Abstract
Calcium deficiency or its inefficient translocation to pepper fruits leads to considerable economic loss by reducing the number of marketable fruits. The present study proposes grafting as an environmentally friendly technique to effectively reduce such loss. A commercial variety (Al-cudia F1; V) was grafted onto two pepper (Capsicum annum L.) accessions (V/A6 and V/A8), a hybrid rootstock (V/N) and was also self-grafted (V/V). All rootstock–scion combinations were cultivated under greenhouse conditions with optimal and suboptimal Ca supply and assessed for fruit yield and biomass production, gas exchange and chlorophyll fluorescence, mineral concentration in leaves and fruits as well as several fruit quality parameters. The V/N plants demonstrated an enhanced capacity for increased biomass, higher yield and number of commercial fruits and greater mean fruit weight compared with the other rootstock–scion combinations. These improvements are attributed primarily to increased intrinsic water efficiency. Additionally, a significantly higher Ca concentration in leaves was found under suboptimal Ca conditions in the V/N combination than that found in the other rootstock–scion combinations indicating a higher capacity for Ca uptake and translocation. Under the same conditions, the concentration of organic acids in fruits, such as citric and tartaric, which impact the organoleptic quality, was also higher in V/N plants. Consequently, we can conclude that grafting pepper onto tolerant rootstocks is a successful tool for ameliorating the negative impact of suboptimal Ca conditions on pepper crop performance and fruit quality.
Keywords:
Capsicum annum L.; grafting; calcium; gas exchange; yield; fruit quality; mineral composition; organic acids 1. Introduction
With a cultivated area of approximately 2 million hectares and an annual global production of almost 35 million tons, peppers (Capsicum annum L.) are currently among the most widely cultivated vegetable crops [1,2,3]. Calcium deficiency in pepper induces “blossom-end rot” (BER) in fruits which is the main physiological disorder that causes a reduction in yield [4,5,6,7]. Interestingly, its incidence has increased significantly, driven primarily by climate change [5]. The well-visible symptomatology of the fruits determines their unsaleability, causing waste and deleterious economic losses.
Calcium, an essential macronutrient in plants, stabilizes cell membranes and walls and plays a crucial role as a secondary messenger in cell signalling [6]. Environmental (temperature and vapour pressure deficit), genetic, and physiological (photosynthetic activity) factors significantly influence the uptake and transport of this element in the plant [5]. The establishment of conditions that limit Ca2+ translocation to developing fruits triggers the development of BER [4].
Genetic improvement programs aimed at developing cultivars tolerant to suboptimal growth conditions, such as calcium deficiency and/or unavailability, have not been successful due to the complexity of multigenic traits involved in response to such stresses [7,8]. Moreover, low genetic availability inexorably reduces conventional breeding programs. However, even the development of genetically modified plants cannot be considered a concrete solution due to stringent EU regulations [9].
Given the above, plant grafting is an effective technique to counteract and overcome stress conditions in Cucurbitaceae and Solanaceae [9]. Furthermore, since no chemical treatment is required, grafting should be considered an environmentally friendly agronomic technique [10]. Although the primary objective of grafting was to limit the damaging effects of soilborne pathogens [11], over time, this practice has expanded remarkably successfully for other purposes [12]. Grafting has been shown to improve nitrogen efficiency and uptake [13], potassium [14] and magnesium [15]. Increased biomass and improved architecture of the hypogeal system of selected rootstocks (wild genotypes of the same species as the scion and hybrids or relatives thereof) due to improved water and macro/microelement uptake ensure better performance under suboptimal abiotic conditions [7,12]. At the same time, improved performance of grafted plants under abiotic stresses is associated with the maintenance of ion homeostasis, enhanced hormonal signalling, a stronger antioxidant defence system, and long-distance and large-scale movement of proteins, small RNAs, and mRNAs [7] associated mainly with robust rootstock able to cope environmental stresses. Indeed, the latter play crucial roles in regulating plant development and growth, influencing their response to different abiotic stresses, including suboptimal temperatures, high salinity, drought, and deprivation of key mineral elements [16].
Considering the high susceptibility of Solanaceae fruits to BER [5,17], increasing uptake and improving calcium translocation in fruits due to grafting could be a viable and practical solution. To date, investigations on the influence of this agronomic technique have focused mainly on tomatoes without highlighting the positive impact of grafting on reducing BER [12]. Unsurprisingly, among Solanaceae, sweet bell pepper grafting has received less attention, probably due to its limited compatibility with other species [18] which limits the availability of commercial rootstocks. However, specific pepper rootstocks are an effective solution, especially against the onset of water, heat, and salt stress [8,19,20].
To the best of our knowledge, there is a lack of information in the scientific literature on the influence of grafting under sub-optimal calcium supply in pepper plants. For this reason, the objective of this research was to find new pepper rootstocks tolerant to suboptimal calcium conditions from an agronomic and physiological point of view. The crop tolerance to suboptimal calcium conditions was evaluated under greenhouse conditions in terms of fruit yield and quality, net photosynthesis, transpiration, water use efficiency, leaf and fruit mineral composition and fruit quality attributes. To reach this objective, we have selected a sweet bell pepper (Alcudia F1) that was grafted onto two accessions (A6 and A8), a hybrid rootstock (NIBER®) and onto its own roots (self-grafted), under optimal and sub-optimal calcium concentration.
2. Materials and Methods
2.1. Plant Material
The species used for this experiment was pepper. The commercial pepper (Capsicum annuum L.) variety Alcudia F1 (Semillas Fitó, Spain, Lamuyo type) was used as a scion (V). The grafting combinations used for this experiment were: (i) self-grafted (V/V); (ii) variety grafted onto two pepper accessions (V/A6 and V/A8, respectively), provided by the germplasm bank placed in the Institute for Conservation and Improvement of Valencian Agrodiversity “COMAV” (Universitat Politècnica de València, Valencia, Spain); (iii) a commercial hybrid pepper rootstock, called NIBER® (N), registered by the “Instituto Valenciano de Investigaciones Agrarias (IVIA, Valencia, Spain) and the “Universitat Politècnica de València”, (V/N). Non-grafted pepper plants were not included in the present study.
