The Effect of the Nitrogen-Fixing Bacteria and Companion Red Clover on the Total Protein Content and Yield of the Grain of Spring Barley Grown in a System of Organic Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Methodology
2.2. Agrotechnological Practices
2.3. Origin and Preparation of Bacterial Inoculates
2.4. Determination of Mutual Interactions between the Bacteria Used in the Construction of Inoculates
- Azotobacter chroococcum on Azospirillum lipoferum
- Azospirillum lipoferum on Azotobacter chroococcum
- Bacillus megaterium var. phosphaticum on Arthrobacter agilis
- Arthrobacter agilis on Bacillus megaterium var. phosphaticum
- Azotobacter chroococcum on Arthrobacter agilis
- Arthrobacter agilis on Azotobacter chroococcum
- Azospirillum lipoferum on Arthrobacter agilis
- Arthrobacter agilis on Azospirillum lipoferum
- Azotobacter chroococcum on Bacillus megaterium var. phosphaticum
- Bacillus megaterium var. phosphaticum on Azotobacter chroococcum
- Azospirillum lipoferum on Bacillus megaterium var. phosphaticum
- Bacillus megaterium var. phosphaticum on Azospirillum lipoferum
- Azotobacter chroococcum on Bacillus subtilis
- Bacillus subtilis on Azotobacter chroococcum
- Azotobacter chroococcum on Bacillus amyloliquefaciens
- Bacillus amyloliquefaciens on Azotobacter chroococcum
- Azotobacter chroococcum on Pseudomonas fluorescens
- Pseudomonas fluorescens on Azotobacter chroococcum
- Azospirillum lipoferum on Bacillus subtilis
- Bacillus subtilis on Azospirillum lipoferum
- Azospirillum lipoferum on Bacillus amyloliquefaciens
- Bacillus amyloliquefaciens on Azospirillum lipoferum
- Azospirillum lipoferum on Pseudomonas fluorescens
- Pseudomonas fluorescens on Azospirillum lipoferum
2.5. Chemical Analyses
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biel, W.; Jaroszwska, A.; Stankowski, S.; Sadkiewicz, J.; Bosko, P. Effects of genotype and weed control on the nutrient composition of winter spelt (Triticum aestivum ssp. spelta) and common wheat (Triticum aestivum ssp. vulgare). Acta Agric. Scand. B Soil Plant Sci. 2016, 66, 27–35. [Google Scholar] [CrossRef]
- Biel, W.; Kazimierska, K.; Bashutska, U. Nutritional value of wheat, triticale, barley and oat grains. Acta Sci. Pol. Zoot. 2020, 19, 19–28. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug. Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushra, Z.I.; Hussain, A.; Dar, A.; Ahmad, M.; Wang, X.; Brtnicky, M.; Mustafa, A. Combined use of novel endophytic and rhizobacterial strains upregulates antioxidant enzyme systems and mineral accumulation in wheat. Agronomy 2022, 12, 551. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezin, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Expr. 2018, 8, 73. [Google Scholar] [CrossRef]
