Abstract
Paeonia emodi is a type of wild herbaceous peony with high ornamental and breeding value. Cold stratification is the only method to break its seed epicotyl dormancy to date, however, the key physiological factors during this process are not clear. In this study, rooted seeds of P. emodi were treated with 4 °C stratification, and the changes of the embryo, four nutrients, and two endogenous hormones in the seeds were investigated. The results showed that the plumule elongated at S6 (i.e., ten weeks of cold stratification), and grew to nearly the same length as the cotyledon at S9. Cold stratification increased starch consumption, significantly decreased soluble sugar content in the later stages, and decreased soluble protein content at S9, but it did not influence crude fat content. The activities of α-amylase and β-amylase increased significantly at S4 and S4 to S6, respectively. At S8 and S9, acid protease activity increased, and the increase in lipase activity continued throughout the whole process. At the same time, the ABA content decreased significantly after S6; from this stage, the ABA/GA3 ratio gradually decreased compared with that of the control, and the difference was significant at S9. Correlation analysis showed that the ABA/GA3 ratio was significantly correlated with starch content and α-amylase activity. It can be concluded that both carbohydrates and proteins were the energy supply for the epicotyl dormancy breaking of P. emodi seeds, rather than crude fat. Cold stratification promoted substance transformation by increasing the corresponding enzyme activities. The balance of ABA and GA3 suggested the key stage for the release of dormancy.
1. Introduction
Paeonia emodi Wall. ex Royle has a very narrow distribution and only exists on both sides of the Himalayas [1]. It is of vital ornamental and breeding value because of its excellent characteristics of up to 150 cm stem height, 3–4 terminal or axillary white flowers, and effective medical source of monoterpene and bioactive compounds [2]. However, it has not been developed and utilized to date, due to limited existing numbers, and difficulties in ex situ cultivation and seed dormancy release. Previous studies have found that the main reason for the long germination time is the physiological dormancy of the epicotyl, similar to other species in Paeonia [3,4,5]. Both the application of growth regulators and radiation treatment failed to release epicotyl dormancy, and 4 °C stratification for 110 days was the only effective method [6].
Dormancy breaking of Paeonia commonly includes two steps: obtaining the radicle first by warm stratification, and then providing cold stratification to release epicotyl dormancy [5]. Many studies have focused on the physiological and metabolic changes in starch degradation, sugar transport, protein hydrolysis and resynthesis, as well as the hormone changes in the first step [7,8,9,10], and only a few studies have investigated the physiological changes during cold stratification. During the breaking process of root-shoot dormancy in P. ostii, it was found that GA3 increased rapidly along with a slow decrease in ABA [3]. In cultivated herbaceous peony (P. lactiflora), researchers determined the hormone content and the differentially expressed genes at the two stages when the radicle and epicotyl protruded from the seed coat [11]. However, these studies were not carried out in P. emodi. In addition, it took up to 110 d to release epicotyl dormancy for P. emodi, and it is difficult to determine when and what changes occurred in those substances if only by comparing changes at the two time points before and after cold treatment or to help accurately affect seed dormancy breaking by more artificial measures.
To solve this problem, rooted seeds of P. emodi were taken as objects, and changes in embryo morphology, four kinds of nutrients, and two kinds of endogenous hormones in seeds during the whole process of cold stratification were investigated in this study. Meanwhile, by comparing the low temperature treatment and the control (warm stratification), the key time stages of the effect of low temperature on various substances were discussed, and the morphological changes of embryos were combined to clarify the importance of low temperature on the internal changes of the epicotyl dormancy process of P. emodi seeds. It is of great significance to reveal the internal regulatory mechanism by which low temperature regulates the release of Paeonia seed dormancy.
2. Materials and Methods
2.1. Plant Materials and Stratification Treatment
Seeds of P. emodi were harvested from Jilong County, Tibet, China (84°35′–86°20′ E, 28°3′–29°3′ N) in August 2020 and were dried in a cool and ventilated room for one week. Then, after 24 h of water absorption, they were stratified in sand with 60% moisture and storage at 15 °C. After 71.67 ± 3.33 d of warm stratification, seeds started rooting, and around seven days later, the root length was 3–4 cm, the materials were randomly divided into two groups, and the sampling at this time was marked as S1. Group one continued to be stratified at 15 °C as a control, and group two underwent cold stratification at 4 °C. The experiment lasted for 16 weeks, and the sand humidity in all pots was kept at 60% ± 10% during this process. It was found in our previous study that seeds of P. emodi need 110 d of cold stratification to break epicotyl dormancy [6], so samples were taken once every two weeks and sampled a total of eight times, samples being marked as S2 to S9 for observation and index determination.
2.2. Morphological Observation of Embryos
After the endosperm was removed from the samples of each stage, the seed embryos were taken and dissected longitudinally along the cotyledons under a stereomicroscope (Leica EZ, Wetzlar, Germany) to observe the embryo morphology and epicotyl. At each stage, at least ten seeds were observed. It should be noted that the radicle was cut off before taking pictures.
2.3. Determination of Nutrient Content, Related Enzyme Activities and Endogenous Hormones
Each sample for these indices’ determination consisted of three seeds, and the determination of each index contained three biological replicates. Except for crude fat measurement, each sample was ground into powder using a high-throughput tissue grinder (Scientz-24, Xinzhi Bio, Ningbo, China) to make it evenly mixed.