Seeds of scion and rootstocks were sown in 104-hole seed trays containing a commercial peat-based substrate (Bril) for germination. Two months after sowing, V was grafted onto the three selected rootstocks herein (V/A6, V/A8, V/N) and self-grafted (V/V). Three weeks after grafting, plants were transplanted at a plant density of 2.7 plants m−2 in a 300 m2 polyethylene greenhouse located in the Experimental Farm ‘Nello Lupori’ of Tuscia University (Viterbo, Italy) into a 15 L pot containing a mixture of sand and peat (1:1; v:v). Plants were grown under natural light conditions. After transplant, plants were irrigated with two nutrient solutions that contained the following nutrients in common: 8.0 mM N-NO3, 1.0 mM N-NH4, 1.0 mM P, 1.0 mM S, 5.0 mM K, 1.5 mM Mg, 20 μM Fe, 9 μM Mn, 0.3 μM Cu, 1.6 μM Zn, 20 μM B, and 0.3 μM Mo. The two solutions were differentiated in the Ca concentration: optimal Ca concentration (OCC; 4.5 mM Ca) and sub-optimal Ca concentration (SCC; 0.5 mM of Ca). CaCl2 was used to reach the highest Ca concentration. The pH and electrical conductivity of the nutrient solutions were on average 4.4 and 1.2 dS m−1, respectively.
The experimental design was a factorial combination of 4 grafting combinations (V/A6, V/A8, V/N, V/V) and 2 nutrient solution concentrations (optimal and sub-optimal Ca concentration) with four replicates for each plant combination and Ca concentration with four plants by replicate.
2.2. Yield Production and Biomass
Fruits of all plants were harvested at 71, 84 and 98 days after the beginning of the calcium treatment (DAT) and individually classified into marketable (FW > 100 g) and non-marketable (FW < 100 g) fruits. After that, fruits were counted and weighed to finally obtain the number of fruits and yield per plant, as well as the average weight of marketable fruits. The shape index (SI) of fruits was determined as the width/length ratio [21].
At the end of the experiment (98 DAT), two plants per replicate (n = 8) were cut, separated fruits from the aerial part and separately weighted. Afterwards, both were dried in an oven at 65 °C and weighed when weight was constant to obtain the dry weight (DW). With both measurements, harvest index (HI) was calculated by the ratio between the total dry fruit weight/total aerial dry weight [19].
2.3. Fluorescence Measurements
Maximum efficiency of photosystem II (Fv/Fm) in dark-adapted conditions was measured at 37, 51, 71, 84 and 98 DAT in all plants, from 12.30–14.00 (UT + 01:00) on saturating light conditions (1000 µmol m−2 s−1) with a Handy PEA portable fluorimeter (Hansatech instruments, King’s Lynn, UK). Measurements were taken on one fully expanded leaf per plant (the 3rd–4th leaf from the apex).
2.4. Gas Exchange Measurements
CO2 fixation rate (AN; µmol CO2 m−2 s−1), stomatal conductance (gs; mol H2O m−2 s−1), substomatal CO2 assimilation rate (Ci; µmol CO2 mol−1 air) and transpiration rate (E; mmol H2O m−2 s−1) were measured with a portable LI-COR 6400 infrared gas analyzer (Li-Cor Inc., United States). Measurements were taken from 12.30–14.00 (UT + 01:00) on saturating light conditions (1000 µmol m−2 s−1), 400 ppm CO2, at a cuvette temperature of 25 °C (1 ± °C) and 75% of relative humidity. Parameters AN/E, AN/gs and AN/Ci were calculated as instantaneous water use efficiency, intrinsic water efficiency and instantaneous carboxylation efficiency, respectively.
Two plants per block replicate were measured (n = 8), on one fully expanded leaf (the 3rd–4th leaf from the apex).
2.5. Mineral Analysis
Macronutrients (total N, P, K, Ca, Mg and S) were measured in dried leaves and fruits of one plant per replicate in the case of leaves and five fruits homogenized together per replicate (n = 4). Total nitrogen content analyses were conducted on dry, milled samples using the Kjeldahl method. Based on [22] protocol, a 250 mg aliquot of milled (model MF10.1, IKA-Werke GmbH & Co., KG, Staufen, Germany) and a dry leaf sample was used for the determination of mineral (P, K, Ca, Mg, and S) composition. Mineral analysis was then carried out after 0.45 µm filtering using an ion chromatographer (model ICS-3000, Dionex, Sunnyvale, CA, USA), quantified using an electrical conductivity detector equipped with an IonPac CS12A and IonPac AS11-HC analytical columns for the analysis of cationic and anionic contents, respectively (Dionex, Sunnyvale, CA, USA).
2.6. Fruit Quality
At 84 DAT, 5 fruits per plant replicate were harvested and used for quality trait determinations (n = 4). Firmness was measured in triplicate on the middle part of the pepper fruits using a manual penetrometer (Bertuzzi FT 011; Brugherio, Milan, Italy) fitted with an 8 mm-diameter round-head probe. Edible (EP; pericarp) and inedible (IP; seeds and central placenta) part of each fruit was then weighed separately and the ratio between EP/IP was calculated
Edible parts of the fruits were separated into two parts: one part was weighed and dried in an oven at 65 °C for 72 h to determine the percentage of dry fruit weight and the other part was used for electrical conductivity (EC; dS m−1), pH, organic acids (malate, tartrate, oxalate, citrate and isocitrate), and total soluble solids (TSS; °Brix) determinations. Organic acids were analyzed using anion exchange HPLC with conductivity detection as reported by [23].