- Hasan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A rewiew. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A. Impact of biofertilizer in enhancing growth and productivity of wheat: A review. Int. J. Chem. Stud. 2018, 6, 360–362. [Google Scholar]
- Yadav, K.K.; Sarkar, S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019, 37, 89–93. [Google Scholar]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Głuchowska, K.; Waraczewska, Z.; Budka, A. An Assessment of the influence of co-inoculation with endophytic bacteria and rhizobia, and the influence of PRP SOL and PRP EBV fertilizers on the microbial parameters of soil and nitrogenase activity in yellow lupine (Lupinus luteus) cultivation. Pol. J. Environ. Stud. 2018, 315, 52–53. [Google Scholar] [CrossRef]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The Good, the Bad, and the Ugly of Rhizosphere Microbiome: Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290. [Google Scholar]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Asad, S.A.; Hafeez, F. Bacillus strains as potential alternate for zinc biofortification of maize grains. Int. J. Agric. Biol. 2018, 20, 1779–1786. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Jamil, M.; Nazli, F.; Iqbal, Z. Field application of ACC-deaminase biotechnology for improving chickpea productivity in Bahawalpur. Soil Environ. 2017, 36, 93–102. [Google Scholar] [CrossRef]
- Kumar, K.; Dasgupta, C.N.; Das, C. Cell growth kinetics of chlorella sorokiniana and nutritional values of its biomass. Bioresour. Technol. 2014, 167, 358–366. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Barry, K.M.; Baker, A.L.; Nichols, D.S.; Ahmad, M.; Zahir, Z.A.; Britz, M.L. Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: A possible mechanism for Zn solubilization. Rhizosphere 2019, 12, 100170. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Hauggard-Nielsen, H.; Mundus, S.; Jensen, E.J. Grass-clover undersowing affects nitrogen dynamics in grain legume-cereal arable cropping system. Field Crops Res. 2012, 136, 23–31. [Google Scholar] [CrossRef]
- Płaza, A.; Gąsiorowska, B.; Rzążewska, E. Wsiewki Roślin Bobowatych i ich Mieszanek z Trawami źródłem azotu Biologicznego dla Ziemniaka Jadalnego; Wydawnictwo UPH Siedlce: Siedlce, Poland, 2020; p. 62. [Google Scholar]
