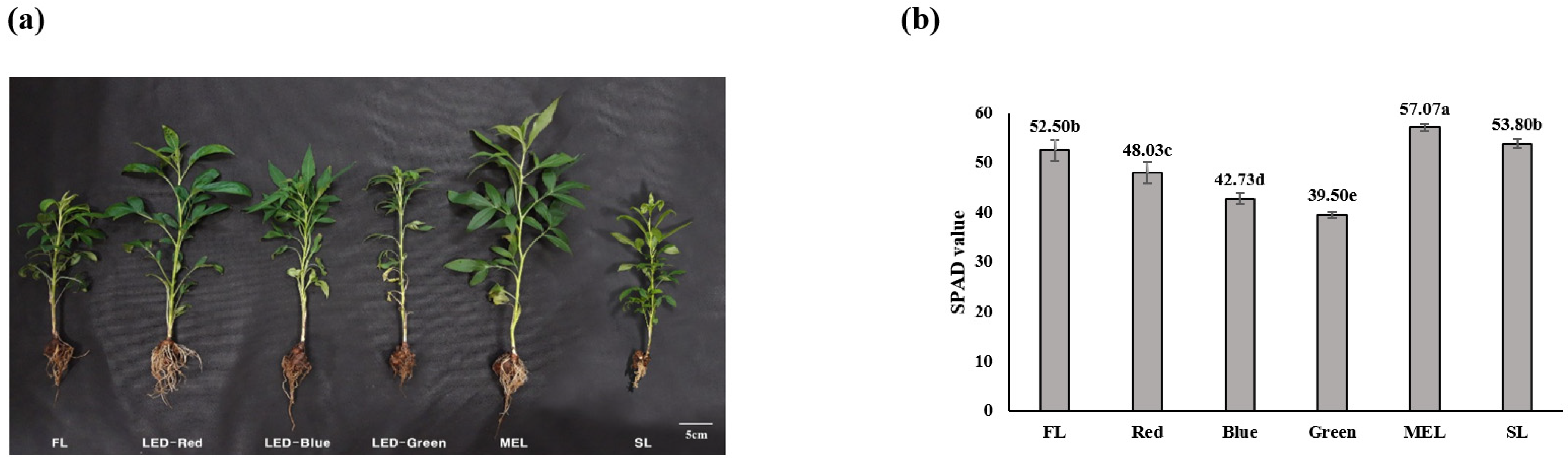
3.1. Growth Characteristics and Chlorophyll Content of A. macrocephala Grown under Artificial Light Source
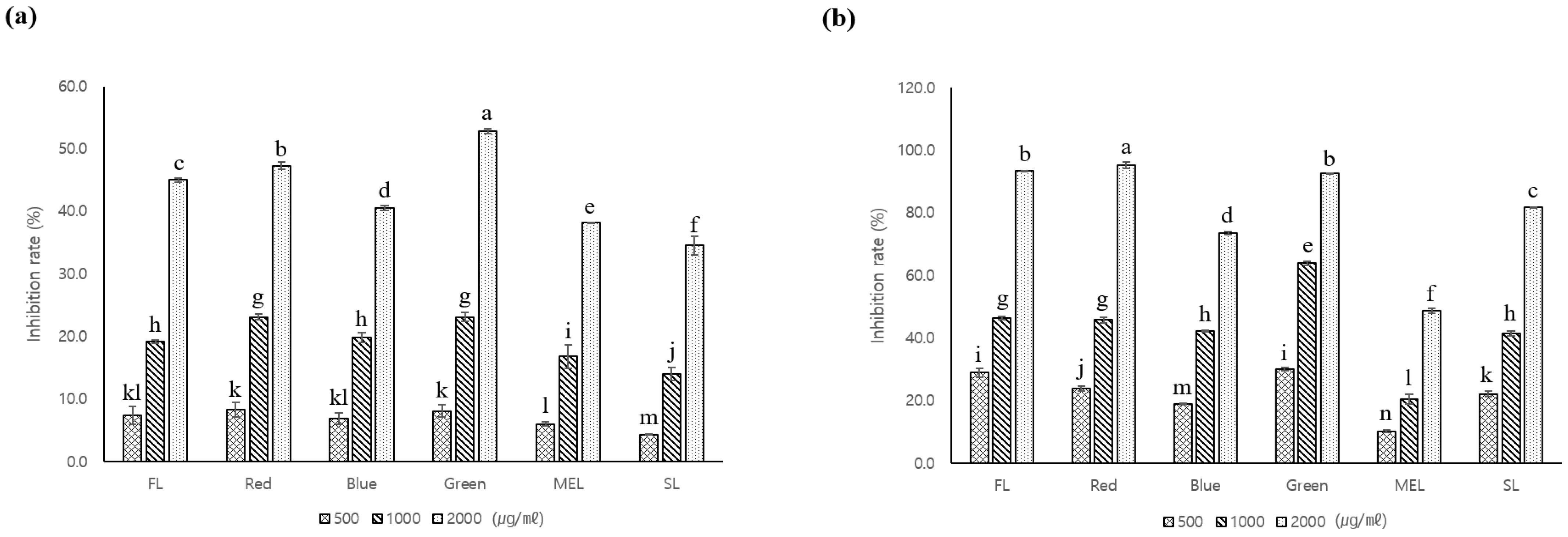
3.2. Antioxidant and Whitening Activity of A. macrocephala Grown under Artificial Light
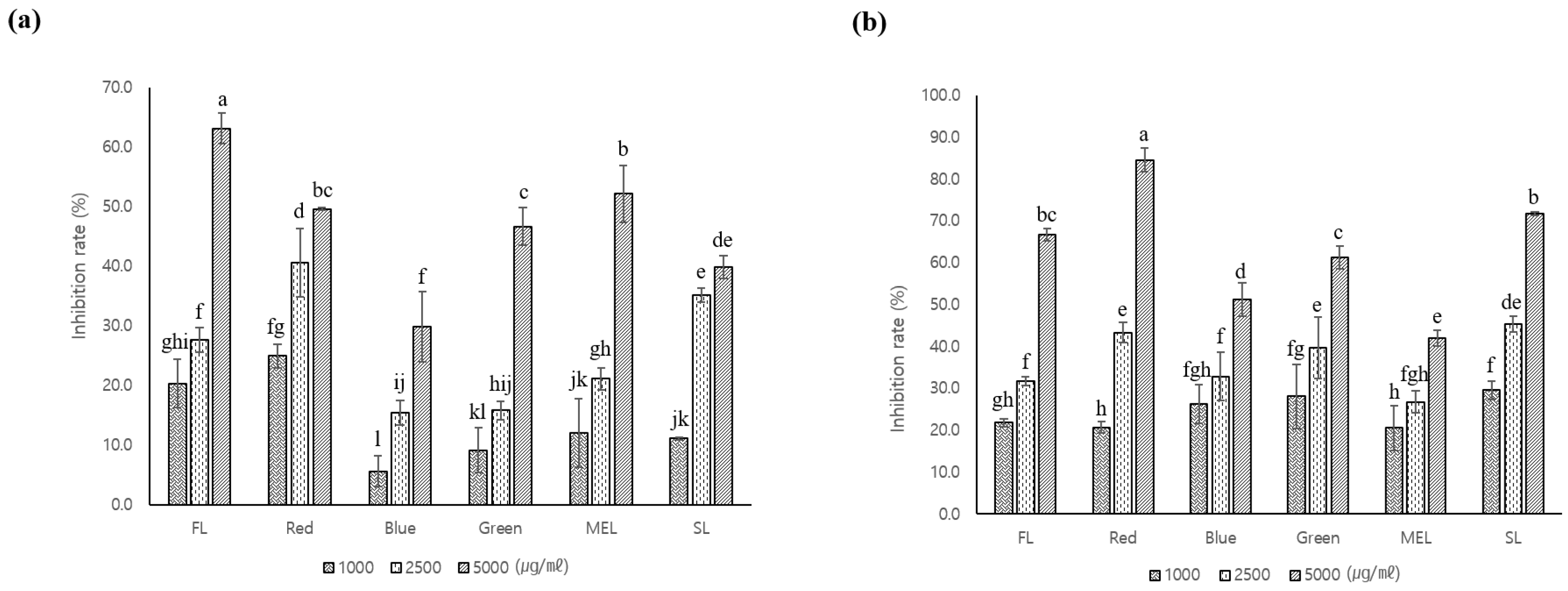
3.3. Tyrosinase Inhibitory Activity of A. macrocephala Grown under Artificial Light
3.4. Antimicrobial Activity of A. macrocephala Grown under Artificial Light
3.5. Anti-Inflammatory Activity of A. macrocephala Grown under Artificial Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Production Results of Special Crops; Ministry of Agriculture, Food and Rural Affairs: Sejong, Korea, 2019; pp. 41, 60.