2.7. Statistical Analysis
Experimental data were subjected to a one-way ANOVA analysis (Statgraphics Centurion for Windows, Statistical Graphics Corp.) where Ca treatment (T) and plant combination (Pc) were joined as individual factors of the analysis. Mean separation was performed by Fisher’s least significance difference (LSD) at P < 0.05.
3. Results
3.1. Yield, Yield Components and Biomass Parameters
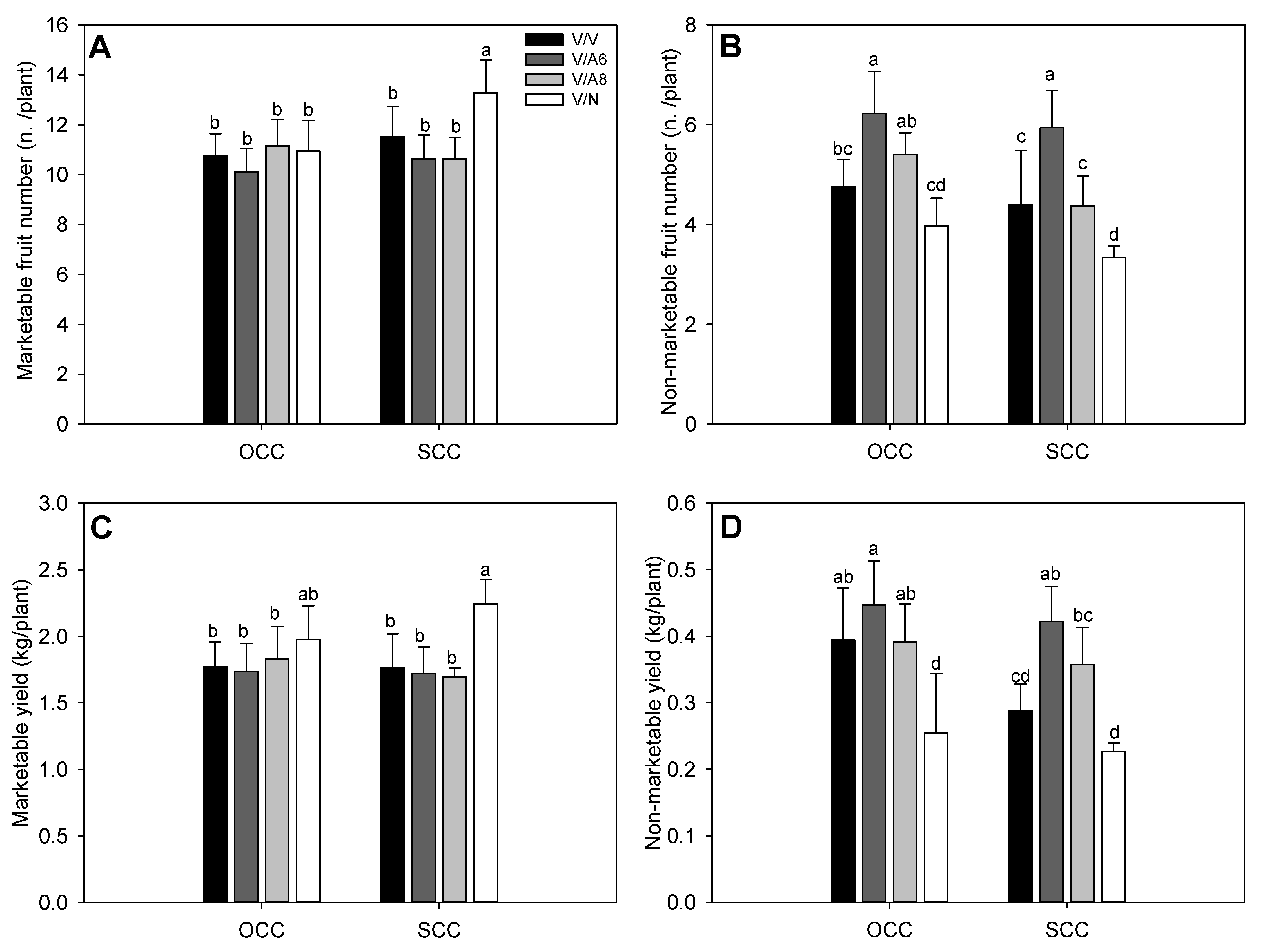
The number of marketable fruits per plant (Figure 1A) was significantly highest for V/N plants under SCC conditions, the rest of the plant’s combinations did not show significant differences between them for any treatment. On the other side, the number of non-marketable fruits (Figure 1B) was significantly affected in V/A6 in both OCC and SCC treatments; the lowest non-marketable fruit number was recorded for V/N plant combination and for SCC.
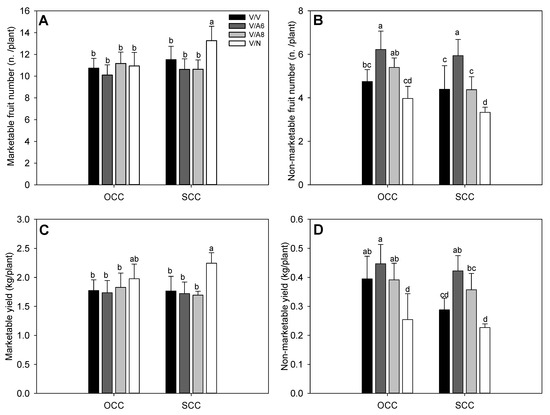
Figure 1.
Number of marketable (A) and non-marketable (B) fruits per plant, marketable (C) and non-marketable (D) fruit yield (Kg/plant) under optimal (OCC) and suboptimal (SCC) calcium conditions. The selected variety (V) was grafted onto the rootstocks A6 (V/A6), A8 (V/A8) and NIBER® (V/N), as well as self-grafted (V/V). Data are the mean values of n = 4 and bars correspond to the standard deviation. Different letters indicate significant differences at P < 0.05 (LSD test).
