Elicitor Induced JA-Signaling Genes Are Associated with Partial Tolerance to Hemibiotrophic Pathogen Phytophthora capsici in Capsicum chinense
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Inoculation Method
2.3. Treatment of Seedlings
2.4. RNA Extraction
2.5. Expression Analysis through qRT-PCR
2.6. Statistical Analysis
3. Results
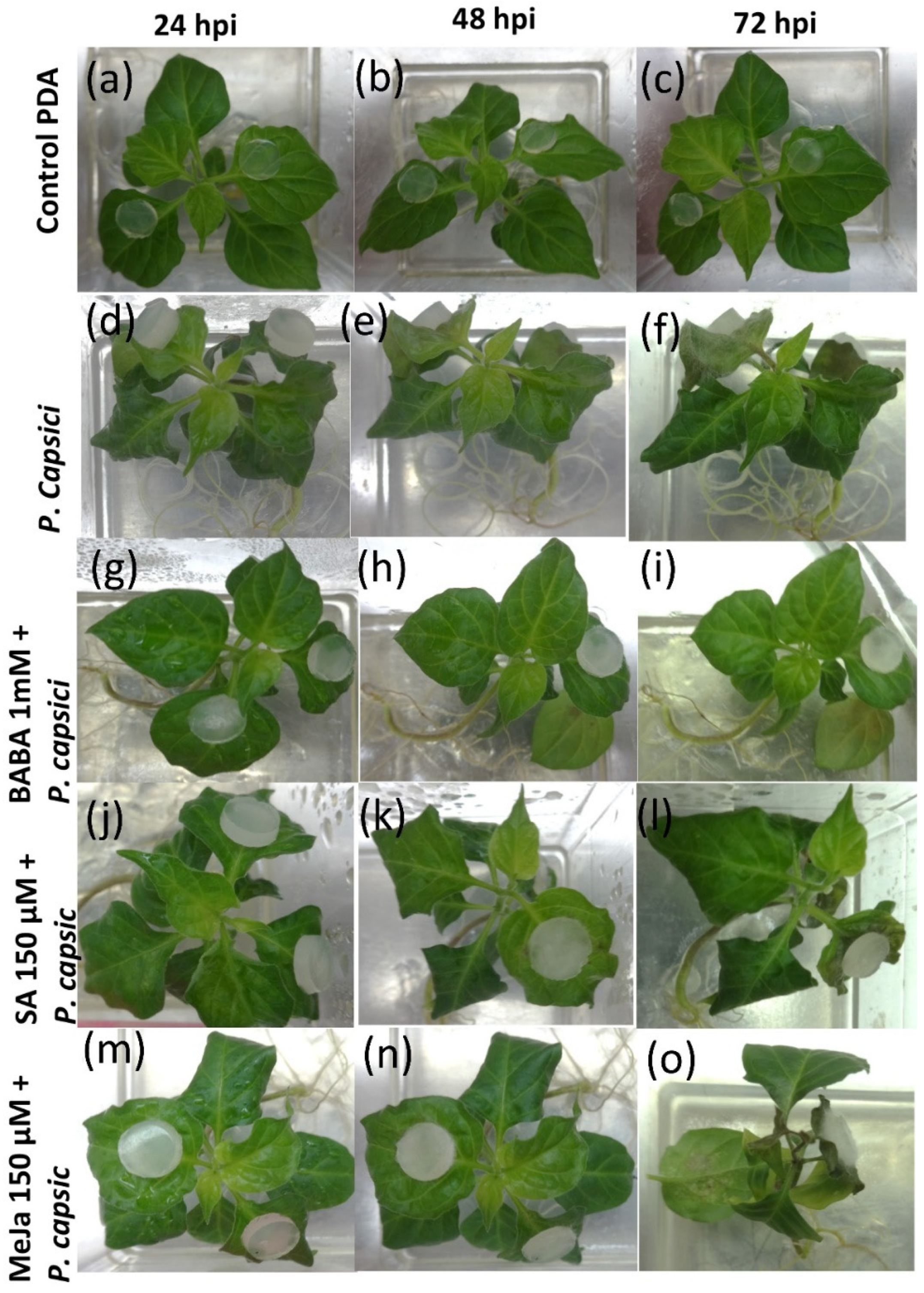
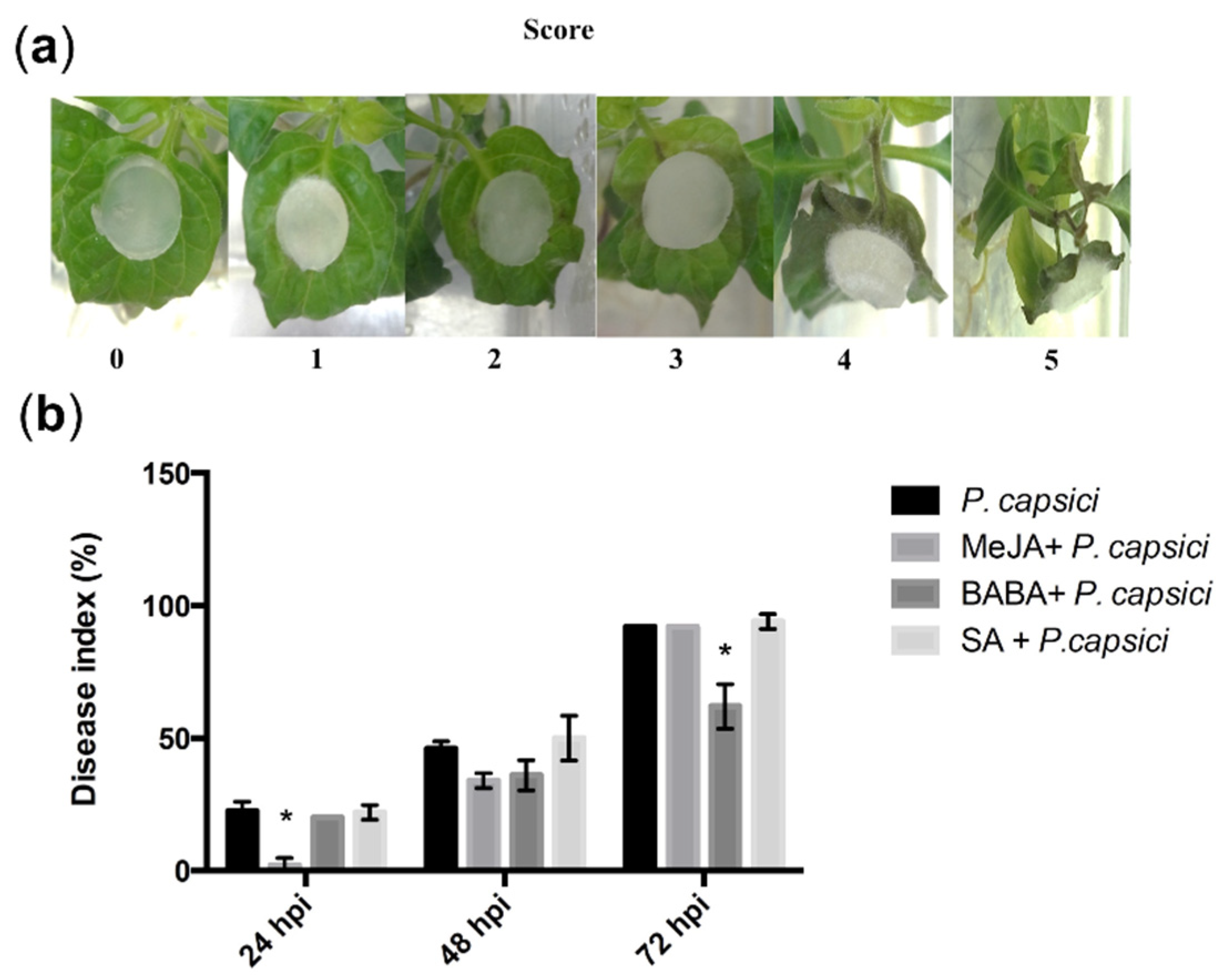
3.1. MeJA and BABA Treatments Modifies the Response of C. chinense Plants by Reducing Severity of Disease Symptoms
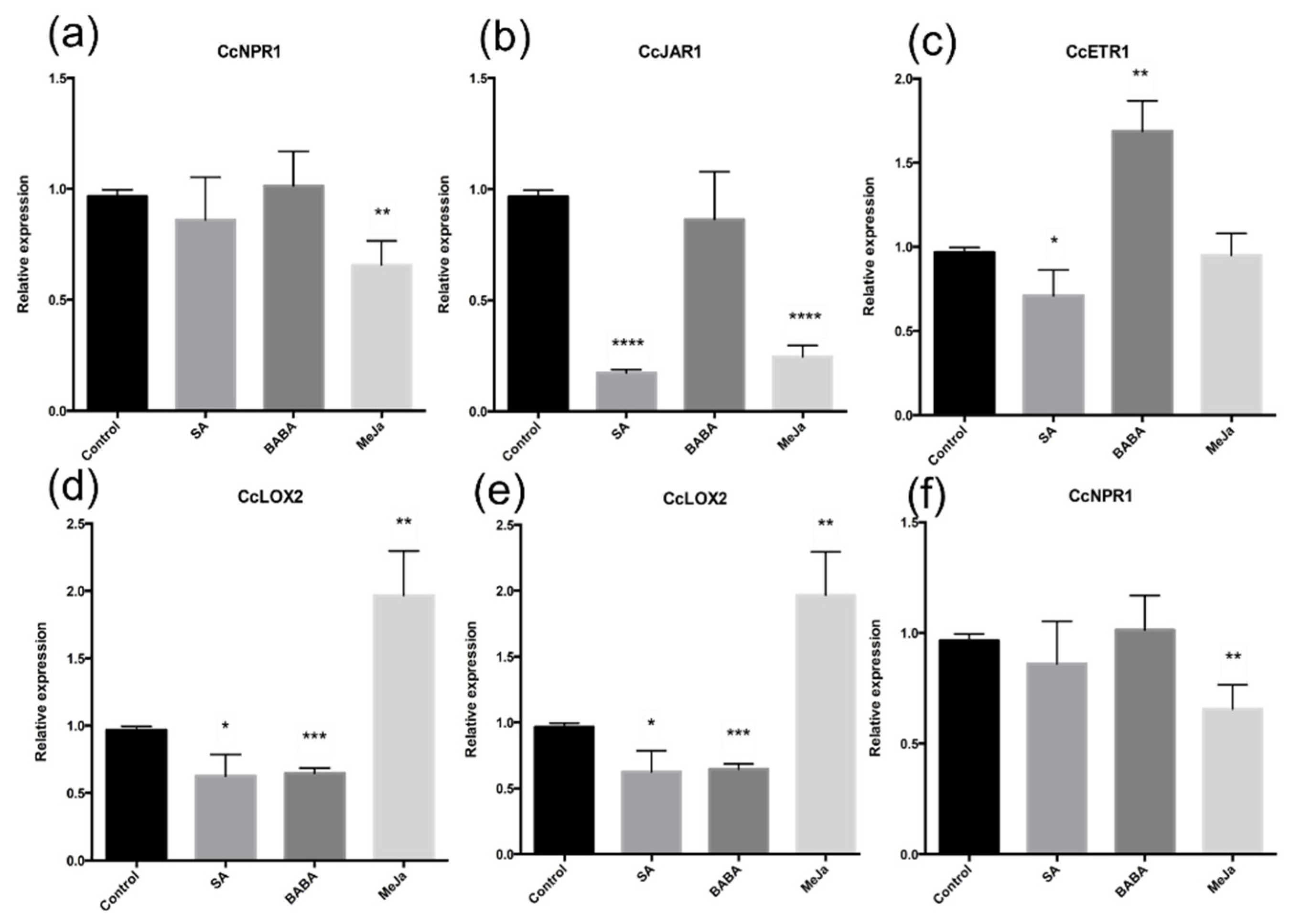
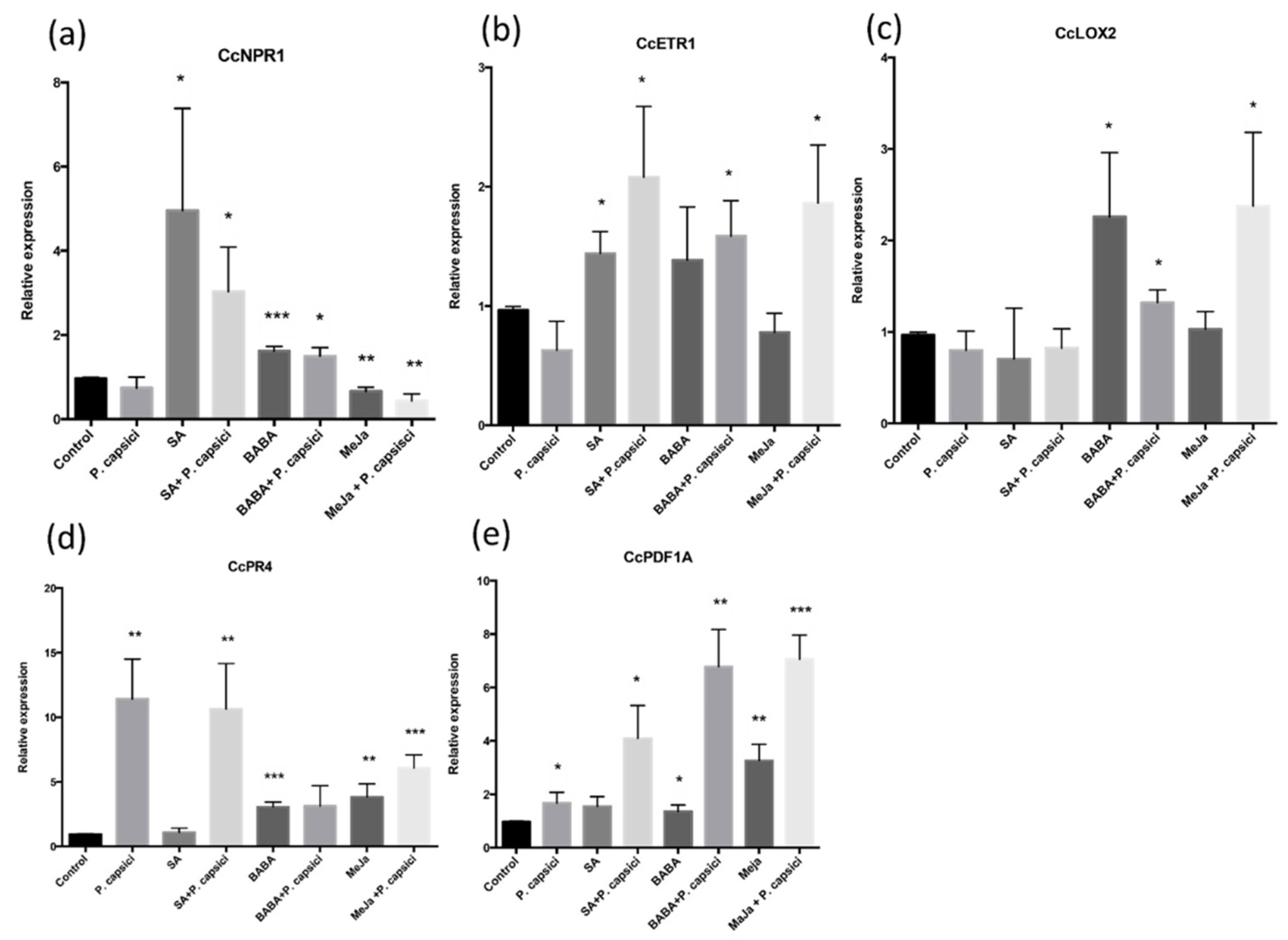
3.2. Effects of Elicitor Application in the Expression of Defense-Related Genes
3.3. Increased Expression of Genes Associated with JA-ET Signaling Is Associated with C. chinense Partial Tolerance to P. capsici
3.4. Expression of NBS–LRR Encoding R Genes in Response to Elicitor Treatment and P. capsici
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moscone, E.; Scaldaferro, M.; Grabiele, M.; Cecchini, N.; Sánchez, Y.; Jarret, R.; Daviña, J.; Ducasse, D.; Barboza, G.; Ehrendorfer, F. The evolution of chili peppers (Capsicum-Solanaceae): Cytogenetic perspective. Acta Hort. 2007, 745, 137–169. [Google Scholar] [CrossRef]
- Kraft, K.; Brown, C.; Nabhan, G.; Luedeling, E.; Luna, J.; Coppens, G.; Hijmans, R.; Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barboza, G.; Carrizo, C.; Scaldaferro, M.; Bohs, L. An amazing new Capsicum (Solanaceae) species from the Andean-Amazonian Piedmont. Phytokeys 2020, 167, 13–29. [Google Scholar] [CrossRef]
- Aguilar-Meléndez, A.; Morrell, P.; Roose, M.; Chul, S. Genetic diversity and structure in semi wild and domesticated chiles (Capsicum annuum; Solanaceae) from Mexico. Am. J. Bot. 2009, 96, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Fabela-Morón, M.; Cueva-Bernardino, J.; Ayora-Talavera, T.; Pacheco, N. Trend in capsaicinoids extraction from habanero chile pepper (Capsicum chinense Jacq.): Recent advanced techniques. Food. Rev. Int. 2019, 36, 105–134. [Google Scholar] [CrossRef]