The content of soluble sugar and starch was determined by the anthrone colorimetric method as previously described [12,13]. In detail, 0.2 g of sample powder was taken in a tube,10–15 mL of distilled water was added, and it was given a boiling water bath for 30 m. The supernatant and residue were used for the determination of soluble sugar content and starch content, separately. After the supernatant was cooled naturally, diluted the solution to 25 mL. Then, 1 mL was taken out of the well-mixed solution, and we added 1 mL of distilled water, 0.5 mL of anthrone-ethyl acetate solution, and 5 mL of concentrated sulfuric acid, mixed these together, and placed it in boiling water for 10 m. After natural cooling, the solution was mixed again, 2 mL of it were taken into a cuvette, its absorbance was measured under 630 nm, and the soluble sugar content was calculated according to the absorbance value. The residue in the first step above was taken, and 8 mL of distilled water were added. Subsequently, it was treated with a boiling water bath for 15–20 m, 2 mL of 9.2 mo1·L−1 HClO4 solution were added, and after 15 min of reaction, it was cooled down, diluted to 25 mL, and mixed well. We next pipetted 0.5 mL of the filtrate into a new tube, added 1.5 mL of distilled water, 0.5 mL of 2% anthrone–ethyl acetate solution and 5 mL of 98% concentrated sulfuric acid in turn, and then put it into a boiling water bath for 1 min after sufficient shaking. Using the cooling solution to measure the absorbance under 630 nm for the calculation of the starch content.
The soluble protein content was determined by a Bradford method [14]. Weighed 0.05 g of sample powder, added 2 mL of phosphate buffer, and grinded on ice. The mixture was centrifuged at 10,000 r/min for 15 m in a 4 °C refrigerated centrifuge. We took 0.1 mL of the supernatant, then added 5 mL of Coomassie brilliant blue G-250 solution, mixed it well, and let it stand for 2 m. We then measured the absorbance under 595 nm for calculation of soluble protein content.
The crude fat content of seeds was determined by Soxhlet extraction, according to the Chinese national standard for the determination of crude fats in cereals and oil crop seeds (NY/T 4-1982). The seeds in each sample were cut into pieces, weighed as 0.3 g of the mixture, and dried to constant weight at 80 °C. The dry weight was recorded as W1. The mixture was ground into powder and wrapped with filter paper, then it was put into the Soxhlet extractor. Petroleum ether was used as the extraction reagent, and the extraction time was approximately 5 h. After the petroleum ether was volatilized, its weight was recorded as W2. Calculated the difference between W2 and W1, the crude fat content was the percentage of it in W1.
The activities of α-amylase, β-amylase, acid protease and lipase were measured via assay kits (Comin Biotechnology Co., Ltd., Suzhou, China), all the steps followed the instructions of the manufacturer. The principle of amylase activities was based on the DNS method, and the measurement of α-amylase activity was performed at 540 nm. β-amylase activity was calculated from total amylase activity minus α-amylase activity. Acid protease activities were measured at 680 nm according to the principle of the Folin-phenol method. Lipase activity was measured at 710 nm based on the principle of the copper soap-spectrophotometry method.
Abscisic acid (ABA) and gibberellin (GA3) levels were determined by the public experimental analysis platform of the China Agricultural University using ELISA kits (China Agricultural University, Beijing, China) according to the manufacturer’s instructions.
2.4. Data Processing and Analysis
The percentages in the data were processed by arcsine transformation, and SPSS 22.0 (The software was placed in Beijing, China) was used for variance analysis. If the difference was significant, the LSD method and independent sample t-test were used for multiple comparisons. Microsoft Excel 2019 and Adobe Illustrator 2018 (These two kinds of software was placed in Beijing, China) were used for charting and layout.
3. Results
3.1. Morphological Changes of Embryos during Cold Stratification
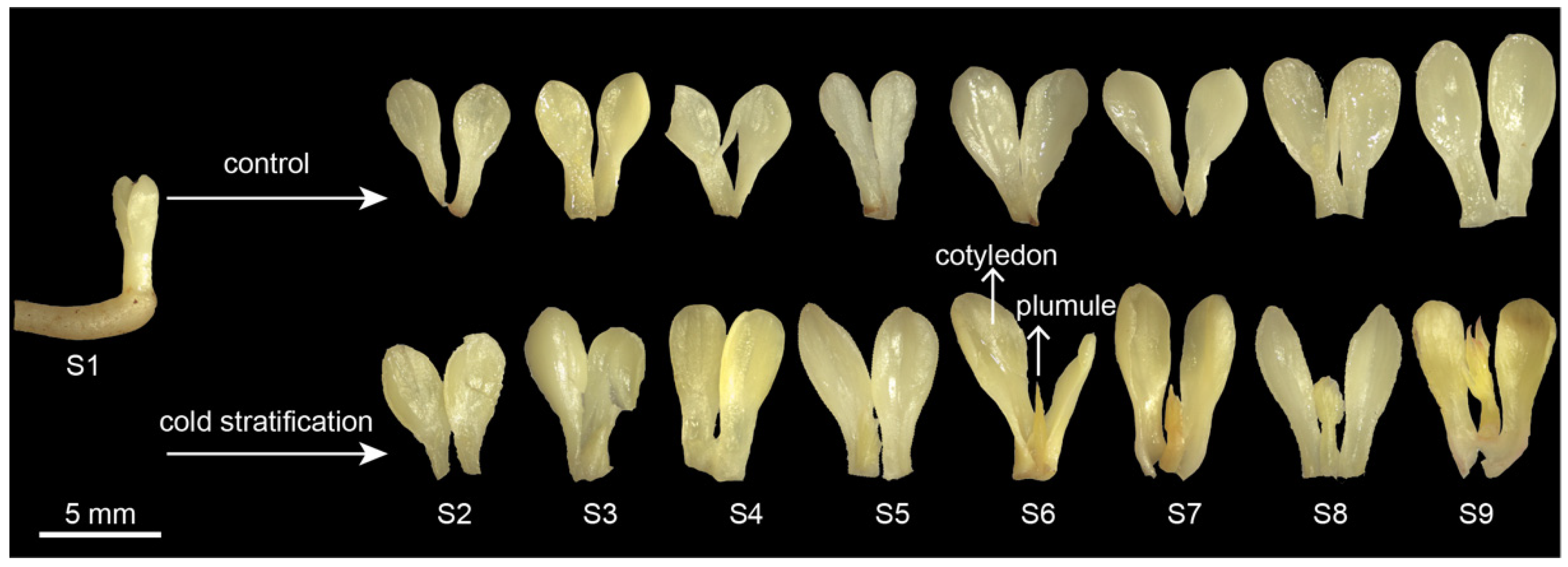
The embryo morphology of P. emodi seeds at nine stages during cold stratification is shown in Figure 1. In the control, the morphology of the cotyledon did not change until S8, at which time the cotyledon began to enlarge. There was no plumule differentiation throughout the process. Under cold stratification, the plumule began to elongate at S6, grew to nearly the same length as the cotyledon, and differentiated into the shape of primary leaves at S9. Therefore, cold stratification treatment made a key change in the shape of the embryos of P. emodi, and S6 was a key stage in this process.