When marketable and non-marketable fruit yields were recorded (Figure 1C,D), a similar pattern to the number of marketable and non-marketable fruits was observed, respectively. V/N reached the highest marketable yield in SCC (Figure 1C). In the case of non-marketable yield (Figure 1D), V/N showed the lowest values in OCC and SCC without significant differences between them. The non-marketable fruits were mainly fruits with blossom-end rot.
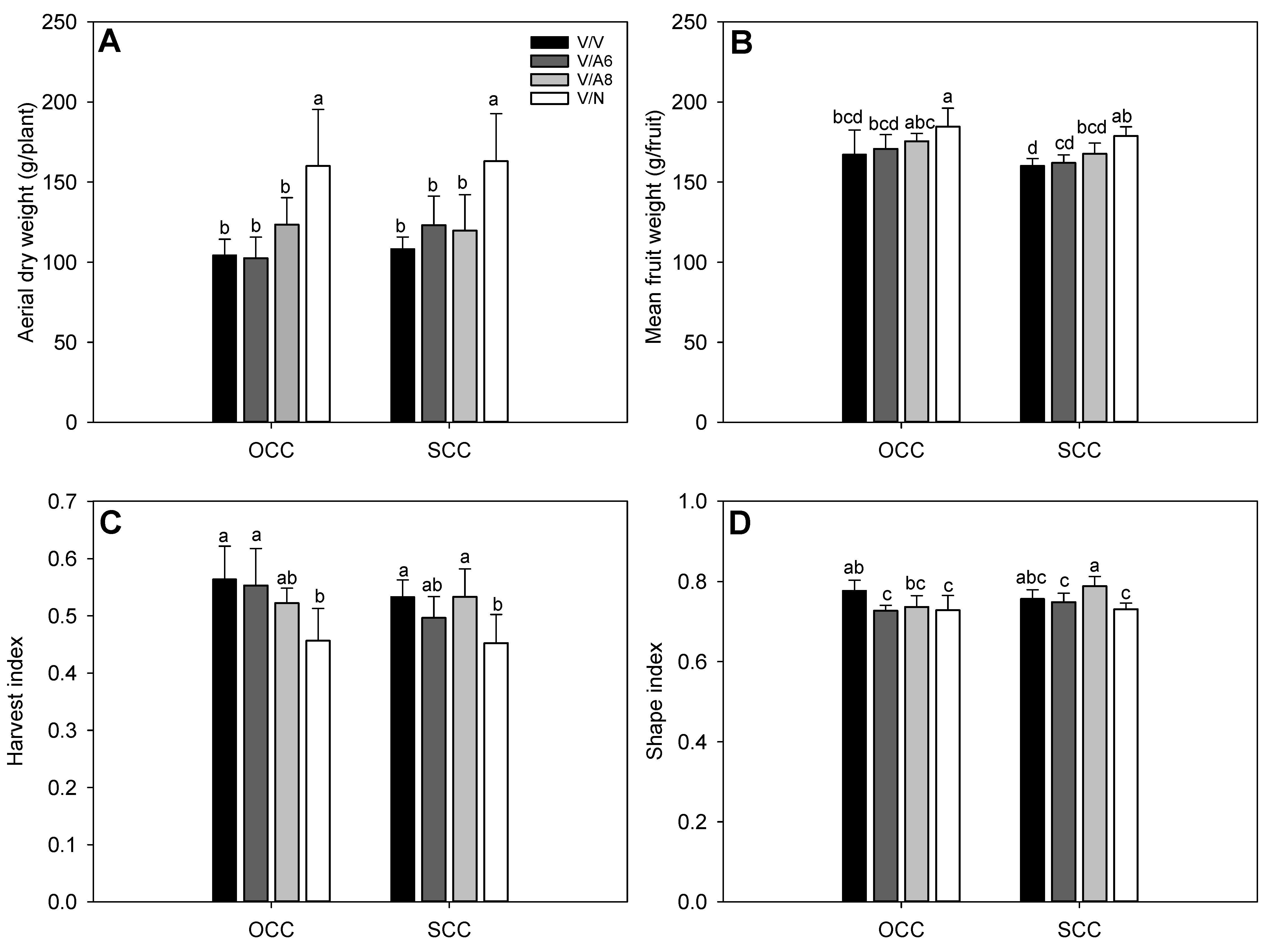
In line with the above results, aerial plant dry weight (ADW) was improved only in V/N (Figure 2A) in OCC and SCC, while the rest of the plant combinations did not display significant differences among them for any treatment. For mean marketable fruit weight (Figure 2B), the heaviest fruits were found in V/N in OCC and SCC with significant differences followed by V/A8 under OCC treatment. HI index (Figure 2C) showed significant differences with the highest values in all plant combinations and treatments except for V/N where the HI obtained the lowest values in both Ca conditions. Finally, SI (Figure 2D) decreased significantly in V/A6 and V/N in OCC and SCC while the highest values were observed in V/A8 under SCC.
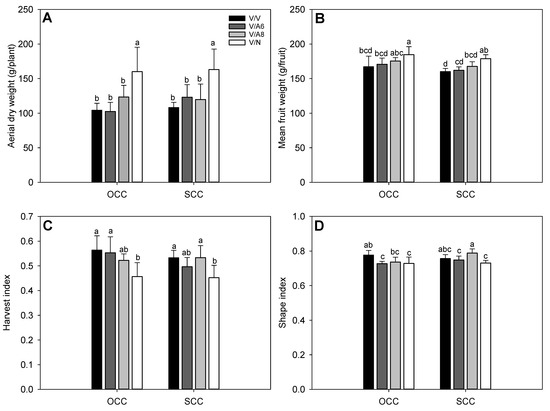
Figure 2.