- Jang, S.; Park, M.; Lee, D.; Lim, J.; Jung, J.; Kang, B. Breeding Capsicum chinense lines with high levels of capsaicinoids and capsinoids in the fruit. Agriculture 2021, 11, 819. [Google Scholar] [CrossRef]
- Lamour, K.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant Pathol. 2011, 13, 329–337. [Google Scholar] [CrossRef]
- Leonian, L. Stem and fruit blight of peppers caused by Phytophthora capsici. Phytopathology 1922, 12, 401–408. [Google Scholar]
- Zuluaga, A.; Vega-Arreguín, J.; Fei, Z.; Matas, A.; Patev, S.; Fry, W.; Rose, J. Analysis of the tomato leaf transcriptome during successive hemibiotrophic stages of a compatible interaction with the oomycete pathogen Phytophthora infestans. Mol. Plant Pathol. 2016, 17, 42–54. [Google Scholar] [CrossRef]
- Wang, W.; Jiao, F. Effectors of phytophthora pathogens are powerful weapons for manipulation host immunity. Planta 2019, 250, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, L.; Jia, Q.; Pan, R.; Oelmüller, R.; Zhang, W.; Wu, C. Arms race: Diverse effector proteins with conserved motifs. Plant Signal Behav. 2019, 14, 1557008. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhao, Z.; Abbas, A.; Qian, C.; Chen, J. Identification and characterization of potential NBS- encoding resistance genes and induction kinetics of a putative candidate gene associated with downy mildew resistance in Cucumis. BMC Plant Biol. 2010, 10, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhwal, M.; Li, P.; Lam, I.; Wang, X.; Cloutier, S.; You, F. Disease resistance gene analogs (RGAs) in plants. Int. J. Mol. Sci. 2015, 16, 19248–19290. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Yuan, W.; Ye, Q.; Wang, R.; Ruan, M.; Li, Z.; Zhou, G.; Yao, Z.; Zhao, J.; Liu, S.; et al. Analysis of TIR- and non-TIR-NBS-LRR disease resistance genes analogous in pepper: Characterization, genetic variation, functional divergence and expression patterns. BMC Genom. 