- Sarunaite, L.; Kadziuline, Z.; Deveikyte, I.; Kadziulis, L. Effect of legume biological nitrogen on cereals grain yield and soil nitrogen budget in double-cropping system. J. Food Agric. Environ. 2013, 11, 528–533. [Google Scholar]
- Shendy, M.Z. Evaluation of four new barley cultivars productivity intercropped with berseem clover at different seeding rate in new lands in Egipt. Bull. Fac. Agric. Cairo Univ. 2015, 66, 29–39. [Google Scholar] [CrossRef]
- Fenglerowa, W. Simple method for counting azotobacter in soil samples. Acta Microbiol. Pol. 1965, 14, 203–206. [Google Scholar]
- Dőbereiner, J. Forage grasses and grain crops. In Methods for Evaluating Biological Nitrogen Fixation; Bergsen, F.J., Ed.; Wiley and Son: New York, NY, USA, 1980; pp. 535–555. [Google Scholar]
- Rodina, A. Mikrobiologiczne Metody Badania Wód; Wydawnictwo PWRiL: Warszawa, Poland, 1968; p. 254. [Google Scholar]
- Hagedorn, C.; Holt, J. Ecology of soil arthrobacters in clation-webster toposequences of Iowa. Appl. Microbiol. 1975, 2, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Burbianka, M.; Pliszka, A. Mikrobiologiczne Badania Produktów Żywnościowych; Wydawnictwo PZWL: Warszawa, Poland, 1963; p. 337. [Google Scholar]
- Niewiadomska, A. Assessment of the Impact of PRP SOL Fertilizer and Coinoculation on the Process Diazotrophy, Biological and Chemical Properties of Soil and Crop Condition under Clover and Alfalfa Cultivation; Rozprawy Naukowe 462: Poznań, Poland, 2013; p. 105. ISBN 978-83-7160-710-3. [Google Scholar]
- Khorshidi, Y.R.; Ardakani, M.R.; Ramezanpour, M.R.; Khavazi, K.; Zargari, K. Response of yield and yield components of rice (Oryza sativa L.) to Pseudomonas flouresence and Azospirillum lipoferum under different nitrogen levels. Am. Euras. J. Agric. Environ. Sci. 2011, 10, 387–395. [Google Scholar]
- Couillerot, O.; Ramírez-Trujillo, A.; Walker, V.; von Felten, A.; Jansa, J.; Maurhofer, M.; Défago, G.; Prigent-Combaret, C.; Comte, G.; Caballero-Mellado, J.; et al. Comparison of prominent Azospirillum strains in Azospirillum–Pseudomonas–Glomus consortia for promotion of maize growth. Appl. Microbiol. Biotechnol. 2013, 97, 4639–4649. [Google Scholar] [CrossRef] [PubMed]
