Abstract
Morchella importuna is a highly priced edible and medicinal mushroom. Crop rotation is an important management technique to improve soil health. In this study, the morphological characteristics, chemical composition, and nutritional quality of the M. importuna fruitbody under five different rotation systems (named RSA to RSE) were investigated. The results showed that the fruitbodies of M. importuna in rotation system C (RSC, Rice–Pea–M. importuna rotation) grew best (with the highest yield of 6804.90 kg/hm2) and were of higher quality, which showed significant increases in crude protein (37.32 g/100 g) and decreases in crude fat (4.04 g/100 g), crude fiber (10.06 g/100 g), and total ash (9.32 g/100 g). The heavy metal contents (Pb, Cd, and Hg) in the fruitbodies from all rotation systems were rare or none, which meets the standards of the Chinese Green Food Standard and the maximum limit in foodstuffs of the European Union Standard. In addition, the free amino acid compositions of morel under different rotation systems were analyzed. The ratio of essential amino acids to total amino acids (EAA/TAA) was highest in RSC (37.11%). The tested morels were abundant in umami, sweet, aromatic, and medicinal amino acids (UAA, SAA, AAA and MAA). Combining amino acid score, chemical score, and other nutrition indexes, RSC had the best impact on the yield and quality of morel. Our results demonstrate the feasibility and effectiveness of the cultivation model of rotating “Rice–Vegetables–Fungi” for the production of M. importuna in a way that develops high-quality agriculture.
1. Introduction
Morels (Morchella spp., Pezizales, Ascomycota) are highly priced edible and medicinal mushrooms [1]. As a food source, the morel is widely known for its “honeycomb-like” cap [2,3], its nutrition and delicate taste, its pharmaceutical importance, and its economic value [4]. Many bioactive components were found in morels, including polysaccharides, polyphenols, proteins, trace elements, vitamins, dietary fiber, and flavonoids [5,6]. Modern phytochemical studies demonstrated that morels have lipid-lowering, anti-microbial, anti-fatigue, anti-oxidative, and immunity enhancing functions [7,8,9]. Therefore, the morels have become more and more popular in recent years.
Morels are a seasonal mushroom, and has a limited growth period during winter. Therefore, naturally grown morels are very rare and insufficient to meet the demands of consumers. Excitingly, in recent years, two species of morel (Morchella importuna and Morchella sextelata) have successfully been domesticated in China, and M. importuna has accounted for >95% of total cultivated area [10,11]. However, the yields are not yet stable in the fields [12]. There have been some studies of M. importuna focusing on cultivation characteristics [13,14,15], heavy metal ion tolerance [16,17], bioactive constituents [18,19,20], germplasm resources [21], genetic information [12,22,23], and microbial communities [24,25]. However, as an edible fungus, culture studies are still warranted for stable and high-quality production of the morel.
Morels are directly cultivated in soil. The growth of mushrooms may be limited by environmental characteristics, such as soil, pH, light, temperature, and humidity [26]. Among these, soil is one of the essential factors for mushroom fruiting. It is known that crop rotation an important management technique that can help improve soil health and reduce erosion. The diverse rotation systems using different crops such as alfalfa, canola, maize, soybean, wheat, etc. cause distinct differences in physicochemical properties [27], bacterial community composition [28], and enzyme activity [29] of soils. Moreover, crop rotation has obvious advantages, including an increase in land use efficiency, greater annual yield per unit land area, and economic benefits [30,31]. As mentioned above, the limited growth period of M. importuna makes it ideal for rotation. However, there is a lack of systematic research on the effect of different rotation systems on the M. importuna fruiting body. In this study, we analyzed the morphological characteristics, yield, nutritional ingredients, and quality of the M. importuna fruitbody under different rotation systems. The purpose of this study was to explore an optimal rotation system suitable for M. importuna, with stable yield and excellent quality of crops.
2. Materials and Methods
2.1. Raw Materials
The M. importuna strain MJC-S6 (preserved in the China National Engineering Research Center of JUNCAO Technology) was initially grown on potato dextrose agar (PDA, 200 g potato extract, 20 g glucose, 20 g agar, 1000 mL distilled water) (sterilized at 121 °C for 20 min) at 20 °C in the dark for 5 d. After growth, mycelial plugs (20 mm in length) were used as inocula for further culturing at a large scale at 20 °C in the dark for 30 d or more, until the mycelium bags were full. The mycelium bags would be used as seeds for planting. The formula for seed cultivation of M. importuna: wheat 45%, sawdust 30%, Dicranopteris pedate powder 7%, soil 15%, lime 1.5%, gesso 1%, and KH2PO4 0.5% (sterilized at 121 °C for 120 min).
The formula for external nutrition bags: wheat 65%, rice husk 26%, Dicranopteris pedate powder 6.5%, lime 1.5%, and gesso 1% (sterilized at 121 °C for 60 min). The external nutrition bags were used during fruiting of M. importuna for special nutritional supplements.
2.2. Experimental Design
The experiments were carried out from 2019 to 2020 in Yuankou Village (Latitude: 25°46′33.61″ N, Longitude: 116°14′43.69″ E), Gucheng Town, Changting County, Fujian province, China. The cultivation land was prepared in rows with a width of 100 cm, height of 15 cm, and spacing of 35 cm. A simple sunshade was built using a six-needle black shade (80–98% shading). M. importuna bags (size of bag = 15 × 30 cm) were planted (4500 bags/per hectare), covered with soil (≈3 cm) and black plastic film to maintain humidity and temperature. The plastic films were removed when the mycelia of M. importuna spread and covered the surrounding soil. The external nutrition bags with a size of 13 × 26 cm were cut (10–12 cm) and placed on the mycelia at 22,500 bags/per hectare, keeping the incision facing down.
The experiment had five different rotation systems, and each rotation used a randomized block method in the field. The area of a small plot was 12 m2, and each rotation system was repeated five times.
2.3. Rotation Systems
“Vegetables–Vegetables–Fungi” and “Rice–Vegetables–Fungi” systems were used. Considering local phenological conditions, five representative rotation systems (RS) were chosen and marked as RSA, RSB, RSC, RSD, and RSE, respectively (Table 1).

Table 1.
The rotation systems.
2.4. Determination Indexes and Methods
2.4.1. Morphological Characteristics
The lengths and diameters of the M. importuna pileus and stipe were measured using a Vernier caliper. The perimeters of the pileus and stipe were calculated from the diameter.
2.4.2. Yield
The weight of the M. importuna fruitbodies were measured using an electronic balance. Fresh weight, dry weight, fresh yield, and dry yield were calculated using the following formula:
Fresh weight of one fruitbody = Total fresh weight of fruitbodies (three harvests)/Total number of fruitbodies (three harvests),
Dry weight of one fruitbody = Total dry weight of fruitbodies (three harvests)/Total number of fruitbodies (three harvests),
Fresh yield of a small plot = Fresh weight of one fruitbody × Total number of fruitbodies in one plot (three harvests),
Dry yield of a small plot = Dry weight of one fruitbody × Total number of fruitbodies in one plot (three harvests).
2.4.3. Nutrients
The M. importuna fruitbodies were smashed after low temperature drying (40 °C for 1.5 h, 55 °C for 2 h, then 65 °C for 3 h or more), then kept in a dryer (RT) prior to analysis.
The ash content of was analyzed according to the method used by the National Standard of China, GB 5009.4-2016 [32]. The crude fiber content was determined according to the method used by the National Standard of China, GB/T 5009.10-2003 [33]. The crude protein content was determined according to the Kjedahl method (National Standard of China, GB 5009.5-2016 [34]). The crude fat content was determined according to the National Standard of China, GB 5009.6-2016 [35].
2.4.4. Heavy Metals
The quality of M. importuna fruitbodies would be affected by heavy metal concentrations, which increases with more soil pollution. The contents of heavy metals (Pb, Cd) were determined using atomic absorption spectrometry (graphite furnace), according to the method used by the National Standard of China, GB 5009.12-2017 [36] and GB 5009.15-2014 [37]. The content of Hg was analyzed according to GB 5009.17-2014 [38] using atomic fluorescence spectrometry.
2.4.5. Amino Acids
Amino acids were analyzed and determined by ion-exchange chromatography with post-column derivatization with ninhydrin [39]. Chemical scores were determined using the methods of the Food and Agriculture Organization of the United Nations [40]. The amino acid score, essential amino acid index, biological value, and nutrition index were determined according to methods described by Bano et al. [41].
2.5. Data Processing
The processing and analysis of the experimental data was conducted using Excel 2010 and SPSS 23.0. One-way ANOVAs and LSD were used to analyze all experimental data.