2012, 13, 502. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jia, Q.; Li, D.; Wang, J.; Yin, Y.; Gong, Z. Characteristic of the pepper CaRGA2 gene in defense responses against Phytophthora capsici Leonian. Int. J. Mol. Sci. 2013, 14, 8985–9004. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Cheng, Y.; Huang, S.; Win, J.; Soards, A.; Jinn, T.; Jones, J.; Kamoun, S.; Chen, S.; Zhang, Y.; et al. Regulation of transcription of nucleotide binding leucine-rich repeat encoding genes SNC1 and RPP4 via H3K4 trimethylation. Plant Physiol. 2013, 162, 1694–1705. [Google Scholar] [CrossRef] [Green Version]
- Spoel, S.; Johnson, J.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, N.; Mahomed, W.; Olivier, N. Transcriptome analysis of an incompatible Persea americana-Phytophthora cinnamomi interaction reveals the involvement of SA and JA pathways in a successful defense response. PLoS ONE 2018, 13, e0205705. [Google Scholar] [CrossRef]
- Núñez-Pastrana, R.; Arcos-Ortega, F.; Souza-Perera, R.; Sánchez-Borges, C.; Nakazawa- Ueji, Y.; Garcia-Villalobos, F.; Guzmán-Antonio, A.; Zúñiga-Aguilar, J. Ethylene, but not salicylic acid or methyl jasmonate, induces a resistance response against Phytophthora capsici in habanero pepper. Eur. J. Plant Pathol. 2011, 131, 669–683. [Google Scholar] [CrossRef]
- Venegas-Molina, J.; Proietti, S.; Pollier, J.; Orozco-Freire, W.; Ramirez-Villacis, D.; Leon- Reyes, A. Induced tolerance to abiotic and biotic stresses of broccoli and Arabidopsis after treatment with elicitor molecules. Sci. Rep. 2020, 10, 10319. [Google Scholar] [CrossRef] [PubMed]
- Kuznicki, D.; Meller, B.; Arasimowicz-Jelonek, M.; Braszewska-Zalewska, A.; Drozda, A.; Floryszak-Wieczorek, J. BABA-induced DNA methylome adjustment to intergenerational defense priming in potato to Phytophthora infestans. Front. Plant Sci. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Veena, M.; Melvin, P.; Prabhu, S.; Shailasree, S.; Shetty, H.; Kini, K. Molecular cloning of a coiled-coil-nucleotide-binding-site-leucine-rich repeat genes from pearl millet and its expression pattern in response to the downy mildew pathogen. Mol. Biol. Rep. 2016, 43, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroy-Barbosa, A.; Bosland, P. A rapid technique for multiple-race disease screening of Phytophthora foliar blight on single Capsicum annuum L. plants. HortScience 2010, 45, 1563–1566. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, N.; Mukherjee, K.; Sarkar, A.; Acharya, K. Interaction between Bean and Colletotrichum gloeosporioides: Understanding Through a Biochemical Approach. Plants 2019, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.X.; Feng, X.H.; Ali, M.; Jin, J.H.; Wei, A.M.; Khattak, A.M.; Gong, Z.H. Identification of Pepper CaSBP08 Gene in Defense Response Against Phytophthora capsici Infection. Front. Plant Sci. 2020, 11, 183. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Guo, J.; Yang, W.; Shen, H. Molecular mapping of a gene conferring resistance to Phytophthora capsici Leonian race 2 in pepper line PI201234(Capsicum annuum L.). Mol. Breed. 2016, 36, 66. [Google Scholar] [CrossRef]
- Siddique, M.; Lee, H.; Ro, N.; Han, K.; Venkatesh, J.; Solomo, A.; Patil, A.; Changkwian, A. Identifying candidate genes for Phytophthora capsici resistance in pepper (Capsicum annuum) via genotyping by sequencing based QTL mapping and genome wide association study. Sci. Rep. 2019, 9, 9962. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, H.; Tan, J.; Yan, M.; Li, D.; Khan, A.; Gong, Z. A new ethylene responsive factor CaPTI1 gene of pepper (Capsicum annuum L.) involved in the regulation of defense response to Phytophthora capsici. Front. Plant Sci. 2016, 6, 1217. [Google Scholar] [CrossRef]