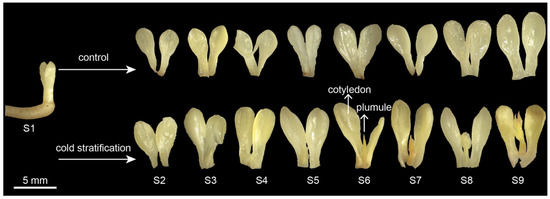
Figure 1.
Morphological anatomy of P. emodi embryos during cold stratification. Note: Radicles have been removed before taking the photo.
3.2. Changes in Nutrient Content during Stratification
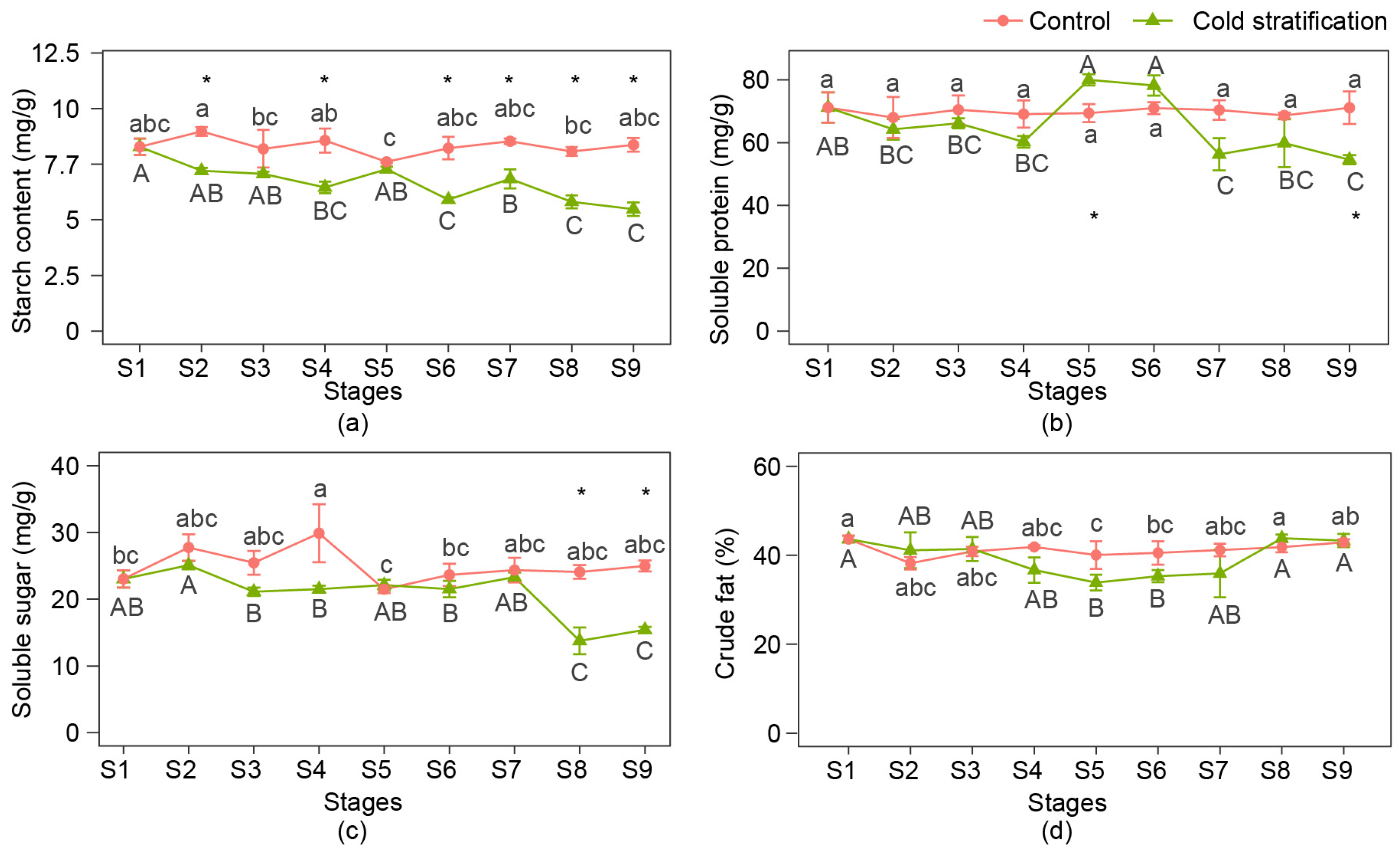
The changes in the starch, soluble protein, soluble sugar, and crude fat contents of seeds at nine stages are shown in Figure 2. During cold stratification, the contents of starch, soluble protein, and soluble sugar decreased at late stages, and crude fat was not significantly consumed. Among them, the starch content began to decrease significantly at S6, then showed a decreasing trend and declined to the bottom at S9. The soluble protein content increased at S5 and S6 compared with the period from S2 to S4, then substantially dropped at S7 and stayed at a relatively low level at S8 and S9. Soluble sugars did not change significantly during the first seven stages but dropped significantly at S8. In the control, the changes in the four nutrients were relatively small and the contents were relatively stable, except that the soluble sugar content suddenly increased at S4.
