Phytophthora sansomeana, an Emerging Threat to Soybean Production
Abstract
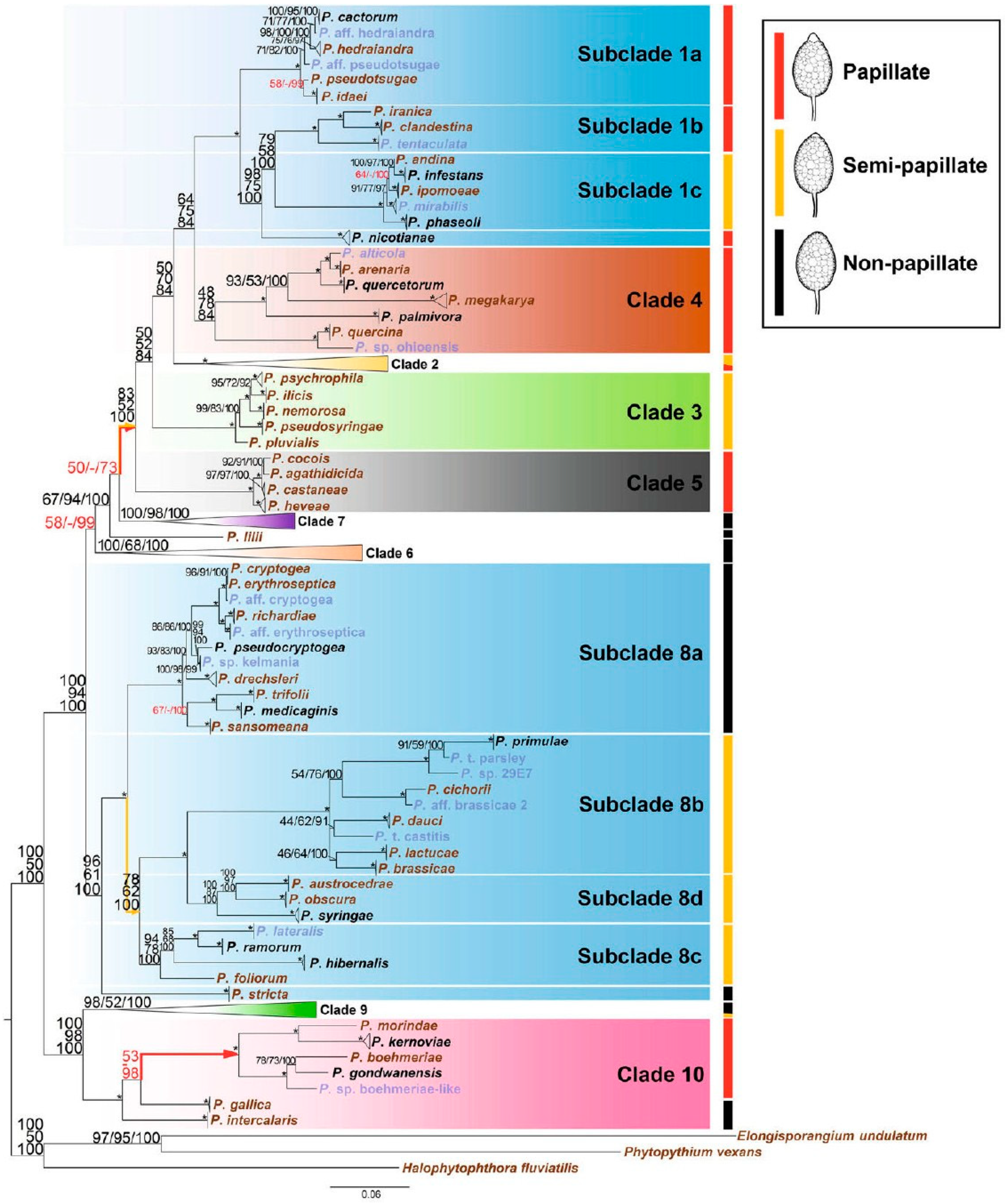
:1. Introduction
2. Nomenclature
3. Morphology and Identification
4. Hosts and Distribution
5. Disease Symptoms and Pathogenicity
5.1. On Soybean
5.2. On Non-Soybean Hosts
6. Genetic Variability
7. Disease Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaufmann, M.J.; Gerdemann, J.W. Root and stem rot of soybean caused by Phytophthora sojae n. sp. Phytopathology 1958, 48, 201–208. [Google Scholar]
- Reeser, P.W.; Scott, D.H.; Ruhl, G.E. Recovery of race non-classifiable Phytopthora megasperma f. sp. glycinea from soybean roots in Indiana in 1990. In Proceedings of the APS Annual Meeting, St. Louis, MO, USA, 17–20 August 1991; p. 1201. [Google Scholar]
- Hansen, E.M.; Wilcox, W.F.; Reeser, P.W.; Sutton, W. Phytophthora rosacearum and P. sansomeana, new species segregated from the Phytophthora megasperma “complex”. Mycologia 2009, 101, 129–135. [Google Scholar] [CrossRef]
- Dreschler, C. A crown-rot of hollyhocks caused by Phytopthora megasperma n. sp. J. Wash. Acad. Sci. 1931, 21, 513–526. [Google Scholar]
- Tompkins, C.M.; Tucker, C.M.; Gardner, M.W. Phytophthora root rot of cauliflower. J. Agric. Res. 1936, 53, 685–692. [Google Scholar]
- Hansen, E.M.; Brasier, C.M.; Shaw, D.S.; Hamm, P.B. The taxonomic structure of Phytophthora megasperma: Evidence for emerging biological species groups. Trans. Br. Mycol. Soc. 1986, 87, 557–573. [Google Scholar] [CrossRef]
- Hansen, E.M.; Hamm, P.B. Morphological differentiation of host-specialized groups of Phytophthora megasperma. Phytopathology 1983, 73, 129–134. [Google Scholar] [CrossRef]
- Förster, H.; Coffey, M.D. Molecular taxonomy of Phytophthora megasperma based on mitochondrial and nuclear DNA polymorphisms. Mycol. Res. 1993, 97, 1101–1112. [Google Scholar] [CrossRef]
- Hamm, P.B.; Hansen, E.M. Host specificity of Phytophthora megasperma from Douglas fir, soybean, and alfalfa. Phytopathology 1981, 71, 65–68. [Google Scholar] [CrossRef]
- Hamm, P.B.; Hansen, E.M. Identification of Phytophthora spp. known to attack conifers in the Pacific Northwest. Northwest Sci. 1987, 61, 103–109. [Google Scholar]
- Wilcox, W.F.; Mircetich, S.M. Lack of host specificity among isolates of Phytophthora megasperma. Phytopathology 1987, 77, 1132–1137. [Google Scholar] [CrossRef] [Green Version]