- Nieto-Garibay, A.; Barraza, A.; Caamal-Chan, G.; Murillo-Amador, B.; Troyo-Dieguez, E.; Burgoa-Cruz, C.; Jaramillo-Limón, J.; Loera-Muro, A. Habanero pepper (Capsicum chinense) adaptation to water deficit stress in a protected agricultural system. Funct. Plant Biol. 2022, 49, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Min, K.; Kwak, A.; Lee, S.; Kang, H. Defense response and suppression of Phytophthora Blight disease of pepper by water extract from spent mushroom substrate of Lentinula edodes. Plant Pathol. J. 2017, 33, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yuan, W.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Zhao, J.; Liu, S.; et al. Identification of reference genes for reverse transcription quantitative real time PCR normalization in pepper (Capsicum annuum L.). Biochem. Biophys. Res. Commun. 2011, 416, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Jupe, J.; Stam, R.; Howden, A.; Morris, J.; Zhang, R.; Hedley, P.; Huitema, E. Phytophthora capsici-tomato interaction features dramatic shifts in gene expression associated with a hemi-biotrophic lifestyle. Genome Biol. 2013, 14, R63. [Google Scholar] [CrossRef] [Green Version]
- Coles, D.; Bithell, S.; Mikhael, M.; Cuddy, W.; Plett, M. Chickpea roots undergoing colonization by Phytophthora medicaginis exhibit opposing jasmonic acid and salicylic acid accumulation and signaling profiles to leaf hemibiotrophic models. Microorganisms 2022, 10, 343. [Google Scholar] [CrossRef]
- Bengtsson, T.; Weighill, D.; Proux-Wéra, E.; Levander, F.; Resjö, S.; Burra, D.; Moushib, L.; Hedley, P.; Liljeroth, E.; Jacobson, D.; et al. Proteomics and transcriptomics of the BABA-induced resistance response in potato using a novel functional annotation approach. BCM Genom. 2014, 15, 315. [Google Scholar] [CrossRef] [Green Version]
- Stamler, R.; Holguin, O.; Dungan, B.; Schaub, T.; Sanogo, S.; Goldberg, N.; Randall, J. BABA and Phytophthora nicotianae induce resistance to Phytophthora capsici in chile pepper (Capsicum annuum). PLoS ONE 2015, 10, e0128327. [Google Scholar]