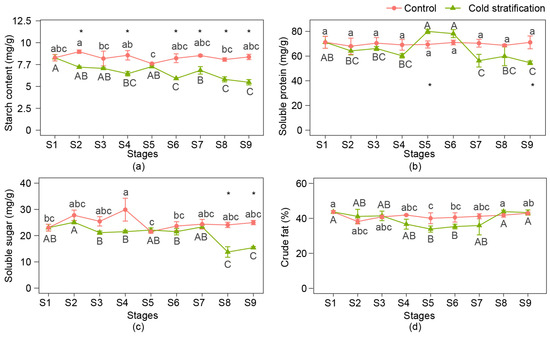
Figure 2.
Nutrient content changes in P. emodi seeds during cold stratification. (a) Starch content; (b) soluble protein content; (c) soluble sugar content; (d) crude fat content. Note: Lowercase letters represent the significance of differences between stages of control (p < 0.05); capital letters represent the significance of differences between stages of cold stratification (p < 0.05); * represents the significant difference between treatments at the same stage (p < 0.05).
Comparing the contents of nutrients at the same stage between the two treatments, it was found that the differences in starch, soluble protein, and soluble sugar content initially appeared at S2, S5, and S8, respectively, while the content of crude fat remained unchanged compared with the control. Combined with the morphological changes of seed embryos, it was shown that the obvious consumption of starch occurred before elongation of the plumule, while that in soluble sugar and protein occurred later than the elongation of the plumule.
3.3. Changes in Enzyme Activities Related to Nutrient Decomposition during Stratification
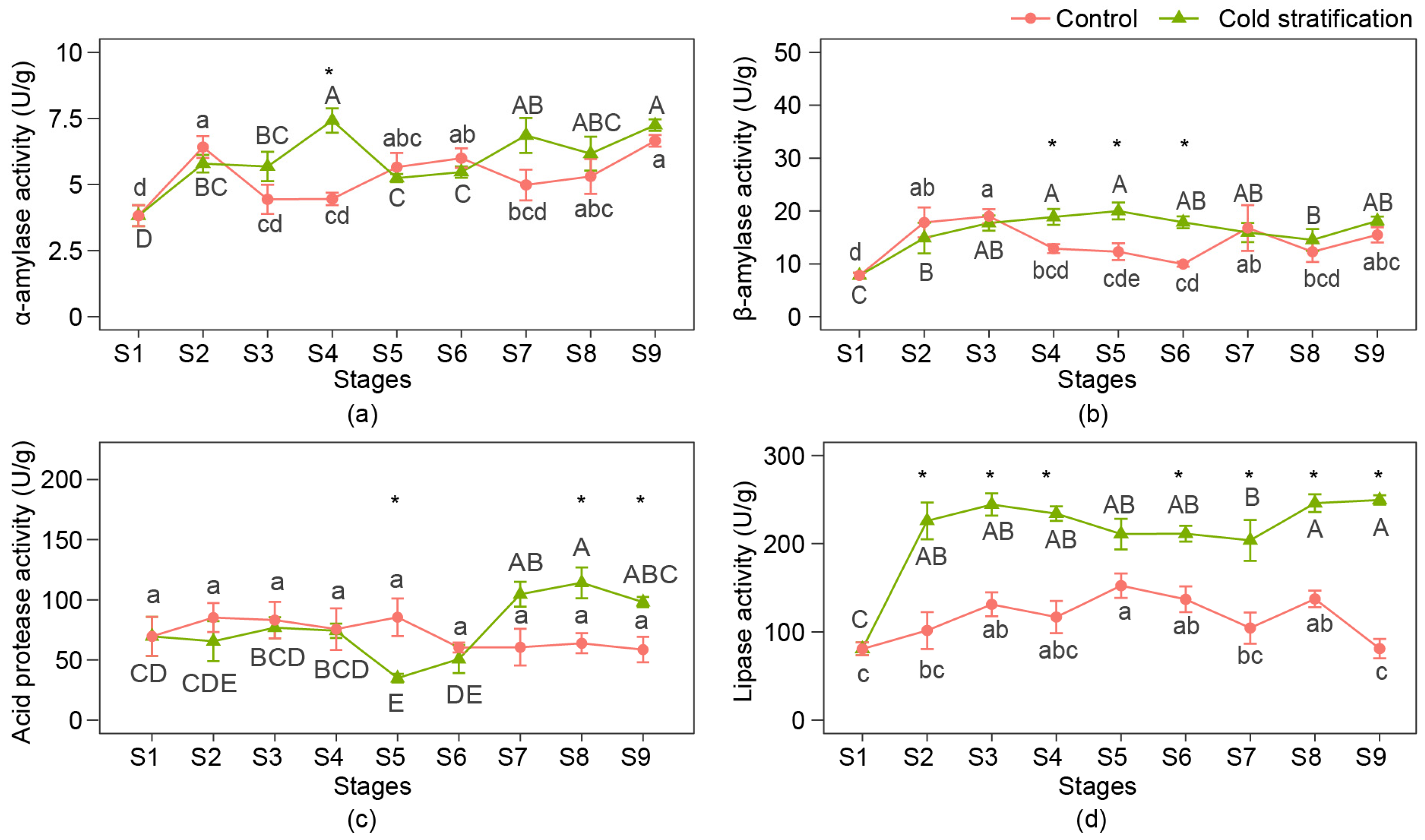
α-amylase, β-amylase, acid protease, and lipase are the key enzymes that decompose carbohydrates, proteins, and fats, and their activities in the seeds of P. emodi at nine stages are shown in Figure 3.