- Cooke, D.E.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Blair, J.E.; Coffey, M.D.; Park, S.Y.; Geiser, D.M.; Kang, S. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 2008, 45, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.N.; Blair, J.E.; Coffey, M.D. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet. Biol. 2014, 66, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tyler, B.M.; Hong, C. An expanded phylogeny for the genus Phytophthora. IMA Fungus 2017, 8, 355–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christova, P.K.; Kostov, K.V.; Lyubenova, A.B.; Slavov, S.B. A new hybrid of Phytophthora from Southeast Europe. Mycologia 2021, 113, 734–747. [Google Scholar] [CrossRef] [PubMed]
- Safaiefarahani, B.; Mostowfizadeh-Ghalamfarsa, R.; Hardy, G.; Burgess, T.I. Species from within the Phytophthora cryptogea complex and related species, P. erythroseptica and P. sansomeana, readily hybridize. Fungal Biol. 2016, 120, 975–987. [Google Scholar] [CrossRef]
- Safaiefarahani, B.; Mostowfizadeh-Ghalamfarsa, R.; Hardy, G.E.S.J.; Burgess, T.I. Re-evaluation of the Phytophthora cryptogea species complex and the description of a new species, Phytophthora pseudocryptogea sp. nov. Mycol. Prog. 2015, 14, 1–12. [Google Scholar] [CrossRef]
- Dorrance, A.E. Management of Phytophthora sojae of soybean: A review and future perspectives. Can. J. Plant Pathol. 2018, 40, 210–219. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocol: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Bonants, P.; Weerdt, M.H.-d.; Gent-Pelzer, M.v.; Lacourt, I.; Cooke, D.; Duncan, J. Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. Eur. J. Plant Pathol. 1997, 103, 345–355. [Google Scholar] [CrossRef]
- Rojas, J.A.; Miles, T.D.; Coffey, M.D.; Martin, F.N.; Chilvers, M.I. Development and application of qPCR and RPA genus- and species-specific detection of Phytophthora sojae and P. sansomeana root rot pathogens of soybean. Plant Dis. 2017, 101, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.A.; Jacobs, J.L.; Napieralski, S.; Karaj, B.; Bradley, C.A.; Chase, T.; Esker, P.D.; Giesler, L.J.; Jardine, D.J.; Malvick, D.K.; et al. Oomycete species associated with soybean seedlings in North America-part II: Diversity and ecology in relation to environmental and edaphic factors. Phytopathology 2017, 107, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, K.; Eyre, M.; McDuffee, D.; Dorrance, A.E. The efficacy of ethaboxam as a soybean seed treatment toward Phytophthora, Phytopythium, and Pythium in Ohio. Plant Dis. 2020, 104, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Phibbs, A. New Phytophthora spp. Causing Root Rot on Soybean in Wisconsin. Available online: https://ipcm.wisc.edu/blog/category/crop-manager/ (accessed on 29 May 2022).