- Martinez-Aguilar, K.; Ramírez-Carrasco, G.; Hernández-Chávez, J.; Barraza, A.; Alvarez-Venegas, R. use of BABA and INA as activators of a primed state in the common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2016, 7, 653. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef] [Green Version]
- Kondratev, N.; Denton-Giles, M.; Bradshaw, R.; Cox, M.; Dijkwel, P. Camellia plant resistance and susceptibility to petal blight disease are defined by the timing of defense responses. Mol. Plant-Microbe Interact. 2020, 33, 982–995. [Google Scholar] [CrossRef]
- Arévalo-Marín, D.; Briceño-Robles, D.; Mosquera, T.; Melgarejo, L.; Sarmiento, F. Jasmonic acid priming of potato uses hypersensitive response dependent defenses and delays necrotrophic phase change against Phytophthora infestans. Physiol. Mol. Plant Pathol. 2021, 115, 101680. [Google Scholar] [CrossRef]
- Chauvin, A.; Lenglet, A.; Wolfender, J.; Farmer, E. Paired hierarchical organization of13- lipoxygenases in Arabidopsis. Plants 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarde, S.; Kumar, A.; Remme, R.; Dicke, M. Genome-wide identification, classification and expression of lipoxygenase gene family in pepper. Plant Mol. Biol. 2018, 93, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Neu, E.; Domes, H.; Menz, I.; Kaufmann, H.; Linder, M.; Debener, T. Interaction of roses with a biotrophic and a hemibiotrophic leaf pathogen leads to differences in defense transcriptome activation. Plant Mol. Biol. 2019, 99, 299–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yu, T.; Wu, T.; Wang, R.; Wang, H.; Du, H.; Xu, X.; Xie, D.; Xu, X. The dynamic transcriptome of pepper (Capsicum annuum) whole roots reveals an important role for the phenylpropanoid biosynthesis pathway in root resistance to Phytophthora capsici. Gene 2020, 728, 144288. [Google Scholar] [CrossRef]