Aerial dry weight (ADW, g; (A)), mean marketable fruit weight (g; (B)), harvest index (HI; (C)) and shape index (SI; (D)) under optimal (OCC) and suboptimal (SCC) calcium conditions. The selected variety (V) was grafted onto the rootstocks A6 (V/A6), A8 (V/A8) and NIBER® (V/N), as well as self-grafted (V/V). data are the mean values of n = 4 and bars correspond to the standard deviation. Different letters indicate significant differences at P < 0.05 (LSD test).
3.2. Photosynthetic Parameters
Table 1 shows the effect of calcium concentration on photosynthetic parameters at the end of the experiment (98 DAT). Attend to AN, AN/E and AN/Ci parameters, non-significant differences were observed On the other side, the highest gS value was for V/A6 under OCC treatment and the lowest values were measured in V/N for OCC and SCC The Ci parameter and AN/gS ratio showed the highest values for V/A6 at OCC and V/N at OCC, respectively.

Table 1.
Effects of calcium concentration and plant combinations on the net CO2 assimilation rate (AN), stomatal conductance (gS), substomatal CO2 concentration (Ci), transpiration rate (E), instantaneous water use efficiency (AN/E), intrinsic water use efficiency (AN/gS) and instantaneous carboxylation efficiency (AN/Ci) of pepper leaves at the end of the experiment (98 DAT).
The maximum quantum yield of chlorophyll fluorescence (Fv/Fm; Table S1) did not show significant differences among plant combinations and Ca treatment, with Fv/Fm values near or high to 0.8 indicating no photoinhibition processes.
3.3. Mineral Composition in Leaves and Fruits
The use of different rootstocks and treatments caused significant differences in mineral concentration in the leaves of the scion (Table 2). Potassium was the mineral with the highest concentration with respect to N, P, Ca, Mg and S, but K did not show significant differences for any plant combination and treatments. Total nitrogen was the second mineral with the highest concentration in leaves; the maximum leaf concentration was found in V/N in OCC with significant differences. Mg and S exhibited the highest concentration for both ions under SCC in V/N and V/A8 plant for Mg and only for V/N in the S. Ca contribution showed the highest significant differences in V/N under SCC, which was the plant combination with the highest capacity for Ca assimilation.

Table 2.
Effects of calcium concentration and plant combinations on mineral composition (N total, P, K, Ca, Mg and S—g/kg DW) of pepper leaves at the end of the experiment (98 DAT).
Like leaves, K concentration had the highest concentration in the fruits (Table 3) but without significant differences in the ANOVA test, as well as S. Total N and Ca showed the highest values in V/N under OCC and V/A6 under SCC. Phosphorus analysis had the lowest concentration in V/A6 plants for both treatments.

Table 3.
Effects of calcium concentration and plant combinations on mineral composition (N total, P, K, Ca, Mg and S—g/kg DW) of pepper fruits at the end of the experiment (98 DAT).
3.4. Organic Acid Contents in Pepper Fruits
The most abundant organic acids in fruits at the end of the experiment were malate and citrate (Table 4), with a mean concentration similar between them (9.80 and 9.94 g Kg DW−1, respectively). For malate, the maximum concentration was in OCC treatment for V/A6 and V/A8 and in SCC for V/N. Conversely, the citrate level was higher in SCC treatment for V/N and V/A8 plants. The highest concentration for isocitrate and tartrate acid content was observed in V/N under SCC treatment.

Table 4.
Effects of calcium concentration and plant combinations on organic acid compositions (malate, tartrate, oxalate, citrate and isocitrate—g/kg DW) of pepper fruits at the end of the experiment (98 DAT).
3.5. Fruit Quality
The analysis of fruit quality parameters (Table 5) revealed that some of them did not show significant differences such as IP%, EP%, EP/IP ratio, firmness and EC. However, fruit dry weight displayed the highest values for V/A8 in OCC and SCC with significant differences. Additionally, calcium supply had a significant effect on TSS, and the highest concentration for V/A8 under SCC treatment followed V/V at SCC and OCC. pH values showed the highest values under SCC in V/A8.

Table 5.
Effects of calcium concentration and plant combinations ondry weight (FDW), percentage of inedible (IP) and edible (EP) part, ratio between edible and inedible parts (EP/IP), firmness, total soluble solids (TSS) and pH of pepper fruits at 84 DAT.
4. Discussion
Vegetable grafting is an eco-friendly technique to improve plant growth and marketable fruits [2,3] enhancing nutrient and water uptake. Among all the demanded macronutrients in plants, calcium deficiency is one of the most important ones, but how grafted plants could improve Ca efficiency uptake is an almost unexplored field, so it claims special attention. In this study, we analysed if the grafting technique can be useful for improving pepper production under suboptimal calcium conditions mediated by agronomic and physiological approaches.
Regarding the agronomical parameters studied herein, we have demonstrated that NIBER® (N) rootstock, previously classified as tolerant to salt and water stresses [19,24], improved commercial production, as well as reduced the non-commercial production when compared to self-grafted (V/V) or V/A6 and V/A8 plants under sub-optimal Ca concentration (SCC) (Figure 1). It is a positive effect of N rootstock given that a low Ca concentration could have resulted in an increase of BER [25,26]. The increase in commercial production in V/N plants was positively joined to an increase in mean commercial fruit weight (Figure 1C and Figure 2B). Additionally, plants grafted onto N rootstock also reached the highest biomass, a symptom of its high vigour (Figure 2A) in OCC and SCC conditions. Our previous results [19,24] using N as rootstock have demonstrated a higher root length under control and abiotic stress indicating that this rootstock could help absorb a larger amount of water and nutrients. Similar studies have also demonstrated that grafting improves production under low nutrient availability, such as in the case of [27], where authors demonstrated that grafting cotton onto a tolerant rootstock improved HI, biomass and K+ uptake under low K+ concentration. Similarly, nitrogen uptake was improved in [28] when high-efficiency tomato rootstock was used, improving aerial and root biomass.