3. Results
3.1. Effect of Different Rotation Systems on the Morphological Characteristics and Yield of M. importuna Fruitbody
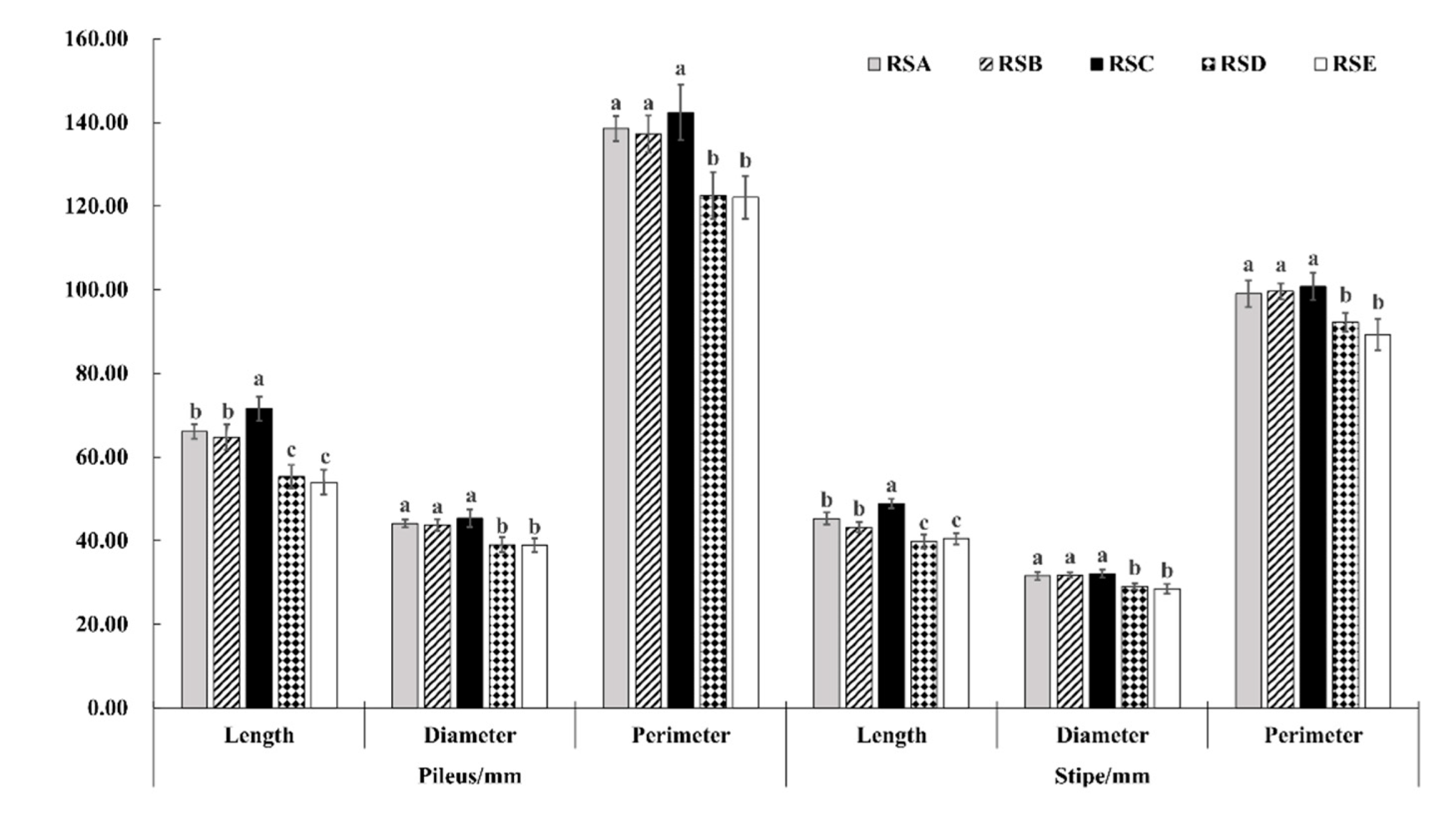
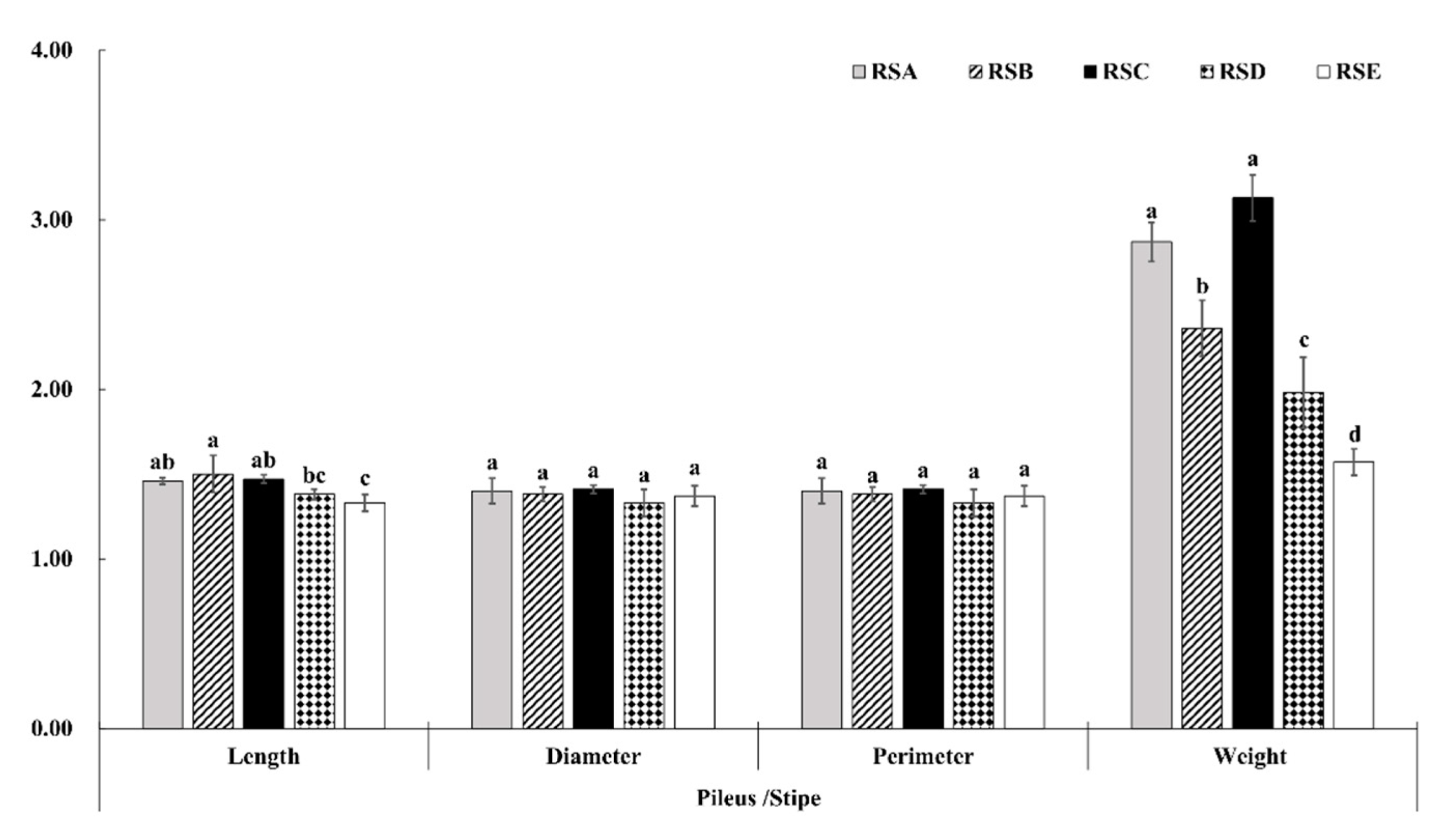
The morphological characteristics and yield of the M. importuna fruitbody appeared to have a significant response to the different rotation systems (Figure 1, Figure 2 and Figure 3). The fruit bodies (pileus and stipe) were significantly larger in RSA and RSC compared with the other rotation systems. The length, diameter, and perimeter of the fruit body (pileus and stipe) were largest in RSC, measuring 71.53, 45.36, and 142.42 mm for the pileus, and 48.86, 32.10, and 100.79 mm for the stipe, respectively (Figure 1). In addition, there was no difference in the growth trend of morphological characteristics between the pileus and stipe in the same rotation system (Figure 2). In contrast, the weight ratio of pileus to stipe (Wp/Ws) showed a significant difference, with RSC being the largest, followed by RSA. The Wp/Ws of RSC and RSA were 3.13 and 2.87, respectively, an increase of 99.36 and 82.80% compared with that of RSE, the rotation system with the lowest weight ratio. The effect of the five rotation systems on the morphological characteristics of the M. importuna fruitbody, in descending order, was RSC > RSA > RSB > RSD > RSE.
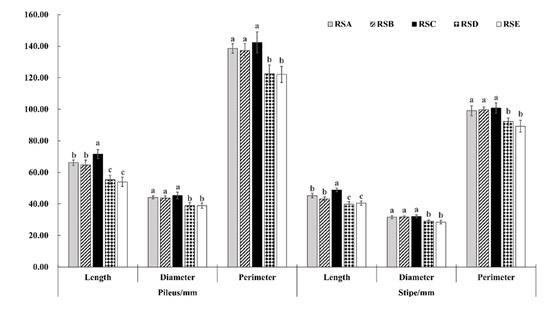
Figure 1.