- Le Berre, J.; Gourgues, M.; Samans, B.; Keller, H.; Panabieres, F.; Attard, A. Transcriptome dynamic of Arabidopsis roots infected with Phytophthora parasitica identifies VQ29, a gene induced during the penetration and involved in the restriction of infection. PLoS ONE 2017, 12, e0190341. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Mabon, R.; Andrivon, D.; Val, F. The effectiveness of induced defense responses in a susceptible potato genotype depends on the growth rate of Phytophthora infestans. Mol. Plant-Microbe Interact. 2019, 32, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Elnahal, A.; Li, J.; Wang, X.; Zhou, C.; Wen, G.; Wang, J.; Lindqvist-Kreuze, H.; Meng, Y.; Shan, W. Identification of natural resistance mediated by recognition of Phytophthora infestans effector gene Avr3aEM in potato. Front. Plant Sci. 2020, 11, 919. [Google Scholar] [CrossRef]
- Zimmerli, L.; Métraux, J.; Mauch-Mani, B. ß-aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol. 2001, 126, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Koen, E.; Trapet, P.; Brulé, D.; Kullk, A.; Kllnguer, A.; Atauri- Miranda, L.; Meunler- Prest, R.; Bonl, G.; Glauser, G.; Mauch-Manl, B.; et al. ß- aminobutyric acid (BABA)-induced resistance in Arabidopsis thaliana: Link with iron homeostasis. Mol. Plant-Microbe Interact. 2014, 27, 1226–1240. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Carrasco, G.; Martínez-Aguilar, K.; Alvarez-Venegas, R. Transgenerational defense priming from crop protection against plant pathogens; a hypothesis. Front. Plant Sci. 2017, 8, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacQueen, A.; Bergelson, J. Modulation of R-genes expression across enviroments. J. Exp. Bot. 2016, 67, 2093–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Genes | Reference Author | Sequence Primer (5′-3′) |
|---|---|---|
| Defense-Related Genes | ||
| NPR1 | [20] Nuñez-Pastrana et al., 2011. | F: GCACAGAGGACAACAGTGGA R: TCAGTGAACGCTTTGGTCAG |
| PDF1.2 | [30] Jin et al., 2016 | F: CAAGGG AGTATGTGCTAGTGAGAC R: TGCACAGCACTATCATTGCATAC |
| LOX2 | [31] Nieto-Garibay et al., 2022 | F: CGAGCTGTAGTTACGGTAAGGAACAAGAACAAGGAAGATCTG R: GTGTTTGGATCGATGTCGGTGCTGATGAGTTCTAAGGCG |