Mineral deficiency affects photosynthetic parameters [15,28]. An optimal ion influx by roots allows increased CO2 fixation and improves the photosynthesis rate in the scion [29,30]. Herein, we focused on the effects of calcium concentration levels on photosynthetic parameters. In V/N under both Ca concentrations, gS and E were reduced with improved instantaneous water use efficiency (AN/gS) compared to the other plant combinations studied herein (Table 1), which in turn was positively correlated with ADW (R = 0.79). It would mean that V/N plants needed a smaller stomatal aperture to maintain photosynthesis, which minimized water losses and thus improved biomass and production. Additionally, the fact that Ci was not significantly modified in SCC with respect to OCC in this plant combination could reveal only stomatal limitations. Improved tolerance to nutrient deficiency by the grafting technique has been reflected in changes in gas exchange parameters in other horticultural species, such as the case of watermelon under low Mg concentration [15] or tobacco under K deficiency [31].
Grafting influences the absorption and translocation of macronutrients and micronutrients mediated by rootstock [32]. In our work, after subjecting pepper plants to SCC during all periods of growing and fructification, differential nutrient accumulation has been detected in both studied organs. The fact that NIBER® rootstock improved Ca concentration, especially in leaves (Table 2), compared to the rest of the plant combinations denoted a better Ca uptake and accumulation due to better plant development. As consequence, processes where Ca is involved (i.e., part of cell membranes and walls, a counter-cation in the vacuole or as a second messenger [33]) were expected to be more active than in the other three plant combinations. Indeed, peach plants grafted onto tolerant rootstocks were demonstrated to have less lipid membrane damage, more phenolic content, and increased cortex and xylem area under low calcium availability, which authors suggested mitigated Ca deficiency [34].
Not only NIBER® has been associated with better Ca uptake under SCC. This is the case of increased nitrogen under OCC in both leaves and fruits and S in V/N leaves under SCC (Table 2 and Table 3). Both nutrients are linked to the synthesis of proteins and amino acids [35], which makes them essential for plant growth and development. In this line, previous studies have demonstrated that improving tolerance to Ca deficiency can improve hypoxia stress tolerance by increasing levels of N content and N-related enzymes in the roots of muskmelon [36]. Similarly, foliar application of both Ca and S improves tolerance to chromium toxicity in tomato and eggplant by the mediation of NO formation [37], which reflects the great importance of looking for mechanisms to improve nutrient uptake.
Nutrient deficiency modifies fruit quality characteristics [38,39], but herein reduced Ca availability did not alter, in general terms, pepper quality parameters when the different rootstock combinations under SCC were compared to OCC or self-grafted plants (Table 5). However, interesting results were found regarding fruit organic acid content evaluated, reflected in the modification of almost all of them under the two Ca concentrations studied (Table 4). Organic acids are abundant constituents of fruits and they determine many fruit characteristics such as sourness and flavour, as well as possess many health-related benefits [40,41]. Since they can be modified by the selected rootstock, their study is remarkable [42].
Malate and citrate, the most abundant organic acids in many fruits, are proposed in the bibliography as responsible for pH regulation [43,44] but herein we did not find a significant correlation between both parameters (R = −0.29 and 0.34, respectively). Consequently, the increased citrate concentration of V/N with respect to the other pepper grafting combinations played alternative roles in this plant combination. High levels of citric acid are proposed as key to improving taste and quality as well as delaying fruit senescence in Citrus spp. fruits [41], as well as improving general fruit quality in the case of tomatoes [45], so similar functions could be attributed to fruits of the tolerant pepper combination of this experiment.
Even if the tartrate concentration was lower than in the case of malate and citrate, it is worth mentioning since its concentration under SCC increased almost 30% in V/N compared to the V/V pepper combination. Its increase may have contributed as well to fruit acidity and improved fruit quality, as has been demonstrated in Vitis vinifera fruits [46].
5. Conclusions
In the present study, we conclude that graft can be a successful technique to overcome Ca deficiency when the appropriate rootstock is used. In our experimental conditions, grafting onto pepper rootstock NIBER® (N) displays higher tolerance to Ca deficiency compared with the other grafted plant combinations. This has been testified by better plant growth and development, as well as enhanced commercial fruit production and a decrease in non-marketable fruits associated with a higher Ca concentration under SCC. These results can be attributed to improved instantaneous water use efficiency (AN/gS), obtaining the maximum photosynthesis efficiency with the minor stomata opening. Differential Ca supply affect the organic acid composition in the fruit where V/N showed higher levels in tartrate, citrate and isocitrate in V/N, which contribute to better fruit quality. However, the specific mechanisms of nutrient uptake and fruit metabolite synthesis and accumulation remain unanswered, so further study is necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12071644/s1, Table S1: Effect of calcium concentration on maximum efficiency of PSII (Fv/Fm) in five periods of measurements (37 DAT, 51 DAT, 71 DAT, 84 DAT, 98 DAT) in the different grafting combinations studied.