Effect of different rotation systems on morphological characteristics of M. importuna, including the length, diameter, and perimeter of the fruitbody (pileus and stipe); different letters indicate the statistical difference (p < 0.05).
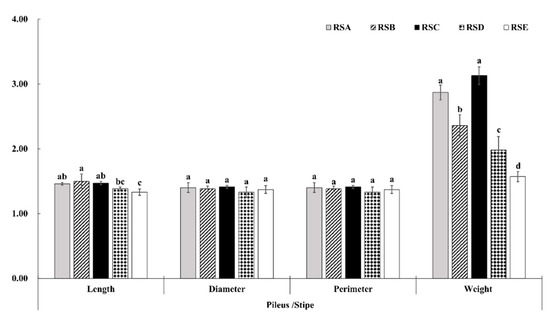
Figure 2.
Effect of different rotation systems to the morphologic proportion of M. importuna, including the length, diameter, perimeter, and weight of the fruitbody (pileus/stipe); different letters indicate a statistical difference (p < 0.05).
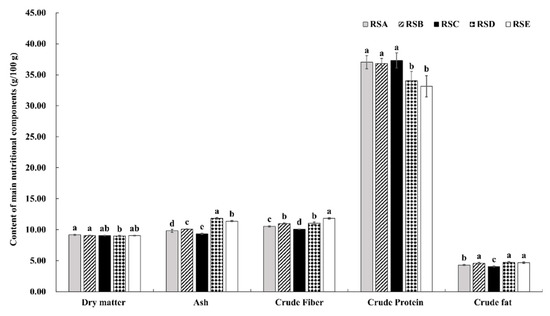
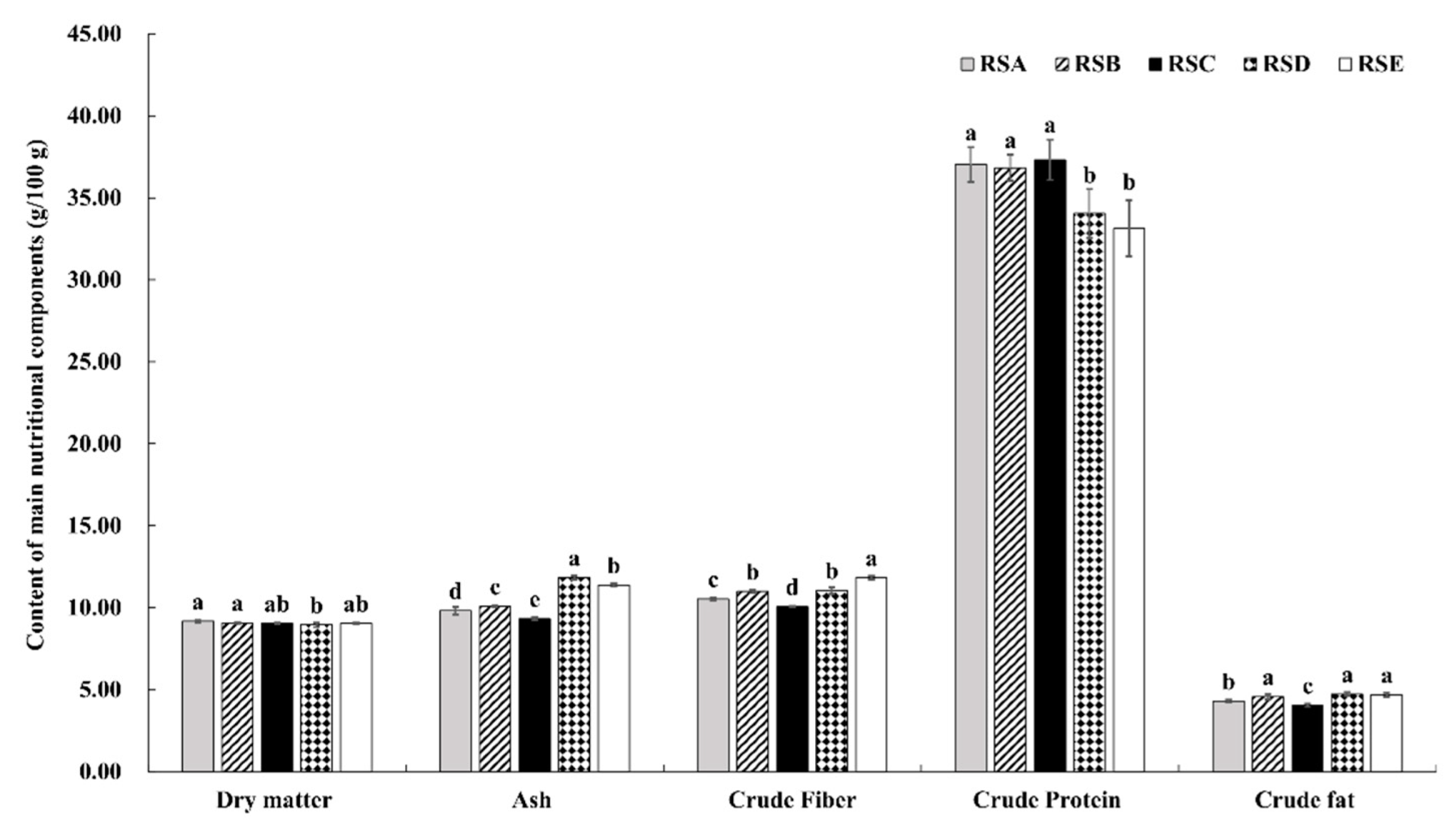
Figure 3.
The content of nutrients (dry matter, ash, crude fiber, crude protein, and crude fat) under different rotation systems; different letters indicate a statistical difference (p < 0.05).
The influence of different rotation systems on the yields of M. importuna was analyzed. As shown in Table 2, the average fresh weight per fruit body was 21.09 g in RSC, with an increase of 2.68, 15.59, 28.44, and 32.98%, respectively, compared with RSA, RSB, RSD, and RSE. In addition, the equivalent yield of fresh M. importuna was the highest (6804.90 kg/hm2) in RSC, with an increase of 2.24, 6.09, 15.43, and 17.36% respectively, compared with those in RSA, RSB, RSD, and RSE. The same trends were found in the equivalent yield of dried M. importuna. These findings indicated that the growth and substance accumulation of the fruit body were affected most significantly in RSC. However, the total number of fruitbody picked in a small plot or per square meter was least in RSC, in contrast with the largest number being picked in RSE, followed by RSD. These data revealed that though RSE and RSD were conducive to fruit body formation, the substance accumulation of fruit bodies in RSE and RSD was less than in the other rotation systems, especially compared with RSC.

Table 2.
Effect of different rotation systems on yield of M. importuna. Different letters indicate a statistical difference (p < 0.05).
3.2. Effect of Different Rotation Systems on Nutritional Quality of M. importuna Fruitbody
3.2.1. Nutrient Analysis
The chemical compositions of the M. importuna fruitbody under different rotation systems were investigated on a dry weight (DW) basis. As shown in Figure 3, relatively little impact of the different rotation systems was seen on dry matter content. Overall, the fruit body of M. importuna was found to be enriched in total protein content, but was lower in crude fat, crude fiber, and total ash (Figure 3). The crude protein content of fruitbodies from the five rotation systems, in descending order, was RSC > RSA > RSB > RSD > RSE. The total crude protein per square kilometer was 227.66 kg in RSC, a respective increase of 0.99, 6.32, 26.80, and 30.76% compared with those in RSA, RSB, RSD, and RSE. In contrast, the lowest crude fat, crude fiber, and total ash (4.04, 10.06, and 9.32 g/100 g, respectively) were found in the fruitbodies from RSC. Therefore, the fruitbody from RSC was of higher quality, with a ratio of crude protein to crude fiber of 3.71, a respective increase of 5.40, 10.42, 20.45, and 32.50% compared with those in RSA, RSB, RSD, and RSE.
3.2.2. Amino Acid Composition Analysis
The free amino acid compositions of M. importuna under different rotation systems were analyzed. As shown in Table 3, the profiles of 17 amino acids: alanine, arginine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine, and valine were determined. The total amino acid (TAA) content in the analyzed samples ranged from 27.41 g/100 g in RSE to 29.09 g/100 g in RSC (Table 3). The TAA content in the fruitbodies from the five rotation systems, in descending order, was RSC > RSA > RSB > RSD > RSE, which was consistent with the influence of rotation systems on crude protein content. Overall, the content of glutamic acid was the highest, followed by aspartic acid, whereas cystine had the lowest content in the fruitbodies under the five rotation systems. Essential amino acid (EAA) content varied from 10.03 g/100 g in RSE to 10.80 g/100 g in RSC (Table 3). Seven kinds of EAA were detected under the five rotation systems (tryptophan could not be determined due to the detection method used). The content of the seven kinds of EAA, in descending order, was lysine > leucine > threonine > valine > isoleucine > phenylalanine > methionine. The ratio of EAA/TAA was 37.42–37.11%, which was highest in RSC. The ratio of EAA/NEAA was 57.29–59.02%, which was close to the Food and Agriculture Organization/World Health Organization (FAO/WHO) model (60%).