- Ministry of Food and Drug Safety (MFDS). The Korea Pharmacopoeia; Ministry of Food and Drug Safety: Seoul, Korea, 2019; p. 44.
- Lee, J.H.; Kim, Y.K.; Hong, S.P.; Kim, C.S. Study of Taxonomic Origins of Atractylodis Rhizoma Alba and Atractylodis Rhizoma. Korean J. Orient. Med. 2002, 8, 55–63. [Google Scholar]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Yoon, C.; Choi, H. A Study on the Various Light Source Radiation Conditions and Use of LED Illumination for Plant Factory. J. Korean Ins. Illum. Electric. Installat. Eng. 2011, 25, 14–22. [Google Scholar]
- Choi, H.L.; Seo, J.W.; Hwang, M.H.; Lee, H.I.; Kim, M.J.; Yu, C.Y. Growth Characteristics and Functional Analysis of Salvia miltiorrhiza Bunge by Artificial Light Sources. Korean J. Med. Crop Sci. 2020, 28, 200–208. [Google Scholar] [CrossRef]
- Duan, Y.; Kim, G.H.; Seong, J.H.; Chung, H.S.; Kim, H.S. Antioxidant Activities of n-Butanol and Ethyl Acetate Extracts from Yam (Dioscorea batatas Decne). J. Korean Oil Chem. Soc. 2015, 32, 599–606. [Google Scholar] [CrossRef]
- Yi, M.R.; Kang, C.H.; Bu, H.J. Antioxidant and Antiinflammatory Activity of Extracts from Kohlrabi(Brassica oleracea var. Gonglodes). J. Korean Oil Chem. Soc. 2017, 34, 189–202. [Google Scholar]
- Lu, Y.; Foo, L.Y. Antioxidant and Radical Scavenging Activities of Polyphenols from Aapple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Ekström, A.; Serafini, M.; Nyren, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary Quercetin Intake and Risk of Gastric Cancer: Results from A Population-based Study in Sweden. Annal. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef]
- Del Marmol, V.; Beermann, F. Tyrosinase and Related Proteins in Mammalian Pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Desmedt, B.; Ates, G.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Hendrickx, B.; Deconinck, E.; De Paepe, K. In Vitro Dermal Absorption of Hydroquinone: Protocol Validation and Applicability on Illegal Skin-Whitening Cosmetics. Skin Pharmacol. Physiol. 2016, 29, 300–308. [Google Scholar] [CrossRef]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as A Skin Whitening Agent: Facts, Myths, Evidence and Controversies. Ind. J. Dermatol. Venereol. Leprol. 2016, 82, 262–272. [Google Scholar] [CrossRef]
- Pestka, J.; Zhou, H. Toll-like Receptor Priming Sensitizes Macrophages to Proinflammatory Cytokine Gene Induction by Deoxynivalenol and Other Toxicants. Toxicol. Sci. 2006, 92, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Saha, P.; Gamre, S.; Bhattacharjee, S.; Hariharan, C.; Ganguly, S.; Sen, R.; Mandal, G.; Chattopadhyay, S.; Majumdar, S.; et al. Anti-inflammatory Effect of Allylpyrocatechol in LPS-induced Macrophages Is Mediated by Suppression of iNOS and COX-2 via the NF-κB Pathway. Int. Immunopharm. 2008, 8, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, L.; Dong, H.; Jin, J. Screening for the Anti-inflammatory Activity of Fractions and Compounds from Atractylodes macrocephala Koidz. J. Ethnopharmacol. 2007, 114, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Yan, Y.; Yang, M.; Li, C.; Li, J.; Zhong, L.; Gong, Q.; Yu, H. Network Pharmacology-Based Strategy to Investigate the Pharmacologic Mechanisms of Atractylodes macrocephala Koidz. for the Treatment of Chronic Gastritis. Front. Pharmacol. 