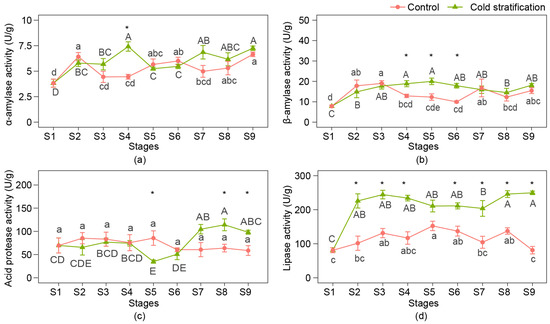
Figure 3.
Enzyme activities related to nutrient consumption of P. emodi seeds during cold stratification. (a) α-amylase activity; (b) β-amylase activity; (c) acid protease activity; (d) lipase activity. Note: Lowercase letters represent the significance of differences between stages of control (p < 0.05); capital letters represent the significance of differences between stages of cold stratification (p < 0.05); * represents the significant difference between treatments at the same stage (p < 0.05).
During cold stratification, amylase activities increased during the first four stages. α-amylase activity decreased at S5 and had a rising trend from S6 to S9, and β-amylase activity remained relatively stable from S6 to S9. In the control, both the α-amylase and β-amylase activity curves were bimodal, with two peaks at S2 or S3 and S6 or S7. Acid protease activities were approximate from S1 to S4, bottomed at S5, having the trend to increase later with the cold stratification, while they did not change during the process in the control. Lipase activities greatly increased after cold treatment, keeping no significant differences from S2 to S6 and S8 to S9. In the control, lipase activities showed a trend of increasing first and then decreasing, and the final activity at S9 was approximate to that at S1.
The time when differences between treatments occurred differed depending on the kinds of enzymes. α-Amylase activities at S4 and β-amylase activities from S4 to S6 in cold stratification were significantly higher than those in the control. Acid protease activities differed at S5, S8, and S9, and the lipase activity was significantly higher than the control from S2 to S9, except for S5.
3.4. Changes in Endogenous Hormone Content during Stratification
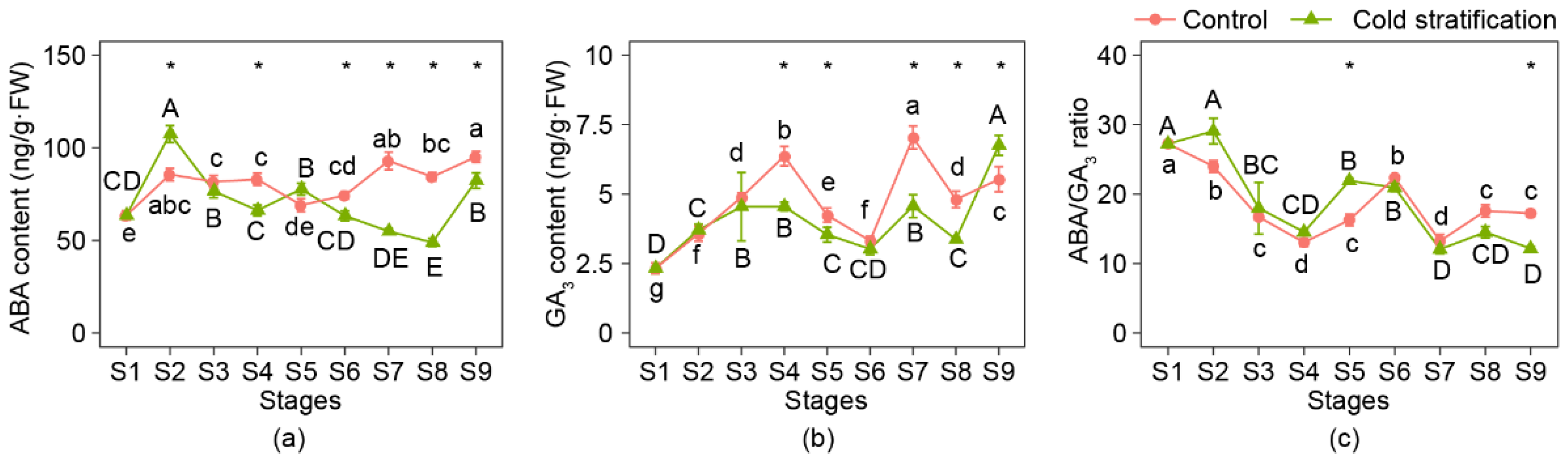
The ABA content, GA3 content, and ABA/GA3 ratio are shown in Figure 4. The ABA content increased significantly at S2 when cold stratification was applied, then showed a decreasing trend from S2 to S8, except at S5, and increased at S9. In the control, ABA content showed a fluctuating upward trend. The change trend of GA3 content was similar in the two treatments; three peaks appeared at S4, S7, and S9; and bottomed at S6 and S8. The ABA/GA3 ratio during this process was similar in the two groups, and the values fluctuated and eventually dropped compared with those at S1.
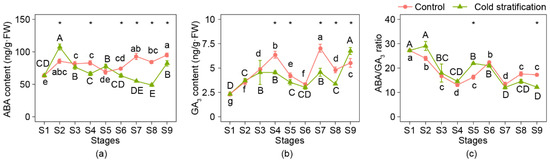
Figure 4.
Content and ratio of ABA and GA3 of P. emodi seeds at nine stages during stratification. (a) ABA; (b) GA3; (c) ABA/GA3 ratio. Note: Lowercase letters represent the significance of differences between stages of control (p < 0.05); capital letters represent the significance of differences between stages of cold stratification (p < 0.05); * represents the significant difference between treatments at the same stage (p < 0.05).