Table 3.
Amino acid composition of M. importuna under different rotation systems.
3.2.3. Flavor Amino Acid Analysis
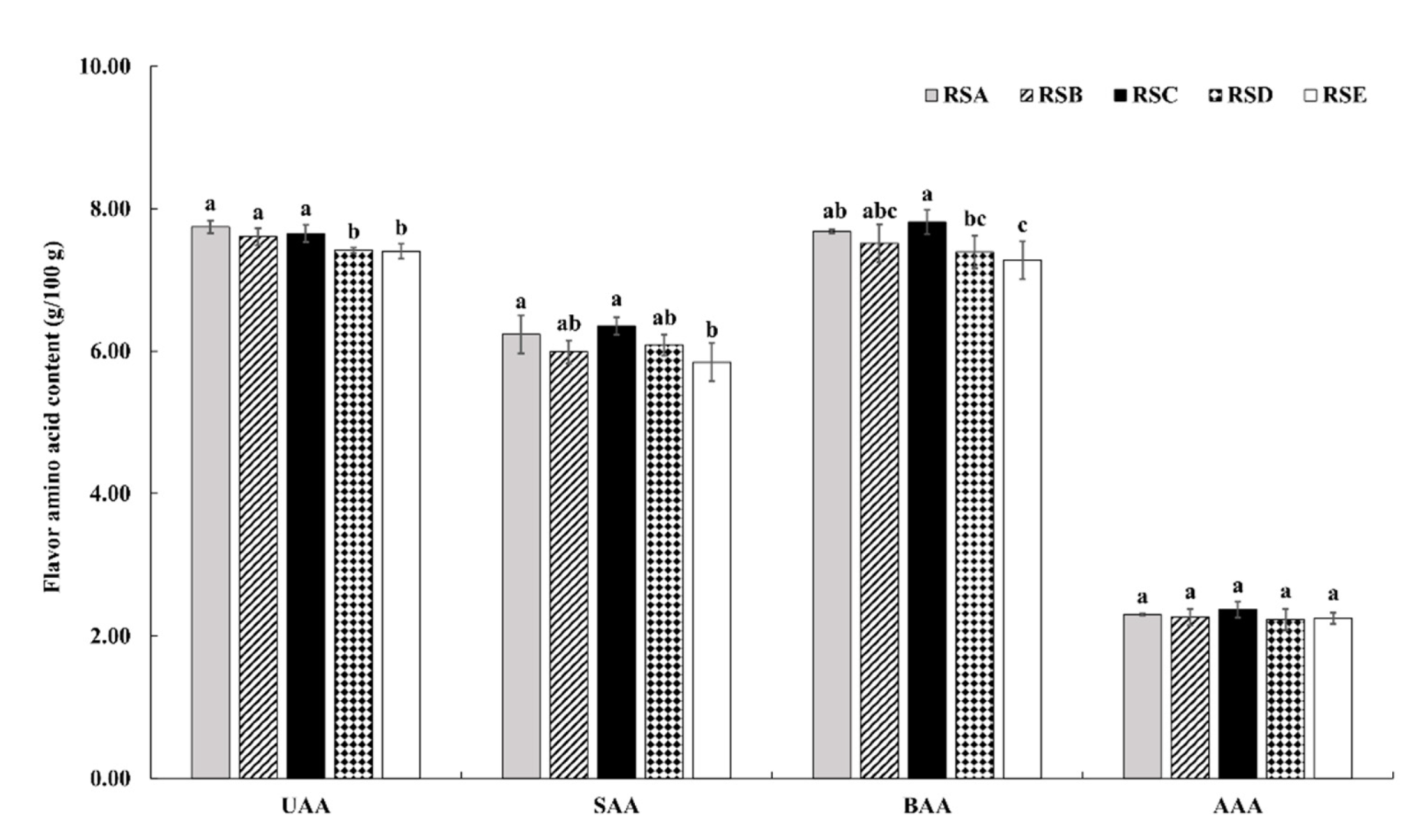
Edible fungi are rich in nutrition and delicious in taste, with umami, sweet, bitter, aromatic, and other components. Amino acids, as the first nutrient element of life activities, also play an important role in food quality and flavor. As shown in Figure 4 and Table 4, the content and proportions of four kinds of flavor amino acids were analyzed in M. importuna under the five rotation systems. As shown in Table 3, glutamate was the most important umami amino acid (UAA; e.g., Asp and Glu), with the highest content in the fruitbodies from the five rotation systems. The ratio of UAA/TAA was higher than 26% (Table 4). The content of sweet amino acids (SAA; e.g., Ser, Ala, Gly, and Pro) in the analyzed fruitbodies ranged from 5.85 g/100 g in RSE to 6.36 g/100 g in RSC, with Ala having the highest content among the SAA. The bitter amino acid (BAA) content ranged from 7.28 g/100 g in RSE to 7.82 g/100 g in RSC, with Arg having the highest content among the BAA. The content of aromatic amino acids (AAA; 2.23–2.37 g/100 g) was determined in all of the analyzed rotation systems. UAA, SAA, and AAA content varied from 15.51 to 16.39 g/100 g, with the ratio of (UAA + SAA + AAA)/TAA being 56.32–56.58%.
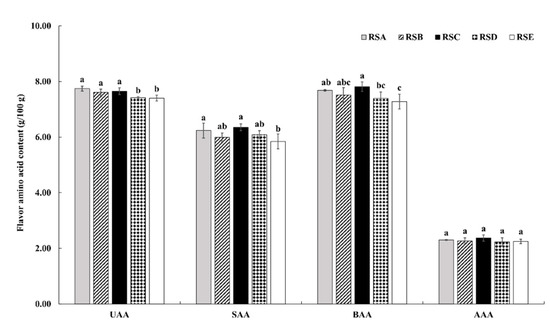
Figure 4.
Flavor amino acid composition of M. importuna under different rotation systems. UAA, umami amino acid; SAA, sweet amino acid; BAA, bitter amino acid; AAA, aromatic amino acid; different letters indicate a statistical difference (p < 0.05).

Table 4.
Flavor amino acid proportion of M. importuna under different rotation systems.
3.2.4. Medicinal Amino Acid Analysis
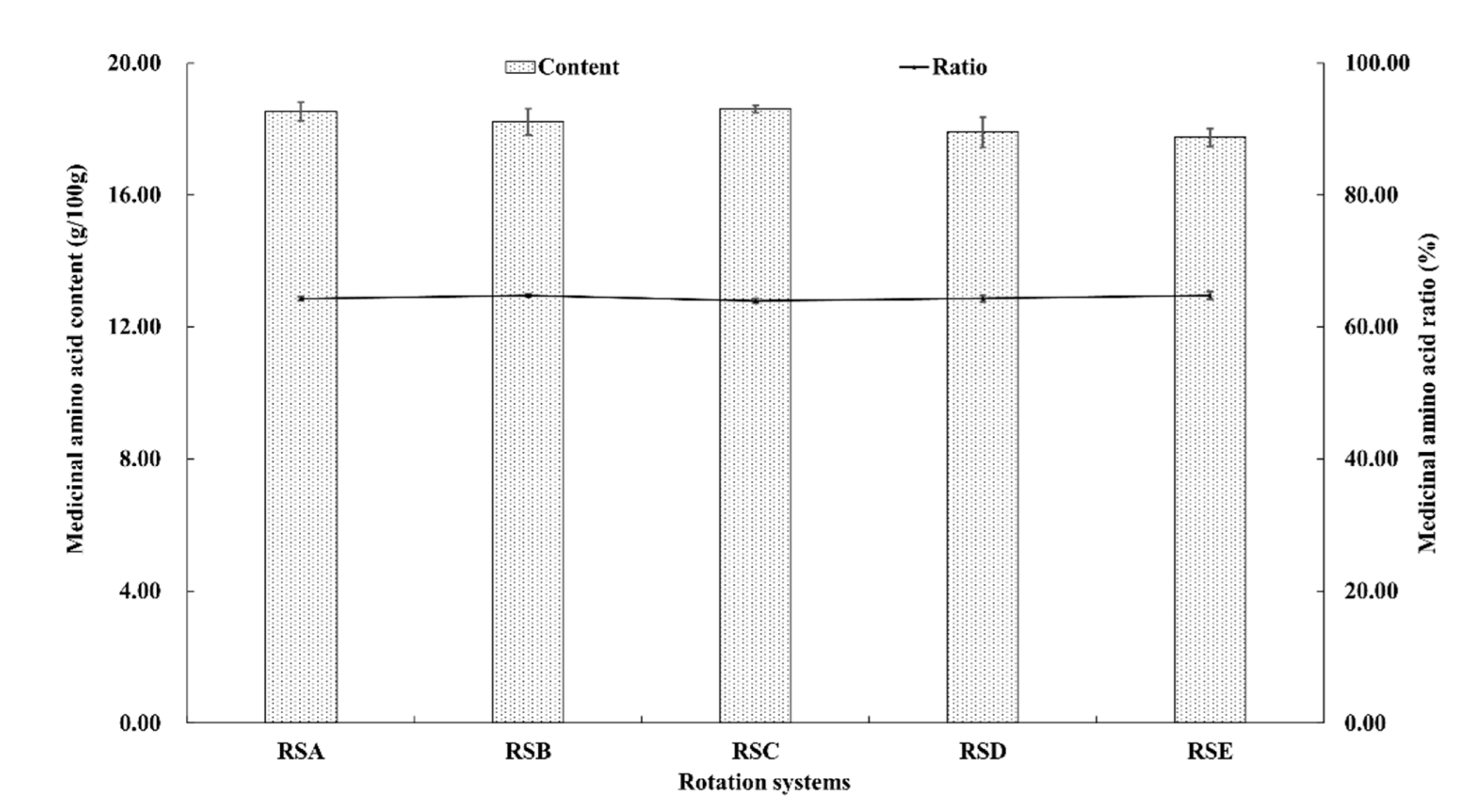
Medicinal amino acids (MAA) are known to be necessary to maintain the nitrogen balance of the body, including aspartic acid, glutamic acid, glycine, methionine, leucine, tyrosine, phenylalanine, lysine, and arginine [42]. The quantification of the identified amino compounds are shown in Figure 5 and Figure 6. The content of the nine kinds of MAA in the fruitbodies were significantly different. The content of glutamic acid was the highest, followed by aspartic acid, whereas methionine had the lowest content in the analyzed fruitbodies from all of the rotation systems (Figure 5). The proportion of MAA in total amino acids was higher than 63%, but there was no significant difference between the different rotation systems.