2020, 10, 1629. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.; Dong, G.Z.; Lee, H.J.; Ryu, J.H. Anti-Inflammatory Compounds from Atractylodes macrocephala. Molecules 2019, 24, 1859. [Google Scholar] [CrossRef] [Green Version]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Ramarathnam, N.; Suzuki, Y.; Ohkubo, T.; Takeuchi, M.; Ochi, H. Varietal Differences in the Phenolic Content and Superoxide Radical Scavenging Potential of Wines from Different Sources. J. Agric. Food Chem. 1996, 44, 37–41. [Google Scholar] [CrossRef]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the Free Radical-Scavenging Activity of Propolis from Several Regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Irith, W.; Kai, H.; Robert, E.W.H. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar]
- Bernard, P.; Berthon, J.Y. Resveratrol: An Original Mechanism on Tyrosinase Inhibition. Inter. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Luis, A.J.M.; Julien, M.; Simona, M.C.; Frans, J.M.H.; Elena, P. Methods of nitric oxide detection in plants: A commentary. Plant Sci. 2011, 181, 509–519. [Google Scholar]
- Park, J.E.; Park, Y.G.; Jeong, B.R.; Hwang, S.J. Growth and Anthocyanin Content of Lettuce as Affected by Artificial Light Source and Photoperiod in a Closed-type Plant Production System. Korean J. Hort. Sci. Technol. 2012, 30, 673–679. [Google Scholar]
- Heo, J.W.; Lee, Y.B.; Lee, D.B.; Chun, C.H. Light Quality Affects Growth, Net Photosynthetic Rate, and Ethylene Production of Ageratum, African Marigold, and Salvia seedlings. Korean J. Hort. Sci. Technol. 2009, 27, 187–193. [Google Scholar]
- Sicora, C.; Zoltan, M.; Imre, V. The Interaction of Visible and UV-B Light During Photodamage and Repair of Photosystem II. Photosyn. Res. 2003, 75, 127–137. [Google Scholar] [CrossRef]
- Kang, S.B.; Jang, H.I.; Lee, I.B.; Park, J.M.; Moon, D.K. Effect of Waterlogging Condition on the Photosynthesis of ‘Campbell Early’ Grapevine. Korean J. Hort. Sci. Technol. 2008, 26, 372–379. [Google Scholar]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hort. Environ. Biotech. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Yoo, J.H.; Choi, J.H.; Kang, B.J.; Jeon, M.R.; Lee, C.O.; Kim, C.H.; Seong, E.S.; Heo, K.; Yu, C.Y.; Choi, S.K. Antioxidant and Tyrosinase Inhibition Activity Promoting Effects of Perilla by the Light Emitting Plasma. Korean J. Med. Crop Sci. 2017, 25, 37–44. [Google Scholar] [CrossRef]
- Choi, J.H.; Seong, E.S.; Yoo, J.H.; Choi, S.K.; Lee, J.G.; Lim, J.D.; Na, J.K.; Yu, C.Y. Enhancement of Growth Characteristics and Biological Activities in Astragalus membranaceus Using Artificial Light Sources. Russ. J. Plant Physiol. 2018, 65, 732–739. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Lim, Y.Y. Antioxidant and Tyrosinase Inhibition Activity of the Fertile Fronds and Rhizomes of Three Different Drynaria species. BMC Res. Notes 2015, 8, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrillo, A.D.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase Inhibition and Antioxidant Properties of Asphodelus microcarpus Extracts. BMC Complement. Alternat. Med. 2016, 16, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Park, H.S.; Yoon, I.J.; Shin, Y.B.; Baik, Y.C.; Kooh, D.H.; Kim, S.K.; Jung, H.K.; Sim, M.O.; Cho, H.W.; et al. Whitening and Anti-wrinkle Effects of Tremella fuciformis Extracts. Korean J. Med. Crop Sci. 2016, 24, 38–46. [Google Scholar] [CrossRef]