- Bulut, S. Evaluation of yield and quality parameter of phosphorus-solubilizing and N-fixing bacteria inoculated in wheat (Triticum aestvum L.). Turk. J. Agric. For. 2013, 37, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.E.; Richardson, A.E.; Simpson, R.J. Components of organic phosphorus in soil extracts that are hydrolysed by phytase and acid phosphatase. Biol. Fertil. Soils 2000, 32, 279–286. [Google Scholar] [CrossRef]
- Xuan, Y.; Xu, L.; Tian-Hui, Z.; Guang-Hai, L.; Cui, M. Co-inoculation with phosphate-solubilizing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur. J. Soil Biol. 2012, 50, 112–117. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Khan, A. Optimization of PGPR and silicon fertilization using response surface methodology for enhanced growth, yield and biochemical parameters of French bean (Phaseolus vulgaris L.) under saline stress. Biocatal. Agric. Biotechnol. 2020, 23, 101–463. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Upadhyay, R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2018, 16, 155–162. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Płaza, A.; Gąsiorowska, B.; Rzążewska, E. Effect of biological preparations and mineral nitrogen fertilization on the content of protein and macroelements in spring wheat grain. J. Elem. 2021, 26, 199–210. [Google Scholar] [CrossRef]
- Suryadi, Y.; Susilowati, D.N.; Fauziah, F. Management of plant diseases by PGPR-mediated induced resistance with special reference to tea and rice crops. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management. Microorganisms for Sustainability; Springer: Singapore, 2019; pp. 65–110. [Google Scholar] [CrossRef]
- Zahran, H.H. Legume-microbe interactions under stressed environments. In Microbes for Legume Improvement; Springer: Cham, Switzerland, 2017; pp. 301–339. [Google Scholar] [CrossRef]
- Toukabri, W.; Ferchichi, N.; Hlel, D.; Jadlaoui, M.; Kheriji, O.; Zribi, F.; Taamalli, W.; Mhamdi, R.; Trabelsi, D. Improvements of durum wheat main crop in weed control, productivity and grain quality through the inclusion of fenugreek and clover as companion plants: Effect of N fertilization regime. Agronomy 2021, 11, 78. [Google Scholar] [CrossRef]
- Naseri, R.; Azadi, S.; Rahimi, M.J.; Maleki, A.; Mirzaei, A. Effect of inoculation with Azotobacter chroococcum and Pseudomonas putida on yield and some of the important agronomic traits in barley (Hordeum vulgare L.). Int. J. Agron. Plant Prod. 2013, 4, 1602–1610. [Google Scholar]

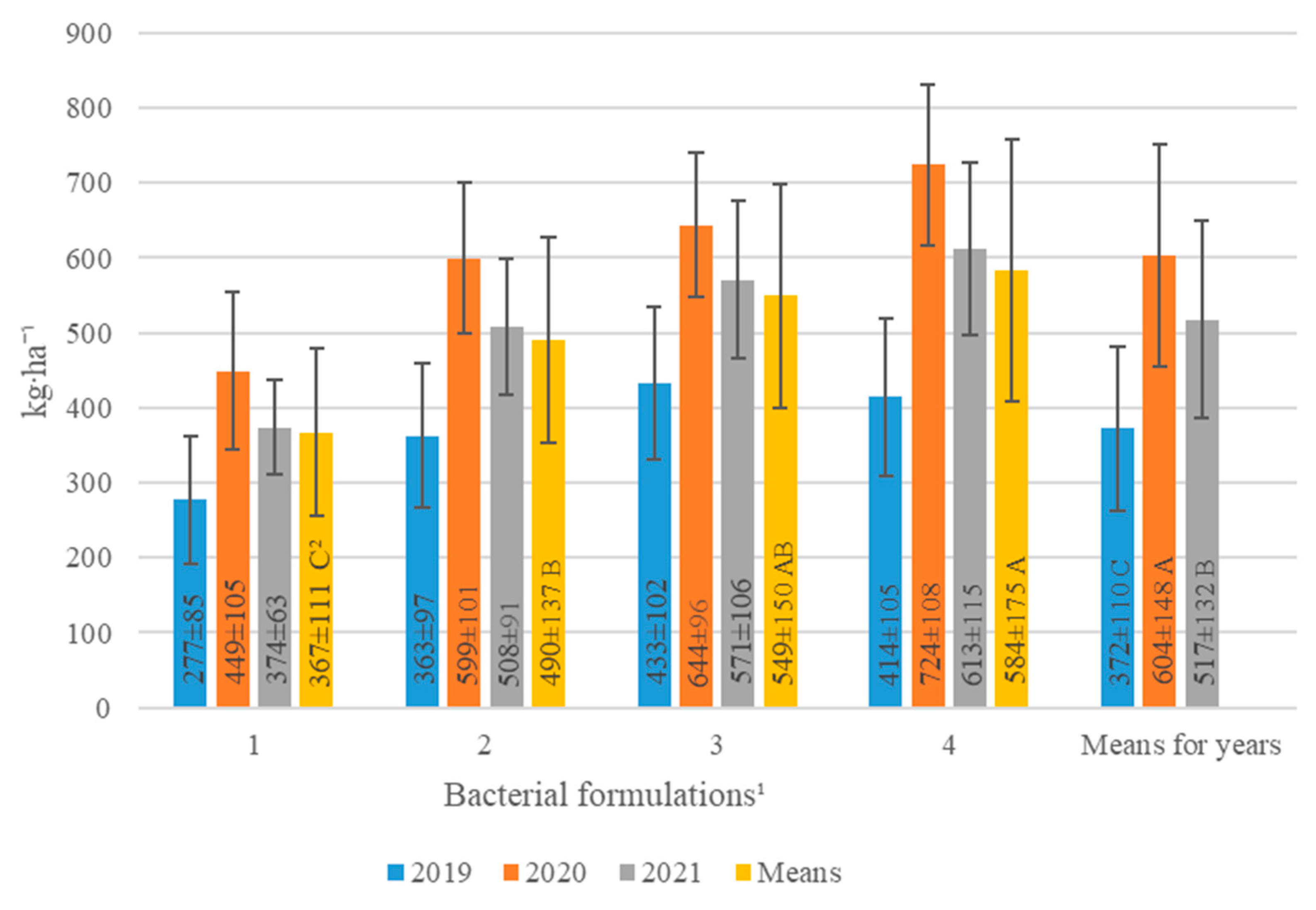

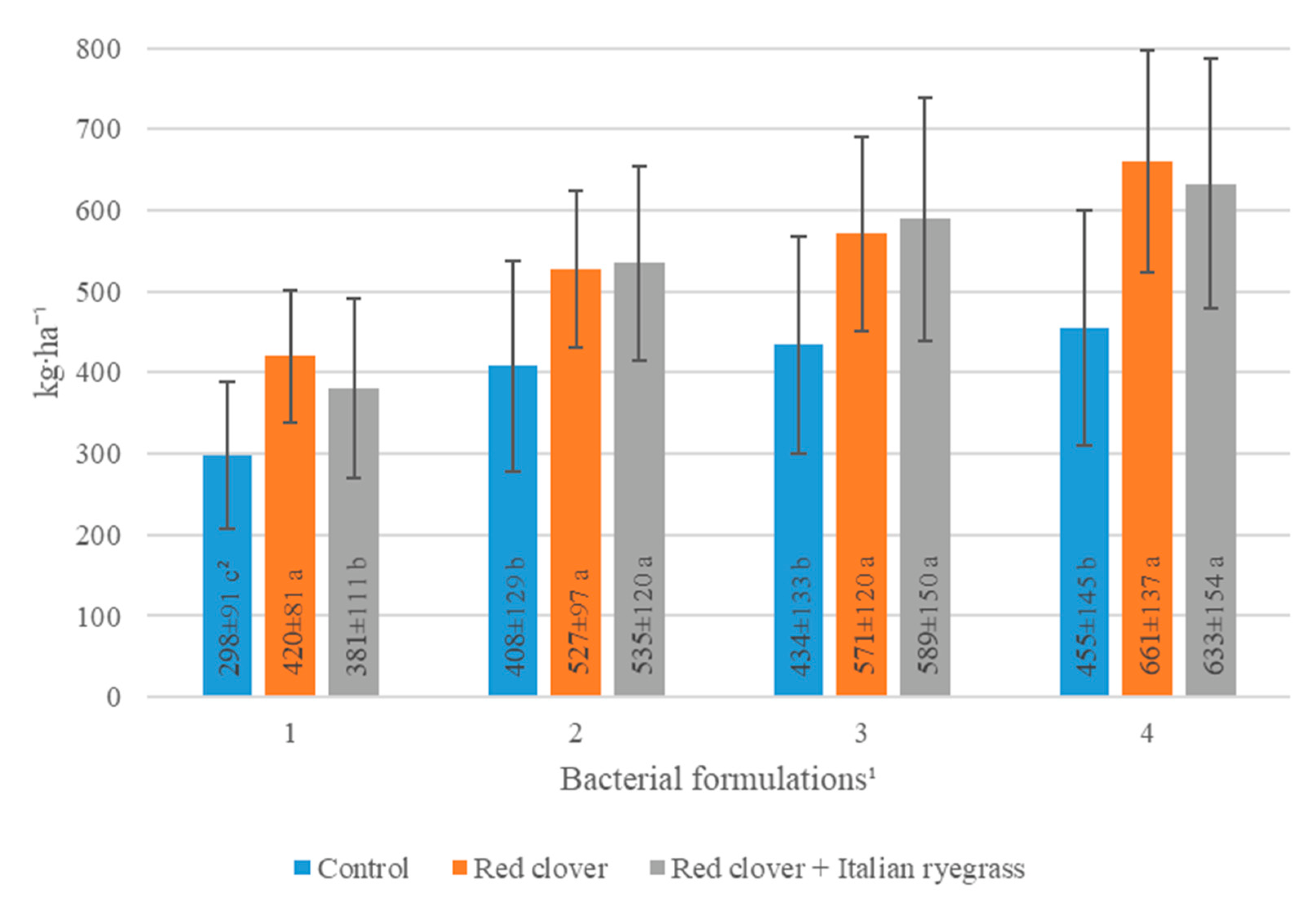
| Bacterial Formulations 1 (A) | Years (Y) | Means | ||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | ||