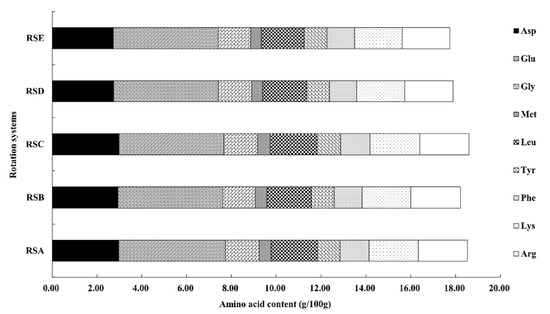
Figure 5.
Medicinal amino acid composition of M. importuna under different rotation systems, including: asparaginic acid (Asp), glutamic acid (Glu), Glycine (Gly), methionine (Met), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), lysine (Lys), and arginine (Arg).
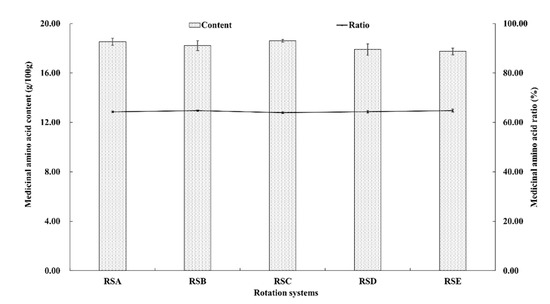
Figure 6.
The total and proportional medicinal amino acid content of M. importuna under different rotation systems.
3.2.5. Amino Acid Correlation Analysis
As shown in Table 5, TAA was positively correlated with EAA, MAA, UAA, SAA, BAA, and AAA, with correlation coefficients of 0.989, 0.987, 0.895, 0.941, 0.994, and 0.878, respectively. Therefore, the content of various amino acids would increase following the increase in TAA.

Table 5.
Correlational analysis of amino acids in M. importuna under different rotation systems.
3.3. Effect of Different Rotation Systems on Heavy Metal Content of M. importuna Fruitbody
The maximum Pb and Cd content in the fruitbodies were determined to be 0.95 and 0.91 μg/g in RSA, respectively (Table 6). And no Hg content was determined in each rotation system. These data met the standard of the Chinese Green Food Standard and the European Union standard (setting maximum levels for certain contaminants in foodstuffs). In addition, the lowest Pb and Cd content was determined to be 0.49 μg/g in RSC and 0.71 μg/g in RSD, respectively (Table 6).

Table 6.
Heavy metal content of M. importuna under different rotation systems. Different letters indicate a statistical difference (p < 0.05).
3.4. Evaluation of Nutritional Value
3.4.1. Amino Acid Score and Chemical Score
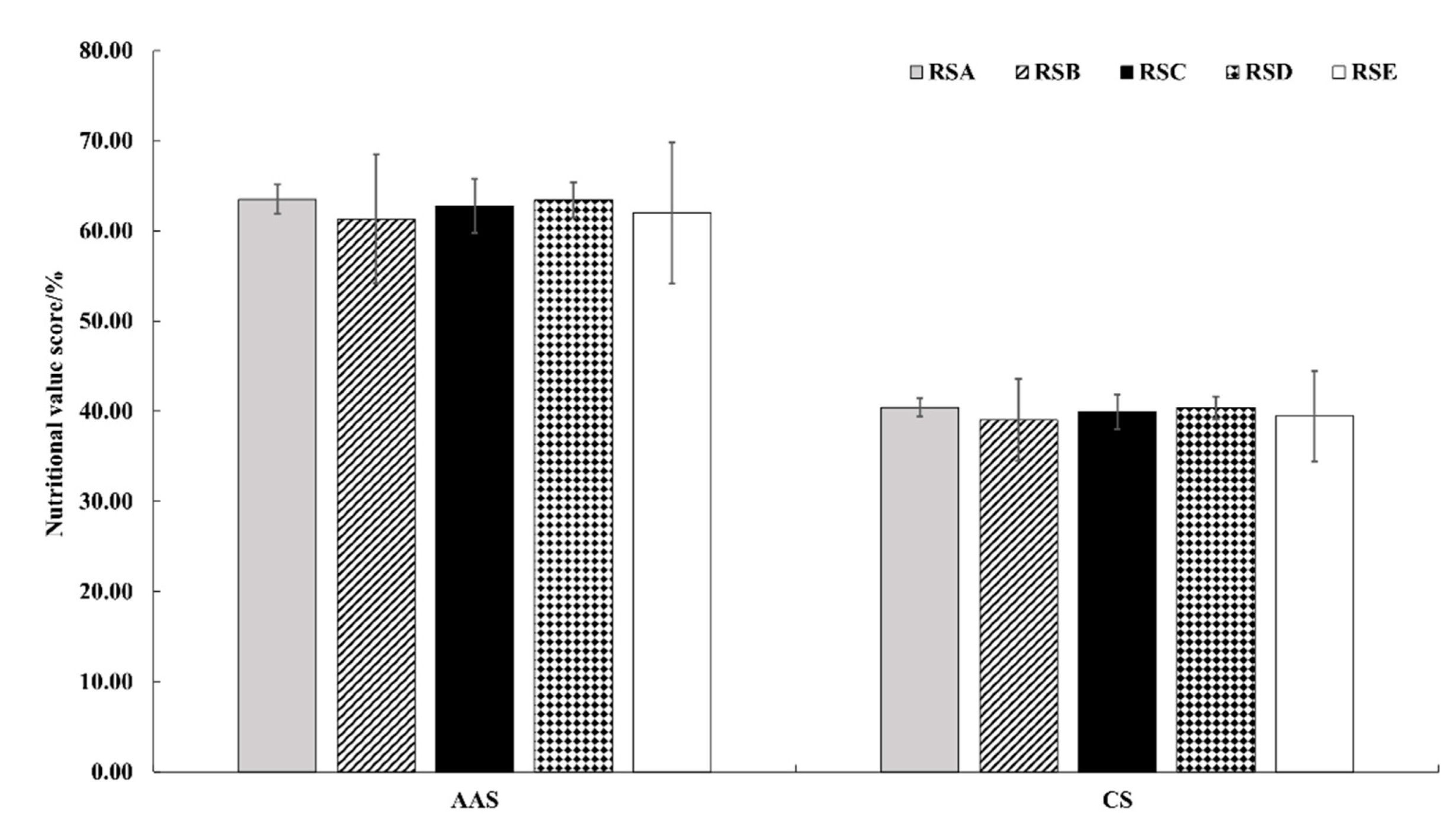
In the FAO/WHO model, the amino acid score (AAS) was used to measure the percentage of essential amino acid to the corresponding amino acid in edible fungi. The nutritional value of edible fungus protein was reflected by the score, with a full score being 100 [43]. As shown in Figure 7, the amino acid scores of the different rotation systems were 63.52 (RSA), 61.33 (RSB), 62.76 (RSC), 63.43 (RSD), and 62.00 (RSE), respectively. The different rotation systems had an effect on the amino acid scores of M. importuna, but the limiting amino acids were consistent, with methionine + cystine as the first limiting amino acid and leucine as the second limiting amino acid.