Author Contributions
Conceptualization, Á.C., G.C. and Y.R.; methodology, L.L.-S.; software, L.L.-S.; validation, L.L.-S.; formal analysis, L.L.-S.; investigation, L.L.-S.; resources, L.L.-S., Á.C., M.C., G.C. and Y.R.; data curation, L.L.-S.; writing—original draft preparation, L.L.-S., Á.C., M.C., G.C. and Y.R.; writing—review and editing, L.L.-S., Á.C., M.C., G.C. and Y.R.; visualization, Á.C., G.C. and Y.R.; supervision, Á.C., G.C. and Y.R.; project administration, Á.C., G.C. and Y.R.; funding acquisition, G.C. and Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
Lidia López-Serrano was beneficiary of a doctoral fellowship (FPI-INIA) associated with the project funded by INIA RTA2017-00030-C02-00 and the European Regional Development Fund (ERDF). A.C. gratefully the Grant PID2020-118824RR-C21 funded by MCIN/AEI/10.13039/501100011033 and, by the “European Union”. The authors gratefully acknowledge MIUR (Minister for education, University and Research) for financial support (Law 232/216, Department of excellence).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the support of Simona Proietti (from the Italian National Research Council) in the photosynthetic measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gisbert-Mullor, R.; Ceccanti, C.; Padilla, Y.G.; López-Galarza, S.; Calatayud, Á.; Conte, G.; Guidi, L. Effect of Grafting on the Production, Physico-Chemical Characteristics and Nutritional Quality of Fruit from Pepper Landraces. Antioxidants 2020, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Penella, C.; Calatayud, A. Pepper Crop under Climate Change: Grafting as an Environmental Friendly Strategy. In Climate Resilient Agriculture—Strategies and Perspectives; Shanker, A., Shanker, C., Srinivasarao, C., Eds.; InTech: London, UK; Houston, TX, USA, 2018; pp. 129–155. [Google Scholar]
- Penella, C.; Nebauer, S.G.; López-Galarza, S.; Quiñones, A.; San Bautista, A.; Calatayud, Á. Grafting pepper onto tolerant rootstocks: An environmental-friendly technique overcome water and salt stress. Sci. Hortic. 2017, 226, 33–41. [Google Scholar] [CrossRef]
- Mayorga-Gómez, A.; Nambeesan, S.U.; Coolong, T.; Carlos Díaz-Pérez, J. Temporal Relationship between Calcium and Fruit Growth and Development in Bell Pepper (Capsicum annuum L.). HortScience 2020, 55, 906–913. [Google Scholar] [CrossRef]
- Hagassou, D.; Francia, E.; Ronga, D.; Buti, M. Blossom end-rot in tomato (Solanum lycopersicum L.): A multi-disciplinary overview of inducing factors and control strategies. Sci. Hortic. 2019, 249, 49–58. [Google Scholar] [CrossRef]
- Thor, K. Calcium—nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable grafting: A toolbox for securing yield stability under multiple stress conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Mullor, R.; Padilla, Y.G.; Martínez-Cuenca, M.R.; López-Galarza, S.; Calatayud, Á. Suitable rootstocks can alleviate the effects of heat stress on pepper plants. Sci. Hortic. 2021, 290, 110529. [Google Scholar] [CrossRef]
- Padilla, Y.G.; Gisbert-Mullor, R.; López-Serrano, L.; López-Galarza, S.; Calatayud, Á. Grafting Enhances Pepper Water Stress Tolerance by Improving Photosynthesis and Antioxidant Defense Systems. Antioxidants 2021, 10, 576. [Google Scholar] [CrossRef]
- Ropokis, A.; Ntatsi, G.; Kittas, C.; Katsoulas, N.; Savvas, D. Effects of Temperature and Grafting on Yield, Nutrient Uptake, and Water Use Efficiency of a Hydroponic Sweet Pepper Crop. Agronomy 2019, 9, 110. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Nawaz, M.A.; Jiao, Y.; Zheng, Z.; Shi, X.; Xie, W.; Yu, Y.; Guo, J.; Zhu, S.; et al. Using rootstock to increase watermelon fruit yield and quality at low potassium supply: A comprehensive analysis from agronomic, physiological and transcriptional perspective. Sci. Hortic. 2018, 241, 144–151. [Google Scholar] [CrossRef]
- Savvas, D.; Colla, G.; Rouphael, Y.; Schwarz, D. Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci. Hortic. 2010, 127, 156–161. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use efficiency traits of mini-watermelon in response to grafting and nitrogen-fertilization doses. J. Plant Nutr. Soil Sci. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Hua, B.; Liu, Z.; Fan, M.; Bie, Z. Grafting onto different rootstocks as a means to improve watermelon tolerance to low potassium stress. Sci. Hortic. 2013, 149, 80–85. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Nawaz, M.A.; Chen, C.; Liu, L.; Lu, Z.; Kong, Q.; Cheng, F.; Bie, Z. Improving magnesium uptake, photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium. Plant Soil 2016, 409, 229–246. [Google Scholar] [CrossRef]
- Li, C.; Yu, X.; Bai, L.; He, C.; Li, Y. Responses of miRNAs and their target genes to nitrogen- or phosphorus-deficiency in grafted cucumber seedlings. Hortic. Environ. Biotechnol. 2016, 57, 97–112. [Google Scholar] [CrossRef]
- Maroto Borrego, J.V.; Baixauli Soria, C. Cultivos Hortícolas al Aire Libre; Maroto Borrego, J.V., Baixauli Soria, C., Eds.; Cajamar Caja Rural: Cajamar, Peru, 2017; ISBN 978-84-95531-82-7. [Google Scholar]
- Gaion, L.A.; Braz, L.T.; Carvalho, R.F. Grafting in Vegetable Crops: A Great Technique for Agriculture. Int. J. Veg. Sci. 2017, 24, 85–102. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Pascual-Seva, N.; Martínez-Gimeno, M.A.; López-Serrano, L.; Marín, E.B.; Pérez-Pérez, J.G.; Bonet, L.; Padilla, Y.G.; Calatayud, Á.; Pascual, B.; et al. Grafting onto an appropriate rootstock reduces the impact on yield and quality of controlled deficit irrigated pepper crops. Agronomy 2020, 10, 1529. [Google Scholar] [CrossRef]