| 1 | 105.7 ± 2.5 c 2 | 125.0 ± 4.5 c | 93.8 ± 2.3 c | 108.2 ± 12.8 C |
| 2 | 111.5 ± 5.0 b | 127.7 ± 2.4 b | 96.5 ± 2.2 b | 111.9 ± 13.4 B |
| 3 | 112.5 ± 5.3 ab | 128.7 ± 2.5 ab | 97.5 ± 2.7 ab | 112.9 ± 13.3 B |
| 4 | 114.1 ± 5.6 a | 130.2 ± 2.4 a | 98.6 ± 3.0 a | 114.3 ± 13.5 A |
| Means | 111.0 ± 5.7 B | 127.9 ± 3.9 A | 96.6 ± 3.1 C | - |
| Companion Crops (B) | Years (Y) | Means | ||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | ||
| Control | 104.9 ± 2.1 c 1 | 125.2 ± 4.3 c | 93.6 ± 2.1 c | 107.9 ± 13.1 C |
| Red clover | 113.3 ± 5.7 a | 128.9 ± 2.6 a | 98.3 ± 2.8 a | 113.5 ± 13.1 A |
| Red clover + Italian ryegrass | 111.2 ± 3.0 b | 127.9 ± 2.5 b | 96.6 ± 2.1 b | 111.9 ± 13.3 B |
| Bacterial Formulations 1 (A) | Companion Crops (B) | ||
|---|---|---|---|
| Control | Red Clover | Red Clover + Italian Ryegrass | |
| 1 | 105.9 ± 12.0 b 2 | 109.3 ± 13.8 a | 109.3 ± 12.8 a |
| 2 | 108.2 ± 13.1 c | 114.9 ± 13.5 a | 112.6 ± 13.4 b |
| 3 | 108.9 ± 13.1 c | 116.3 ± 13.1 a | 113.5 ± 13.1 b |
| 4 | 110.1 ± 13.4 c | 118.1 ± 13.3 a | 114.6 ± 13.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płaza, A.; Niewiadomska, A.; Górski, R.; Rudziński, R.; Rzążewska, E. The Effect of the Nitrogen-Fixing Bacteria and Companion Red Clover on the Total Protein Content and Yield of the Grain of Spring Barley Grown in a System of Organic Agriculture. Agronomy 2022, 12, 1522. https://doi.org/10.3390/agronomy12071522
Płaza A, Niewiadomska A, Górski R, Rudziński R, Rzążewska E. The Effect of the Nitrogen-Fixing Bacteria and Companion Red Clover on the Total Protein Content and Yield of the Grain of Spring Barley Grown in a System of Organic Agriculture. Agronomy. 2022; 12(7):1522. https://doi.org/10.3390/agronomy12071522
Chicago/Turabian StylePłaza, Anna, Alicja Niewiadomska, Rafał Górski, Robert Rudziński, and Emilia Rzążewska. 2022. "The Effect of the Nitrogen-Fixing Bacteria and Companion Red Clover on the Total Protein Content and Yield of the Grain of Spring Barley Grown in a System of Organic Agriculture" Agronomy 12, no. 7: 1522. https://doi.org/10.3390/agronomy12071522
APA StylePłaza, A., Niewiadomska, A., Górski, R., Rudziński, R., & Rzążewska, E. (2022). The Effect of the Nitrogen-Fixing Bacteria and Companion Red Clover on the Total Protein Content and Yield of the Grain of Spring Barley Grown in a System of Organic Agriculture. Agronomy, 12(7), 1522. https://doi.org/10.3390/agronomy12071522