Comparing the hormone contents at the same stage between the treatments, the ABA contents from S6 to S9 were significantly lower than those of the control, which was the time when the plumule began to elongate. Differences in GA3 content between the two treatments appeared at S4, S5, S7, to S9, while only at S9 was the GA3 content under cold stratification higher than that under the control. It should be noted that the ABA/GA3 ratio under cold stratification was significantly higher than that of the control at S5 but significantly lower at S9.
3.5. Correlation Analysis between Hormones and Nutrients under Cold Stratification
The correlation analysis results of the two endogenous hormones with the activities of nutrient contents and metabolic enzymes under cold stratification are shown in Table 1. The GA3 content in seeds was significantly negatively correlated with soluble protein content and positively correlated with α-amylase activity. The ABA/GA3 ratio was significantly positively correlated with starch content and negatively correlated with α-amylase activity. None of the four kinds of nutrients or their metabolic enzymes showed relationships with ABA contents.

Table 1.
Pearson correlation coefficients of hormones and nutrients at each stage during cold stratification.
4. Discussion
In our previous study on methods of P. emodi seed dormancy breaking, it was found that the emergence rate of seeds with 16 weeks of cold treatment reached 67.50%, while shortening the time to 12 weeks did not result in seedlings [6]. In this study, plumule began to elongate from S6 (i.e., ten weeks of cold treatment), which occurred in the middle of cold stratification. Similar results were observed in cultivated herbaceous peony, in which more than 28 d of cold stratification was needed for seedlings [15]. It was indicated that the vital period of the embryo-to-seedling transition was from S6 to S9. Future studies can focus on this period, making shorter time divisions, utilizing scanning electron microscopy to observe shoot apical meristem during epicotyl–plumule development to accurately determine the key time point of transformation.
During seed germination, starch decomposes starch granules in the endosperm and aleurone layer into soluble sugars, which directly provide energy for seed respiration and metabolism [16,17]. However, the starch–soluble sugar metabolic switch was not observed in P. emodi; starch degradation started from the very beginning of cold treatment, and the soluble sugar content remained stable first and then dropped in the last two stages. One explanation is that sugar production and consumption varied among the types. In the 50 days for dormancy release of Pyrus calleryana seeds, sucrose content was always stable, while it decreased at 60 d, and the content of glucose and fructose were not changed in the first 20 d but increased at 50 d [18,19]. The increase in amylase activities is important for seed germination, and exogenous intervention of their activities and gene expression can inhibit germination [20,21,22]. In this study, the period of rising amylase activities was basically the same as the stage of plumule elongation, showing its role in embryo-to-seedling transition.
Paeonia are considered potential oil plants [23,24]. As an energy storage substance in seeds, lipids were thought to play an important role in the process of dormancy and germination in P. suffruticosa, Juglans regia, and Suaeda salsa by converting to sugars via glyoxylic acid metabolism or producing signal molecules related to dormancy release [25,26,27,28]. However, crude fat content did not significantly decrease under stratification in P. emodi, although the lipase activity increased. Some studies suggested that fatty seeds may utilize more starch during germination than starchy seeds [29]. In this study, starch content decreased significantly at S7 to S9, while α-amylase and β-amylase activities did not change in this period. α-Amylase is generally the most concerned enzyme in endosperm starch degradation, which can degradate starch to linear and branched glucans, but there are also DBE (specifically LDA) and α-glucosidase which play important roles in this progress and which are not yet clearly understood [16]. These wild seeds from the Himalayas may have their own characteristics in starch degradation and deserve future attention. In general, lipase is the first key enzyme in lipid metabolism, which can convert fat into glycerol and then provide energy for seed germination by the way of the tricarboxylic acid cycle [30]. It is worth noting that part of lipid was hydrolyzed by lipase to produce unsaturated fatty acids and then re-decomposed to malondialdehyde, which inhibits seed germination [31]. In addition, in some species, the metabolism of fat is initiated by lipoxygenases [32]. Therefore, it is necessary to systematically explore the key enzymes in the lipid metabolism of seeds of P. emodi in future studies. Moreover, it was thought in some studies that the decomposition of a large amount of starch in tree peony seeds is to meet the demand for raw materials for oil synthesis [33]. Another possible explanation is that the reduced starch content at S7 to S9 may be used for oil synthesis, and its synthetic amount was consumed by lipase, so that the final crude fat content did not change.
ABA accumulation represses seed germination, and GA3 is an antagonist of ABA [34]. The ABA content decreased with the prolonged cold days validating the importance of ABA in P. emodi. Before S6, the ABA/GA3 ratio in the cold group was higher than that of the control, and the difference was significant at S5, during plumule elongation, the ABA/GA3 ratio was lower than that of the control, and the difference was significant at S9. It seems that the key transition time of the ABA/GA3 ratio has some potential relation to the elongation of seed plumule and the cold treatment weeks at which seedlings could be obtained. P. ludlowii is also distributed in a narrow area of Tibet and has similar seed dormancy characteristics with P. emodi. It was found that cold stratification can only break the epicotyl dormancy of the P. ludlowii seeds with a root length of 6 cm, but not the seeds with a shorter root length; the GA3 content in the epicotyls between the different experimental groups was similar, and the differences between groups were mainly reflected in the ABA/GA3 ratio [35]. In this experiment, only the hormone content in the whole seed was measured, future measurement should focus on the content in the cotyledon, epicotyl, and radicle separately for more accurately correlating with the time of plumule elongation. In various cultivars of rice, seeds with a deeper degree of dormancy had a high level of ABA/GA3 at early development, decreased along with the release of dormancy, and changed to a similar level of seeds with a low degree of dormancy later [36]. The balance of ABA and GA3 has great reference significance for the key stage for indicating the release of seed dormancy [37]. In addition, it was found that the change of ratio of ABA/GA3 was consistent with the change in amylases activities in this study. Soaking wheat seeds with GA3 can significantly improve the germination rate, and increased α-amylase activity for 1–3 days after the seeds were imbibed [38]. Studies have found that transcripts of α-amylase increased after giving GA treatment, and ABA can directly delay the GA-mediated the increase [39]. The relationship between hormones and changes in nutrient metabolism deserves further exploration in P. emodi.