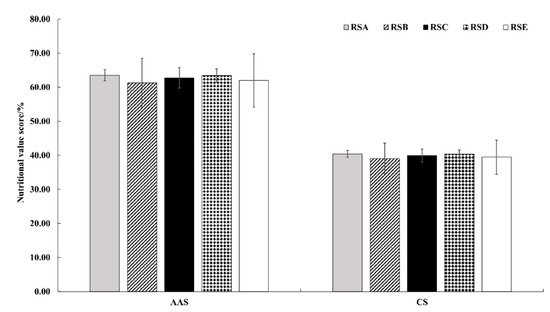
Figure 7.
Effect of different rotation systems on amino acid scores and chemical scores of M. importuna. AAS, amino acid scores; CS, chemical scores.
Chemical score (CS) was used to measure the percentage of an essential amino acid to the corresponding amino acid of edible fungi in standard egg albumen [43]. The lowest chemical score of M. importuna was in RSB, with methionine + cystine as the first limiting amino acid (Figure 7).
3.4.2. Essential Amino Acid Index, Biological Value, and Nutrition Index
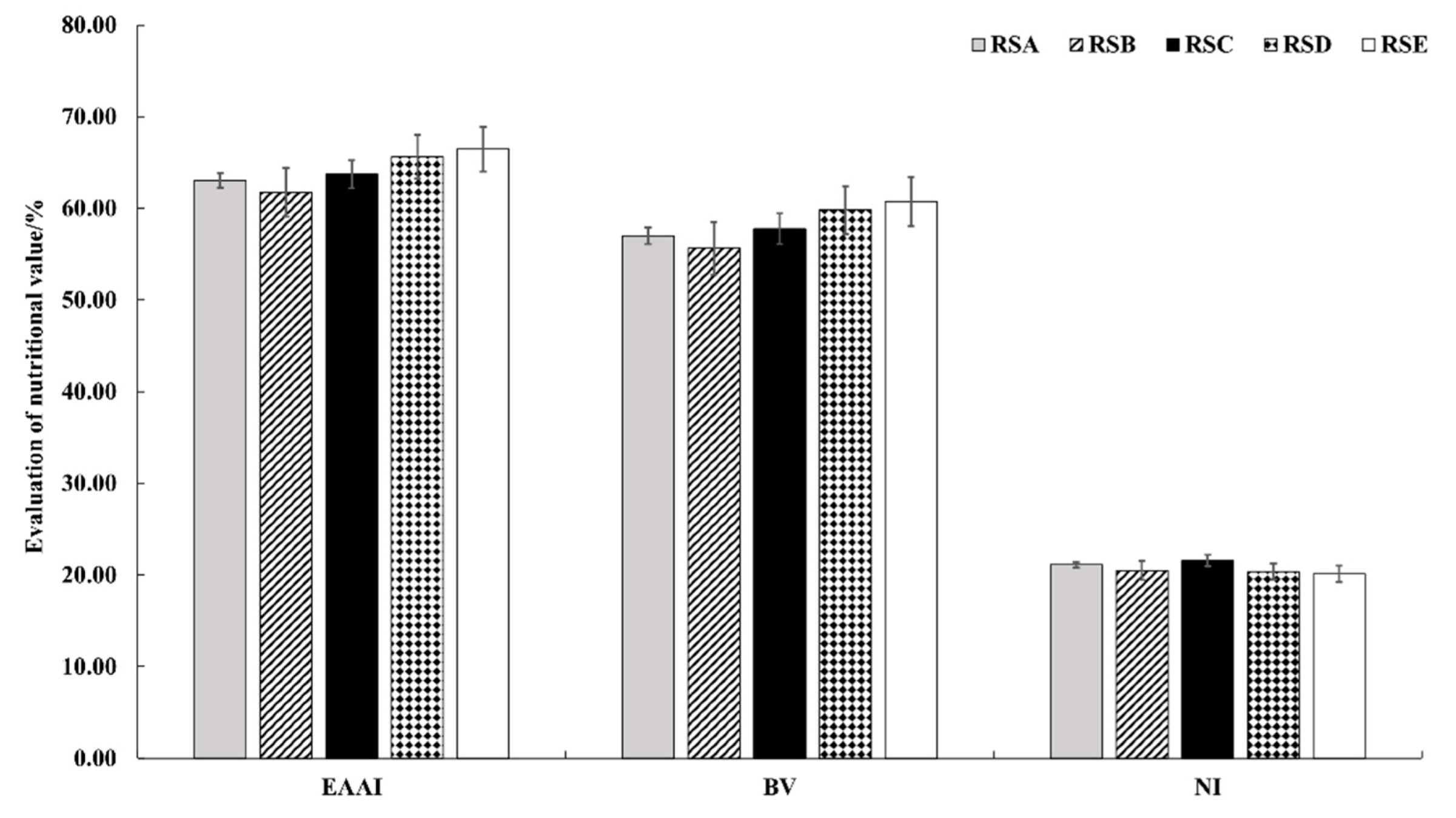
The essential amino acid index (EAAI) was first proposed by Oser B. L. to evaluate the ratio of all essential amino acids in food protein to those in standard egg albumen [44]. The biological value (BV) was the utilization efficiency of the protein after being digested and absorbed. The numerical value was proportional to the utilization efficiency. As shown in Figure 8, the EAAI under the different rotation systems varied from 61.78 in RSB to 66.47 in RSE, with the BV of 55.64–60.75 in the same trend.

Figure 8.
Effect of different rotation systems on essential amino acid indexes, biological values, and nutritional indexes of M. importuna. EAAI, essential amino acid indexes; BV, biological values; NI, nutrition indexes.
The nutrition index (NI) is a comprehensive evaluation of protein content and amino acid composition of food. The NI under the different rotation systems varied from 20.13 in RSE to 21.56 in RSC, which, in descending order, was RSC > RSA > RSB > RSD > RSE (Figure 8).
3.4.3. Correlational Analysis of Main Nutritional Indexes of M. importuna
As shown in Table 7, the NI of M. importuna was negatively correlated with ash, crude fiber, crude fat, EAAI, and BV, but had significant positive correlations with crude protein, TAA, and EAA, with correlation coefficients of 0.808152, 0.979229, and 0.987158, respectively. Overall, the NI of M. importuna would increase, following the increase in crude protein, TAA, and EAA.

Table 7.
The correlational analysis of nutritional indicators of M. importuna under different rotation systems.
4. Discussion
Previous studies have shown that crop rotation can improve land use efficiency, adjust soil structure and microenvironment [45,46], balance soil nutrients, and accumulate soil carbon [47,48], and has proven to be an important measure to promote the high-quality sustainable development of agriculture. Morels grow in soil; therefore, the soil environment is extremely important. In particular, the main nutrients needed for fruitbody formation are absorbed, transmitted, and synthesized from soil. Based on our results, different rotation systems did have an influence on the fruitbody formation of M. importuna. The effect of the five rotation systems on the morphological characteristics and yields of M. importuna fruitbody showed consistency, and in descending order was RSC > RSA > RSB > RSD > RSE. In terms of crop rotation, RSA, RSB, and RSC belong to the “Rice–Vegetables–Fungi” system, whereas RSD and RSE belong to the “Vegetables–Vegetables–Fungi” system. Thus, for morels, the “Rice–Vegetables–Fungi” system was better than the “Vegetables–Vegetables–Fungi” system. This may be related to the advantages of paddy-upland rotation, which promotes microbial diversity and improves soil quality and fertility [27,49].
In terms of chemical composition, the crude protein content from the five rotation systems showed the same trends as morphological characteristics and yields. In contrast, the content of crude fat, crude fiber, and total ash in RSC was the lowest. These findings showed that RSC had greater impact on the total nutrient content and ratio of nutrients than the rotation systems. The fruitbodies in RSC were of apparent higher quality due to obvious increases in crude protein, and reductions in crude fat, crude fiber, and total ash. The content of heavy metals was an important index to measure the quality of this edible and medicinal fungi, especially those covered with soil during fruiting. The growth and development of M. importuna needs to be completed in soil; therefore, the soil environment would directly affect its quality. The maximum Pb, Cd, and Hg content in the fruitbodies of M. importuna from all five rotation systems met the standards of the Chinese Green Food Standard and the European Union Standard. In addition, the lowest Pb content was found in RSC.
The quality of protein in foods mainly depend on the variety, content, and composition proportion of EAA, and the composition proportion is a key factor [50,51]. The composition proportion of EAA varied. There was no single food source that was found to be completely consistent with the FAO/WHO model. The highest EAA/TAA ratio of 37.11% was found in RSC.