- López-Serrano, L.; Penella, C.; San-Bautista, A.; López-Galarza, S.; Calatayud, A. Physiological changes of pepper accessions in response to salinity and water stress. Spanish J. Agric. Res. 2017, 15, 15. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Temperini, O.; Rea, E.; Salerno, A.; Pierandrei, F. Influence of grafting on yield and fruit quality of pepper (Capsicum annuum L.) grown under greenhouse conditions. Acta Hortic. 2008, 782, 359–363. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Moing, A.; Renaud, C.; Gaudillère, M.; Raymond, P.; Roudeillac, P.; Denoyes-Rothan, B. Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 2001, 126, 394–403. [Google Scholar] [CrossRef]
- López-Serrano, L.; Canet-Sanchis, G.; Vuletin Selak, G.; Penella, C.; San Bautista, A.; López-Galarza, S.; Calatayud, Á. Physiological characterization of a pepper hybrid rootstock designed to cope with salinity stress. Plant Physiol. Biochem. 2020, 148, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Shono, M.; Egawa, Y. Localization of calcium in the pericarp cells of tomato fruits during the development of blossom-end rot. Protoplasma 2003, 222, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.S.; García-Sánchez, F.; Rubio, F.; Martínez, V. Yield, blossom-end rot incidence, and fruit quality in pepper plants under moderate salinity are affected by K+ and Ca2+ fertilization. Sci. Hortic. 2009, 119, 79–87. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, C.; Wang, X.; Chen, F. Studies on potassium uptake and use efficiency of different cotton (Gossypium hirsutum L.) genotypes by grafting. J. Food Agric. Environ. 2013, 11, 472–476. [Google Scholar]
- Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Grafting Enhances the Photosynthesis and Nitrogen Absorption of Tomato Plants Under Low-Nitrogen Stress. J. Plant Growth Regul. 2021, 41, 1714–1725. [Google Scholar] [CrossRef]
- Therby-Vale, R.; Lacombe, B.; Rhee, S.Y.; Nussaume, L.; Rouached, H. Mineral nutrient signaling controls photosynthesis: Focus on iron deficiency-induced chlorosis. Trends Plant Sci. 2022, 27, 502–509. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, S.; Bian, T.; Wu, T.; Li, X.; Fu, H.; Sun, Z.; Li, T. Photosynthetic characteristics combined with metabolomics analysis revealed potential mechanisms of cucumber (Cucumis sativus) yield reduction induced by different phosphorus stresses. Sci. Hortic. 2022, 302, 111156. [Google Scholar] [CrossRef]
- Hu, W.; Di, Q.; Wei, J.; Zhang, J.; Liu, J. Grafting Tobacco onto Nutrient-efficient Rootstocks Improves Photosynthesis. J. Am. Soc. Hortic. Sci. 2021, 146, 286–293. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz Juan, M. Romero L Role of grafting in horticultural plants under stress condition. Food Agric. Environ. 2003, 1, 70–74. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Keles, H.; Bozkurt, E. Physiological and histological responses of peach plants grafted onto different rootstocks under calcium deficiency conditions. Sci. Hortic. 2021, 281, 109967. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T. Calcium (Ca) and Sulfur (S) for Citrus Trees. EDIS 2013, 7, 1–5. [Google Scholar] [CrossRef]
- Gao, H.; Jia, Y.; Guo, S.; Lv, G.; Wang, T.; Juan, L. Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J. Plant Physiol. 2011, 168, 1217–1225. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M. Regulation of chromium toxicity tolerance in tomato and brinjal by calcium and sulfur through nitric oxide: Involvement of enzymes of sulfur assimilation and the ascorbate-glutathione cycle. Environ. Exp. Bot. 2019, 166, 103789. [Google Scholar] [CrossRef]
- Asao, T.; Asaduzzaman, M.; Mondal, M.F.; Tokura, M.; Adachi, F.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T. Impact of reduced potassium nitrate concentrations in nutrient solution on the growth, yield and fruit quality of melon in hydroponics. Sci. Hortic. 2013, 164, 221–231. [Google Scholar] [CrossRef]
- Àlvarez-Fernàndez, A.; Abadía, J.; Abadía, A. Iron Deficiency, Fruit Yield and Fruit Quality. In Iron Nutrition in Plants and Rhizospheric Microorganism; Springer: Dordrecht, The Netherlands, 2006; pp. 85–101. [Google Scholar] [CrossRef]
- Duarte, A.M.; Caixeirinho, D.; Miguel, M.G.; Sustelo, V.; Nunes, C.; Fernandes, M.M.; Marreiros, A. Organic acids concentration in citrus juice from conventional versus organic farming. Acta Hortic. 2012, 933, 601–606. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.Y.; Guo, L.X.; Kamran, H.M.; Sadka, A.; Liu, Y.Z. Recent Advances in the Regulation of Citric Acid Metabolism in Citrus Fruit. CRC. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Habran, A.; Commisso, M.; Helwi, P.; Hilbert, G.; Negri, S.; Ollat, N.; Gomès, E.; Van Leeuwen, C.; Guzzo, F.; Delrot, S. Roostocks/scion/nitrogen interactions affect secondary metabolism in the grape berry. Front. Plant Sci. 2016, 7, 1134. [Google Scholar] [CrossRef] [PubMed]
- Lobit, P.; Génard, M.; Wu, B.H.; Soing, P.; Habib, R. Modelling citrate metabolism in fruits: Responses to growth and temperature. J. Exp. Bot. 2003, 54, 2489–2501. [Google Scholar] [CrossRef][Green Version]
- Bastías, A.; López-Climent, M.; Valcárcel, M.; Rosello, S.; Gómez-Cadenas, A.; Casaretto, J.A. Modulation of organic acids and sugar content in tomato fruits by an abscisic acid-regulated transcription factor. Physiol. Plant. 2011, 141, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; An, J.; Deng, W.; Gao, Y.; Chen, Z.; Li, Z. Metabolic and transcriptional regulatory mechanism associated with postharvest fruit ripening and senescence in cherry tomatoes. Postharvest Biol. Technol. 2020, 168, 111274. [Google Scholar] [CrossRef]
- De Angeli, A.; Baetz, U.; Francisco, R.; Zhang, J.; Chaves, M.M.; Regalado, A. The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta 2013, 238, 283–291. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).