5. Conclusions
The embryo of P. emodi seeds began to elongate in the tenth week (S6) of cold stratification, indicating that its epicotyl dormancy began to release. Starch, soluble sugar, and soluble protein were the main energy supply substances in the process of releasing epicotyl dormancy. The activities of α-amylase, β-amylase, and acid protease were significantly increased in different stages during cold stratification. The endogenous hormones ABA and GA3 played a key role in the process, and the change of the balance indicated the release of seed dormancy. Future studies could focus on the relationship between sugar and lipid metabolism and determine the hormone levels in different parts of seeds separately, and combine with multi-omics to analyze the underlying mechanism of epicotyl dormancy of P. emodi.
Author Contributions
Y.W.: conceptualization, investigation, formal analysis, visualization, writing—original draft preparation, funding acquisition; M.Z.: investigation, methodology, formal analysis, validation, writing—review and editing; A.L.: resources, supervision, validation; Q.Y.: visualization; Y.L.: conceptualization, writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities (grant number BLX202113) and the National Natural Science Foundation of China (grant number 32071825).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hong, D. Peonies of the World: Taxonomy and Phytogeography; Royal Botanic Gardens: Richmond, UK, 2010; pp. 118–122. [Google Scholar]
- Ahmad, M.; Malik, K.; Tariq, A.; Zhang, G.; Yaseen, G.; Rashid, N.; Sultana, S.; Zafar, M.; Ullah, K.; Khan, M.P.Z. Botany, ethnomedicines, phytochemistry and pharmacology of Himalayan paeony (Paeonia emodi Royle.). J. Ethnopharmacol. 2018, 220, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pan, H.; Baskin, C.C.; Baskin, J.M.; Xiong, Z.; Cao, W.; Yao, L.; Tang, B.; Zhang, C.; Tao, J. Epicotyl morphophysiological dormancy in seeds of Paeonia ostii (Paeoniaceae): Seasonal temperature regulation of germination phenology. Environ. Exp. Bot. 2022, 194, 104742. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Sequential temperature control of multi-phasic dormancy release and germination of Paeonia corsica seeds. J. Plant Ecol. 2015, 9, 464–473. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, R.; Cheng, F. Seed germination of tree and herbaceous peonies: A mini-review. Seed Sci. Biotechnol 2014, 1, 11–14. [Google Scholar]
- Zhang, M.; Wan, Y.; Li, B.; Gao, J.; Zhou, H.; Liu, Y. Methods of breaking dormancy in Paeonia emodi seed. In Proceedings of the China Ornamental Horticulture Symposium, Beijing, China, 2020; pp. 260–263, (In Chinese with English abstract). [Google Scholar]
- Ren, X.-X.; Xue, J.-Q.; Wang, S.-L.; Xue, Y.-Q.; Zhang, P.; Jiang, H.-D.; Zhang, X.-X. Proteomic analysis of tree peony (Paeonia ostii ‘Feng Dan’) seed germination affected by low temperature. J. Plant Physiol. 2018, 224–225, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2019, 249, 291–303. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Tang, H.; He, L. Effect of exogenous gibberellic acid on Paeonia rockii seeds germination. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1819–1826. [Google Scholar]
- Chen, S.-Y.; Chou, S.-H.; Tsai, C.-C.; Hsu, W.-Y.; Baskin, C.C.; Baskin, J.M.; Chien, C.-T.; Kuo-Huang, L.-L. Effects of moist cold stratification on germination, plant growth regulators, metabolites and embryo ultrastructure in seeds of Acer morrisonense (Sapindaceae). Plant Physiol. Biochem. 2015, 94, 165–173. [Google Scholar] [CrossRef]
- Li, X.; Fei, R.; Chen, Z.; Fan, C.; Sun, X. Plant hormonal changes and differential expression profiling reveal seed dormancy removal process in double dormant plant-herbaceous peony. PLoS ONE 2020, 15, e0231117. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, M.; Hong, A.; Lan, X.; Yang, H.; Liu, Y. Transcriptome and weighted correlation network analyses provide insights into inflorescence stem straightness in Paeonia lactiflora. Plant Mol. Biol. 2020, 102, 239–252. [Google Scholar] [CrossRef]
- Yu, X.; Yu, H.; Zhang, J.; Shao, S.; Xiong, F.; Wang, Z. Endosperm structure and physicochemical properties of starches from normal, waxy, and super-sweet maize. Int. J. Food Prop. 2015, 18, 2825–2839. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, P. Ameliorative effect of trichoderma, rhizobium and mycorrhiza on internodal length, leaf area and total soluble protein in mung bean (Vigna radiata [L.] R. Wilazek) under drought stress. J. Pharmacogn. Phytochem. 2020, 9, 971–977. [Google Scholar]
- Fei, R.; Sun, X.; Yang, P.; Chen, Z.; Ma, Y. Anatomical observation of Paeonia lactiflora seeds during stratification process. J. Shenyang Agric. Univ. 2017, 48, 354–359, (In Chinese with English abstract). [Google Scholar]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef]