Among amino acids, the flavor amino acids are those that contribute to the typical mushroom taste [52]. The delicious taste of mushrooms is primarily due to the presence of UAA and other small molecules [53]. The ratio of (UAA + SAA + AAA)/TAA was 56.32–56.58% in all five rotation systems, without significant differences. The high content of flavor amino acids were similar to results obtained with Lentinula edodes [54,55]. The fresh, sweet, and aromatic amino acids from the fruitbody of M. importuna accounted for their unique flavor, which give it huge potential for market development.
There was no significant difference of MAA content in the fruitbodies from the five rotation systems, but the ratio of MAA/TAA was higher than 63.95% in all tested fruitbodies, which was higher than Lycium barbarum of 61.87% [56], Eriobotrya japonica of 56.00% [57], and Hedysarum semenovii of 63.20% [58]. Glutamic acid is not an essential amino acid, but it plays an important role in the development and growth of the nervous system in organisms [59]. Involved biosynthesis, aspartic acid is mainly used in the treatment of heart disease, liver disease, hypertension, and has been proven to have an effect on relieving fatigue and enhancing liver function [60]. In our results, the content of glutamic acid was the highest, followed by aspartic acid, in the fruitbodies from all of the rotation systems. Therefore, M. importuna shows great development potential in the field of functional foods.
Our data also showed that different rotation systems did have a certain effect on amino acid scores and the chemical score of morels, but the limiting amino acids were consistent. Combining amino acid score and chemical score, methionine + cystine was the limiting amino acid. Therefore, morels can be mixed with other foods to achieve nutritional complementarity. In addition, a comprehensive evaluation of the protein content and amino acid composition under the five rotation systems were analyzed (other indexes included EAAI, BV, and NI). Overall, RSC had greater impact on production performance and production advantages.
5. Conclusions
We demonstrated the effect of different rotation systems on the fruitbody formation of M. importuna in Southeast China. Different rotation systems did have an influence on the morphological characteristics, yield, nutritional ingredients, and quality of the fruitbody. Overall, RSA, RSB, and RSC rotation systems (Rice–Vegetables–Fungi) were found to be more favorable than RSD and RSE rotation systems (Vegetables–Vegetables–Fungi), with higher yields and better qualities of fruitbodies, especially for RSC. These results demonstrate the feasibility and effectiveness of the cultivation model of “Rice–Vegetables–Fungi” for M. importuna and provide a practical basis for the development of high-quality agriculture in Southeast China.
Author Contributions
Conceptualization, D.-W.S. and F.-F.S.; methodology, D.-W.S., F.-F.S., and G.-D.L.; software, X.-S.L. and L.-L.Z.; formal analysis, D.-W.S., F.-F.S., and L.-L.Z.; investigation, H.L. and P.-H.L.; resources, H.-L.L. and D.-M.L.; data curation, D.-W.S.; writing—original draft preparation, D.-W.S. and F.-F.S.; writing—review and editing, D.-W.S., F.-F.S., and G.-D.L.; visualization, Z.-X.L.; supervision, X.-S.L. and L.-L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Major Special Project of Fujian Province “Research and Application of Key Technologies for Innovation and Industrialized Utilization of JUNCAO”, grant number 2021NZ029009 and the Interdisciplinary Integration to Promote the High-Quality Development of JUNCAO Science and Industry, grant number XKJC-712021030.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sambyal, K.; Singh, R.V. A Comprehensive Review on Morchella Importuna: Cultivation Aspects, Phytochemistry, and Other Significant Applications. Folia Microbiol. 2021, 66, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Masaphy, S. Biotechnology of Morel Mushrooms: Successful Fruiting Body Formation and Development in a Soilless System. Biotechnol. Lett. 2010, 32, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Wang, Q.S.; Baiyintala; Wuhanqimuge. The Whole-Genome Sequence Analysis of Morchella Sextelata. Sci. Rep. 2019, 9, 15376. [Google Scholar]
- Phanpadith, P.; Yu, Z.; Li, T. High Diversity of Morchella and a Novel Lineage of the Esculenta Clade from the North Qinling Mountains Revealed by GCPSR-Based Study. Sci. Rep. 2019, 9, 19856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, X.; Tang, L.; Xie, G.; Deng, K.; Xie, L. Chemical Composition of Aromas and Lipophilic Extracts from Black Morel (Morchella importuna) Grown in China. Mycobiology 2020, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Peng, D.; Li, C.; Hu, X.; Bi, S.; Song, L.; Peng, B.; Zhu, J.; Chen, Y.; Yu, R. A New Polysaccharide Isolated from Morchella Importuna Fruiting Bodies and Its Immunoregulatory Mechanism. Int. J. Biol. Macromol. 2019, 137, 8–19. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, L.; Tang, J.; He, X.; Zhang, Z.; Chen, Y.; Zhou, J.; Gan, B.; Peng, W. Morchella Importuna Polysaccharides Alleviate Carbon Tetrachloride-Induced Hepatic Oxidative Injury in Mice. Front. Physiol. 2021, 12, 669331. [Google Scholar] [CrossRef]
- Peng, D.; Wen, Y.; Bi, S.; Huang, C.; Yang, J.; Guo, Z.; Huang, W.; Zhu, J.; Yu, R.; Song, L. A New Glcnac-Containing Polysaccharide from Morchella Importuna Fruiting Bodies: Structural Characterization and Immunomodulatory Activities In Vitro and In Vivo. Int. J. Biol. Macromol. 2021, 192, 1134–1149. [Google Scholar] [CrossRef]
- Xu, N.; Lu, Y.; Hou, J.; Liu, C.; Sun, Y. A Polysaccharide Purified from Morchella Conica Pers. Prevents Oxidative Stress Induced by H2O2 in Human Embryonic Kidney (HEK) 293T Cells. Int. J. Mol. Sci. 2018, 19, 4027. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Li, C.; Cai, Y.; Zhang, Y.; Bian, Y.; Liu, W. First Report of Pileus Rot Disease on Cultivated Morchella Importuna Caused by Diploöspora Longispora in China. J. Gen. Plant Pathol. 2017, 84, 65–69. [Google Scholar] [CrossRef]
- Chen, X.H. Impact of ≥0 °C Accumulated Temperature on the Growth Development of Morchella Importuna. Mycosystema 2018, 37, 1717–1722. [Google Scholar]
- Hao, H.; Zhang, J.; Wang, H.; Wang, Q.; Chen, M.; Juan, J.; Feng, Z.; Chen, H. Comparative Transcriptome Analysis Reveals Potential Fruiting Body Formation Mechanisms in Morchella importuna. AMB Express 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of Biomass and Chitin Production of Morchella Mushrooms Grown on Starch-Based Substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Ma, X.; Zhang, Q.; Yu, F.; Zhao, Q.; Huang, W.; Song, J.; Liu, W. Physiological Characteristics and Comparative Secretome Analysis of Morchella importuna Grown on Glucose, Rice Straw, Sawdust, Wheat Grain, and MIX Substrates. Front. Microbiol. 2021, 12, 636344. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J.; et al. Multi-omic Analyses of Exogenous Nutrient Bag Decomposition by the Black Morel Morchella importuna Reveal Sustained Carbon Acquisition and Transferring. Environ. Microbiol. 2019, 21, 3909–3926. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zhao, M.; Xie, J.Y.; Wang, Y.; Wen, X.M.; He, X.S. Tolerance of Morchella importuna towards Heavy Metals. Mycosystema 2017, 36, 367–375. [Google Scholar]
- Chen, X.; Lv, S.Y.; Mou, C.Y.; Bian, Y.B.; Kang, H. Functions of Gene ATX1 under Cadmium Stress in Morchella importuna. Mycosystema 2020, 39, 827–838. [Google Scholar]
- Yang, C.; Zhou, X.; Meng, Q.; Wang, M.; Zhang, Y.; Fu, S. Secondary Metabolites and Antiradical Activity of Liquid Fermentation of Morchella sp. Isolated from Southwest China. Molecules 2019, 24, 1706. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xie, L.; Tang, J.; He, X.; Zhang, Z.; Chen, Y.; Zhou, J.; Gan, B.; Peng, W. Morchella importuna Flavones Improve Intestinal Integrity in Dextran Sulfate Sodium-Challenged Mice. Front. Microbiol. 2021, 12, 742033. [Google Scholar] [CrossRef]