- Thong, H.Y.; Maibach, H.I. Irritant Dermatitis as A Model of Inflammation. Drug Discov. Today Dis. Mech. 2008, 5, 85. [Google Scholar] [CrossRef]
- You, S.H.; Pyo, Y.H. Antioxidant and Anti-inflammatory Activities of Ethanol Extracts from Wheat Sprout. J. Investig. Cosmetol. 2015, 11, 231–238. [Google Scholar]



| Light Source | Aerial Part | Underground Part | |||||
|---|---|---|---|---|---|---|---|
| Plant Length (cm) | Leaf Length (cm) | Leaf Width (cm) | Number of Leaves | Dry Weight (g) | Root Length (cm) | Dry Weight (g) | |
| FL (1) | 27.77 ± 0.50 c | 8.27 ± 1.08 bc | 7.73 ± 0.25 bc | 21.67 ± 0.58 a | 1.16 ± 0.15 cd | 14.23 ± 3.88 a | 0.29 ± 0.03 ab |
| LED-Red | 35.17 ± 0.98 ab | 10.77 ± 1.15 a | 8.80 ± 1.28 abc | 19.33 ± 1.53 ab | 1.81 ± 0.13 b | 14.73 ± 1.94 a | 0.29 ± 0.09 ab |
| LED-Blue | 33.57 ± 4.39 abc | 9.60 ± 0.75 ab | 9.40 ± 0.40 ab | 21.67 ± 4.73 a | 1.29 ± 0.16 c | 12.90 ± 1.31 a | 0.26 ± 0.07 ab |
| LED-Green | 30.97 ± 2.95 bc | 7.70 ± 0.26 c | 7.57 ± 1.10 bc | 13.67 ± 2.52 b | 0.96 ± 0.05 d | 10.97 ± 1.29 a | 0.18 ± 0.04 b |
| MEL (2) | 37.30 ± 5.90 a | 9.73 ± 0.23 ab | 10.30 ± 2.35 a | 21.33 ± 4.93 a | 2.03 ± 0.15 b | 12.50 ± 0.89 a | 0.17 ± 0.07 b |
| SL (3) | 28.90 ± 1.31 bc | 7.60 ± 0.56 c | 6.57 ± 0.59 c | 14.33 ± 1.53 b | 2.35 ± 0.06 a | 12.60 ± 1.47 a | 0.33 ± 0.08 a |
| Minimal Inhibitory Concentration (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Dachul | S. aureus | V. litoralis | B. subtilis | E. coli | S. typhimurium | P. aeruginosa | |
| Light Source | Plant Parts | ||||||
| FL | Aerial | ND (1) | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥1 | ND | ≥1 | ND | ND | |
| LED-Red | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.5 | ND | ≥0.5 | ND | ND | |
| LED-Blue | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥1 | ND | ≥0.5 | ND | ND | |
| LED-Green | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.25 | ND | ≥0.25 | ND | ND | |
| MEL | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.5 | ND | ≥0.5 | ND | ND | |
| SL | Shoot | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.5 | ND | ≥0.5 | ND | ND | |
| Tetracycline | ≥0.007 | ≥0.007 | ≥0.007 | ≥0.007 | ≥0.007 | ≥0.007 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, M.H.; Seo, J.W.; Han, K.J.; Kim, M.J.; Seong, E.S. Effect of Artificial Light Treatment on the Physiological Property and Biological Activity of the Aerial and Underground Parts of Atractylodes macrocephala. Agronomy 2022, 12, 1485. https://doi.org/10.3390/agronomy12071485
Hwang MH, Seo JW, Han KJ, Kim MJ, Seong ES. Effect of Artificial Light Treatment on the Physiological Property and Biological Activity of the Aerial and Underground Parts of Atractylodes macrocephala. Agronomy. 2022; 12(7):1485. https://doi.org/10.3390/agronomy12071485
Chicago/Turabian StyleHwang, Myeong Ha, Ji Won Seo, Kyeong Jae Han, Myong Jo Kim, and Eun Soo Seong. 2022. "Effect of Artificial Light Treatment on the Physiological Property and Biological Activity of the Aerial and Underground Parts of Atractylodes macrocephala" Agronomy 12, no. 7: 1485. https://doi.org/10.3390/agronomy12071485