- Shao, C.; Wang, G.; Ding, X.; Yang, C.; Yan, M. Physiological and biochemical characteristics of cold stratification to overcome morphophysiological dormancy in Glehnia littoralis seed. Seed Sci. Technol. 2021, 49, 19–24. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, J.-Y.; Bian, Y.-H.; Liu, X.; Wang, C.-L. Transcriptome and metabolite conjoint analysis reveals the seed dormancy release process in callery pear. Int. J. Mol. Sci. 2022, 23, 2186. [Google Scholar] [CrossRef]
- Liu, X.; Huang, X.; Kong, X.-X.; Zhang, J.; Wang, J.-Z.; Yang, M.-L.; Wang, C.-L. Sucrose synthase is involved in the carbohydrate metabolism-based regulation of seed dormancy release in Pyrus calleryana Decne. J. Hortic. Sci. Biotechnol. 2020, 95, 590–599. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity Inhibits Rice Seed Germination by Reducing α-Amylase Activity via Decreased Bioactive Gibberellin Content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef]
- Sheng, Y.; Xiao, H.; Guo, C.; Wu, H.; Wang, X. Effects of exogenous gamma-aminobutyric acid on α-amylase activity in the aleurone of barley seeds. Plant Physiol. Biochem. 2018, 127, 39–46. [Google Scholar] [CrossRef]
- Zaynab, M.; Pan, D.; Fatima, M.; Sharif, Y.; Chen, S.; Chen, W. Proteomics analysis of Cyclobalanopsis gilva provides new insights of low seed germination. Biochimie 2021, 180, 68–78. [Google Scholar] [CrossRef]
- Yan, Z.G.; Xie, L.H.; Wang, N.; Sun, D.Y.; Bai, Z.Z.; Niu, L.X.; Zhang, Y.L.; Ji, X.T. Phenotypic characteristics and fatty acid composition of seeds from different herbaceous peony species native to China. Chem. Biodivers. 2019, 16, e1800589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, J.; Zeng, F. Volatile composition analysis of tree peony (Paeonia section Moutan DC.) seed oil and the effect of oxidation during storage. J. Food Sci. 2021, 86, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, S.; Zhang, P.; Zhu, F.; Ren, X.; Liu, C.; Zhang, X. On the role of physiological substances, abscisic acid and its biosynthetic genes in seed maturation and dormancy of tree peony (Paeonia ostii ‘Feng Dan’). Sci. Hortic. 2015, 182, 92–101. [Google Scholar] [CrossRef]
- Gerivani, Z.; Vashaee, E.; Sadeghipour, H.R.; Aghdasi, M.; Shobbar, Z.-S.; Azimmohseni, M. Short versus long term effects of cyanide on sugar metabolism and transport in dormant walnut kernels. Plant Sci. 2016, 252, 193–204. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, Q.; Yang, Y.; Guo, J.; Song, J. Utilisation of stored lipids during germination in dimorphic seeds of euhalophyte Suaeda salsa. Funct. Plant Biol. 2018, 45, 1009–1016. [Google Scholar] [CrossRef]
- Sun, H.; Li, Q.; Zhu, M.; Li, Y.; Li, S. Dynamic changes of nutrients of Paeonia ostii ‘Feng Dan’ seed during its dormancy breaking. J. Nanjing For. Univ. 2021, 45, 70–78, (In Chinese with English abstract). [Google Scholar]
- Zhao, M.; Zhang, H.; Yan, H.; Qiu, L.; Baskin, C.C. Mobilization and role of starch, protein, and fat reserves during seed germination of six wild grassland species. Front. Plant Sci. 2018, 9, 234. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Eastmond, P.J. Seed storage oil catabolism: A story of give and take. Curr. Opin. Plant Biol. 2012, 15, 322–328. [Google Scholar] [CrossRef]
- Yue, J.; Li, M. Review of the mobilization and change of seed storage materials during germination. Seed 2021, 40, 56–62, (In Chinese with English abstract). [Google Scholar]
- Rudolph, M.; Schlereth, A.; Körner, M.; Feussner, K.; Berndt, E.; Melzer, M.; Hornung, E.; Feussner, I. The lipoxygenase-dependent oxygenation of lipid body membranes is promoted by a patatin-type phospholipase in cucumber cotyledons. J. Exp. Bot. 2010, 62, 749–760. [Google Scholar] [CrossRef]
- Zou, Y.; Zhu, M.; Li, Y.; Zhai, J.; Li, S. Daynamic changes in nutrients content and related enzymes activity during Paeonia ostii ‘Feng Dan’ seeds development. J. Nanjing For. Univ. 2021, 45, 62–70, (In Chinese with English abstract). [Google Scholar]
- Iwasaki, M.; Penfield, S.; Lopez-Molina, L. Parental and Environmental Control of Seed Dormancy in Arabidopsis thaliana. Annu. Rev. Plant Biol. 2022, 73, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.-P.; He, Z.; Li, H.; Shi, L.; Tang, Y.-D. Effect of root length on epicotyl dormancy release in seeds of Paeonia ludlowii, Tibetan peony. Ann. Bot. 2013, 113, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, J.; Xu, F.; Chu, J.; Yan, C.; Schläppi, M.R.; Wang, Y.; Chu, C. Expression patterns of ABA and GA metabolism genes and hormone levels during rice seed development and imbibition: A comparison of dormant and non-dormant rice cultivars. J. Genet. Genom. 2014, 41, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Yang, Y.; Wang, Z.; Xiong, F. Effects of exogenous gibberellic acid and abscisic acid on germination, amylases, and endosperm structure of germinating wheat seeds. Seed Sci. Technol. 2016, 44, 64–76. [Google Scholar] [CrossRef]
- Shahpiri, A.; Talaei, N.; Finnie, C. Spatio-temporal appearance of α-amylase and limit dextrinase in barley aleurone layer in response to gibberellic acid, abscisic acid and salicylic acid. J. Sci. Food Agric. 2015, 95, 141–147. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).