- Xiong, C.; Luo, Q.; Huang, W.L.; Li, Q.; Chen, C.; Chen, Z.Q.; Yang, Z.R. The Potential Neuritogenic Activity of Aqueous Extracts from Morchella importuna in Rat Pheochromocytoma Cells. Food Sci. Biotechnol. 2017, 26, 1685–1692. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Peng, W.H.; Gan, B.C.; Huang, Z.Q.; Wang, Y.; Liu, T.H.; Liu, L.X. Variation and Probability Grading of Six Quantitative Traits Associated with Morchella importuna Fruit Bodies. Acta Edulis Fungi 2017, 24, 27–32. [Google Scholar]
- Liu, W.; Chen, L.; Cai, Y.; Zhang, Q.; Bian, Y. Opposite Polarity Monospore Genome De Novo Sequencing and Comparative Analysis Reveal the Possible Heterothallic Life Cycle of Morchella importuna. Int. J. Mol. Sci. 2018, 19, 2525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, G.; Wei, J.; Dong, C. Comparative Transcriptome Analysis of Cells from Different Areas Reveals ROS Responsive Mechanism at Sclerotial Initiation Stage in Morchella importuna. Sci. Rep. 2021, 11, 9418. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and Bacterial Community Dynamics in Substrates during the Cultivation of Morels (Morchella rufobrunnea) Indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Longley, R.; Zhang, P.; Zhao, Q.; Bonito, G.; Yu, F. Microbial Communities Associated with the Black Morel Morchella sextelata Cultivated in Greenhouses. PeerJ 2019, 7, e7744. [Google Scholar] [CrossRef] [Green Version]
- Karwa, A.; Rai, M.K. Tapping into the Edible Fungi Biodiversity of Central India. Biodivers. J. Biol. Divers. 2009, 11, 97–101. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.F.; Chen, Y.; Westby, A.P.; Ren, W.J. Soil Physicochemical and Biological Properties of Paddy-Upland Rotation: A Review. Sci. World J. 2014, 2014, 856352. [Google Scholar] [CrossRef] [Green Version]
- Koyama, A.; Dias, T.; Antunes, P.M. Application of Plant-Soil Feedbacks in the Selection of Crop Rotation Sequences. Ecol. Appl. 2021, 32, e2501. [Google Scholar] [CrossRef]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley-Smith, L. Light-Fraction Organic Matter in Soils from Long-Term Crop Rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Agomoh, I.V.; Drury, C.F.; Yang, X.; Phillips, L.A.; Reynolds, W.D. Crop Rotation Enhances Soybean Yields and Soil Health Indicators. Soil Sci. Soc. Am. J. 2021, 85, 1185–1195. [Google Scholar] [CrossRef]
- Wang, X.; Duan, Y.; Zhang, J.; Ciampitti, I.A.; Cui, J.; Qiu, S.; Xu, X.; Zhao, S.; He, P. Response of Potato Yield, Soil Chemical and Microbial Properties to Different Rotation Sequences of Green Manure-Potato Cropping in North China. Soil Tillage Res. 2022, 217, 105273. [Google Scholar] [CrossRef]
- GB 5009.4-2016; National Standard for Food Safety Determination of Ash in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.10-2003; National Standard for Food Safety Determination of Crude Fiber in Plant Foods. National Health and Standard Management Commission of the People’s Republic of China: Beijing, China, 2003.
- GB 5009.5-2016; National Standard for Food Safety Determination of Protein in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China; Food and Drug Administration: Beijing, China, 2016.
- GB 5009.6-2016; National Standard for Food Safety Determination of Fat in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China; Food and Drug Administration: Beijing, China, 2016.
- GB 5009.12-2017; National Standard for Food Safety Determination of Lead in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2017.
- GB 5009.15-2014; National Standard for Food Safety Determination of Cadmium in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2015.
- GB 5009.17-2014; National Standard for Food Safety Determination of Total Mercury and Organic Mercury in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2015.
- Zahra, R.; Hamid, R.S.; Shideh, M.; Kambiz, J. Statistical Optimization of Culture Conditions for Protein Production by a Newly Isolated Morchella fluvialis. Biomed. Res. Int. 2019, 2019, 7326590. [Google Scholar]
- FAO. Nutritional Studies: Amino-Acid Content of Foods and Biological Data on Proteins. FAO Nutr. Stud. 1970, 24, 1–285. [Google Scholar]
- Bano, Z.; Rajarthram, S.; Steinkraus, K.H. Pleurotus mushrooms. Part II—Chemical Composition, Nutritional Value, Post-Harvest Physiology, Preservation, and Role as Human Food. Food Sci. Nutr. 1988, 27, 87–158. [Google Scholar] [CrossRef]
- Feng, X.X.; Li, J.; Chen, Q.Q.; Li, M.P.; Zhang, S.W. Amino Acid Composition and Nutritional Evaluation of Proteins Extracted from Elaeagnus mollis Diels Seed Kernels. Food Sci. 2016, 37, 160–165. [Google Scholar]
- Luo, Z.M.; Liu, X.L.; Jia, Y.Q.; Hao, R.L. Protein Nutritional Assessments of Four Kinds of Wild Edible Fungi in Mount Wutai. Sci. Technol. Food Ind. 2015, 36, 349–354. [Google Scholar]
- Oser, B.L. Method for Integrating Essential Amino Acid Content in the Nutritional Evaluation of Protein. J. Am. Diet. Assoc. 1951, 27, 396–402. [Google Scholar] [CrossRef]
- Zhang, F.H.; Wang, J.J. Effect of Planting Patterns on Organic Carbon and Soil Aggregate Composition. Agric. Res. Arid. Areas 2014, 32, 113–116, 139. [Google Scholar]
- Trabelsi, D.; Ammar, H.B.; Mengoni, A.; Mhamdi, R. Appmisal of the Crop-rotation Effect of Rhizobial Inoculation on Potato Cropping Systems in Relation to Soil Bacterial Communities. Soil Biol. Biochem. 2012, 54, 1–6. [Google Scholar] [CrossRef]
- Moore, J.M.; Susanne, K.; Tabatabai, M.A. Soil Microbial Biomass Carbon and Nitrogen as Affected by Cropping Systems. Biol. Fertil. Soils 2000, 31, 200–210. [Google Scholar] [CrossRef]
- Song, M.H.; Xu, X.L.; Hu, Q.W.; Tian, Y.Q.; Hua, O.Y.; Zhou, C.P. Interactions of Plant Species Mediated Plant Competition for Inorganic Nitrogen with Soil Microorganisms in an Alpine Meadow. Plant Soil 2007, 297, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.F.; Chien, C.H.; Chiang-Hsieh, Y.F.; Tseng, K.C.; Chow, C.N.; Huang, H.J.; Chang, W.C. Paddy-upland rotation for sustainable agriculture with regards to diverse soil microbial community. Sci. Rep. 2018, 8, 7966. [Google Scholar] [CrossRef]
- Li, P.; He, W.; Wu, G. Composition of Amino Acids in Foodstuffs for Humans and Animals. Adv. Exp. Med. Biol. 2021, 1332, 189–210. [Google Scholar]
- Wiedemair, V.; Scholl-Bürgi, S.; Karall, D.; Huck, C.W. Amino Acid Profiles and Compositions of Different Cultivars of Panicum miliaceum L. Chromatographia 2020, 83, 829–837. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Q.; Bao, C.; Fan, J. Comparison of Free Total Amino Acid Compositions and Their Functional Classifications in 13 Wild Edible Mushrooms. Molecules 2017, 22, 350. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.; Andrade, P.B.; Silva, B.M.; Baptista, P.; Seabra, R.M.; Valentao, P. Comparative Study on Free Amino Acid Composition of Wild Edible Mushroom Species. J. Agric. Food Chem. 2008, 56, 10973–10979. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Huang, Z.; Feng, X.; Bian, Y.; Huang, W.; Liu, Y. Bioconversion of Rice Straw Agro-Residues by Lentinula edodes and Evaluation of Non-Volatile Taste Compounds in Mushrooms. Sci. Rep. 2020, 10, 1814. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, X.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes. Foods 2021, 10, 2836. [Google Scholar] [CrossRef]
- Wang, J. Comparative analysis on amino acid content in wolfberry dried fruit among different growing regions. Hubei Agric. Sci. 2015, 54, 3411–3413. [Google Scholar]
- Gao, H.Y.; Jiang, F.; Zhang, L.J.; Chen, X.M.; Zheng, S.Q. Analysis of the Compositions and Contents of Amino Acids in Five Late Ripening Loquat Fruits. Fujian Fruits 2009, 2, 37–41. [Google Scholar]
- Wang, X.Q.; Hailiqian, T.; Abuddu, X.; Guo, X.F. Analysis on Amino Acids in Hedysarum Semenovii Regelet Herd. Lishizhen Med. Mater. Med. Res. 2007, 18, 1310–1311. [Google Scholar]
- Cauli, O.; Rodrigo, R.; Llansola, M.; Montoliu, C.; Monfort, P.; Piedrafita, B.; Mlili, N.E.; Boix, J.; Agustí, A.; Felipo, V. Glutamatergic and Gabaergic Neurotransmission and Neuronal Circuits in Hepatic Encephalopathy. Metab. Brain Dis. 2009, 24, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yan, S.W.; Ma, W.S.; Yang, B. Amino Acid Composition and Nutritional Evaluation Oflaggera Pterodonta (DC.) Benth. from Different Areas in Yunnan. J. Food Saf. Qual. 2015, 6, 4173–4180. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).