| ETR1 | [31] Nieto-Garibay et al., 2022 | F: CCACATCATTCCTGATTTACTTAGCGTCAAAACTAGGGAG R: CATTCTAACATGTCTACCTGTCTCCTCTTGAGTCCGAATAATACCCA |
| PR4 | [32] Kang et al., 2017 | F: ATCCAAGGTACATATAGAGCTTCC R: AACTGGGATTTGAGAACTGCCAGC |
| R Genes | ||
| ARGA2 | [16] Zhang et al., 2013 | F: TGCTAGGCGGGAAACAGGTTATG R: CAAGCCGAGTAGTGGTTAGAACAG |
| ARGA18 | [15] Wan et al., 2012 | F: TCGGCAGAATGAAATAGAGG R: ACAATAGGGACAACAGCCG |
| ARGA23 | [15] Wan et al., 2012 | F: AAGAGCGATTGATTGACCGT R: CCAACAATAGGGACAACAGT |
| ARGA36 | [15] Wan et al., 2012 | F: AGAGTGTTGCCTTGATGATG R: TTCCACCCTTCAACCTCTG |
| ARGA38 | [15] Wan et al., 2012 | F: TTCTGGATGATGTGTGGA GT R: ATCATCAAGGCAACACTCTC |
| RPP13 | [27] Wang et al., 2016 | F: GGA GAA GGG GCG AGT AAT AGG T R: CATCCTGAAAGCCAACAAA |
| Reference gene | ||
| GADPH | [33] Wan et al., 2011 | F: ATGATGATGTGAAAGCAGCG R: TTTCAACTGGTGGCTGCTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraza, A.; Núñez-Pastrana, R.; Loera-Muro, A.; Castellanos, T.; Aguilar-Martínez, C.J.; Sánchez-Sotelo, I.S.; Caamal-Chan, M.G. Elicitor Induced JA-Signaling Genes Are Associated with Partial Tolerance to Hemibiotrophic Pathogen Phytophthora capsici in Capsicum chinense. Agronomy 2022, 12, 1637. https://doi.org/10.3390/agronomy12071637
Barraza A, Núñez-Pastrana R, Loera-Muro A, Castellanos T, Aguilar-Martínez CJ, Sánchez-Sotelo IS, Caamal-Chan MG. Elicitor Induced JA-Signaling Genes Are Associated with Partial Tolerance to Hemibiotrophic Pathogen Phytophthora capsici in Capsicum chinense. Agronomy. 2022; 12(7):1637. https://doi.org/10.3390/agronomy12071637
Chicago/Turabian StyleBarraza, Aarón, Rosalia Núñez-Pastrana, Abraham Loera-Muro, Thelma Castellanos, Carlos Julián Aguilar-Martínez, Isaac Salvador Sánchez-Sotelo, and María Goretty Caamal-Chan. 2022. "Elicitor Induced JA-Signaling Genes Are Associated with Partial Tolerance to Hemibiotrophic Pathogen Phytophthora capsici in Capsicum chinense" Agronomy 12, no. 7: 1637. https://doi.org/10.3390/agronomy12071637