- Tande, C.; Dorrance, A.E.; Schwarzrock, D.; Mahecha, E.; Byamukama, E. First report of Phytophthora sansomeana causing root rot of soybean in South Dakota. Plant Dis. 2020, 104, 1877. [Google Scholar] [CrossRef]
- Tang, Q.H.; Gao, F.; Li, G.Y.; Wang, H.; Zheng, X.B.; Wang, Y.C. First Report of Root Rot Caused by Phytophthora sansomeana on Soybean in China. Plant Dis 2010, 94, 378. [Google Scholar] [CrossRef]
- Malvick, D.K.; Grunden, E. Traits of soybean-Infecting Phytophthora populations from Illinois agricultural fields. Plant Dis. 2004, 88, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Hamm, P.B.; Hansen, E.M. Pathogenicity of Phytopthora species to Pacific Northwest conifers. Eur. J. Plant Pathol. 1982, 12, 167–174. [Google Scholar]
- McKeever, K.M.; Chastagner, G.A. A survey of Phytophthora spp. associated with Abies in U.S. Christmas tree farms. Plant Dis. 2016, 100, 1161–1169. [Google Scholar] [CrossRef]
- Pettersson, M.; Frampton, J.; Rönnberg, J.; Shew, H.D.; Benson, D.M.; Kohlway, W.H.; Escanferla, M.E.; Cubeta, M.A. Increased diversity of Phytophthora species in Fraser fir Christmas tree plantations in the Southern Appalachians. Scand. J. For. Res. 2017, 32, 412–420. [Google Scholar] [CrossRef]
- An, T.J.; Park, M.S.; Jeong, J.T.; Kim, Y.G.; Kim, Y.I.; Lee, E.S.; Chang, J.K. Occurrence of the Phytophthora blight caused by Phytophthora sansomeana in Atractylodes macrocephala Koidz. Korean J. Med. Crop Sci. 2019, 27, 404–411. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.Z.; Uematsu, S.; Coffey, M.D.; Uzuhashi, S.; Suga, H.; Kageyama, K. Re-evaluation of Japanese Phytophthora isolates based on molecular phylogenetic analyses. Mycoscience 2013, 55, 314–327. [Google Scholar] [CrossRef]
- Grígel, J.; Černý, K.; Mrázková, M.; Havrdová, L.; Zahradník, D.; Jílková, B.; Hrabetová, M. Phytophthora root and collar rots in fruit orchards in the Czech Republic. Phytopathol. Mediterr. 2019, 58, 261–275. [Google Scholar] [CrossRef]
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Fu, H.; Zhou, Q.; Strelkov, S.E.; Turnbull, G.D. First report of Phytophthora sansomeana causing root rot in field pea in Alberta, Canada. Crop Prot. 2017, 101, 1–4. [Google Scholar] [CrossRef]
- Betz, E.C.; MacDonald, J.L.; Punja, Z.K. New and recurring pathogens of wasabi in British Columbia. In Proceedings of the BC Regional Meeting, Summerland, BC, Canada, 27 October 2016. [Google Scholar]
- Zelaya-Molina, L.X.; Ellis, M.L.; Berry, S.A.; Dorrance, A.E. First report of Phytophthora sansomeana causing wilting and stunting on corn in Ohio. Plant Dis. 2010, 94, 125. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.A.; Witte, A.; Noel, Z.A.; Jacobs, J.L.; Chilvers, M.I. Diversity and characterization of Oomycetes associated with corn seedlings in Michigan. Phytobiomes J. 2019, 3, 224–234. [Google Scholar] [CrossRef]
- Phibbs, A.; Fieweger, S.; Barta, A.; Lueloff, S. Early Season Soybean Root Rot Survey. Available online: https://datcp.wi.gov/Documents/EarlySeasonSoybeanSurvey.pdf (accessed on 28 June 2022).
- Bienapfl, J.C.; Malvick, D.K.; Percich, J.A. Specific molecular detection of Phytophthora sojae using conventional and real-time PCR. Fungal Biol. 2011, 115, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ugwuja, F.N.; Chiejina, N.V.; Ugwuoke, K.I.; Akanwa, F.E.; Onifade, A.K.; Tatfeng, M. First report of Phytophthora sansomeana (E.M. Hansen) causing leaf blight of taro (Colocasia esculenta (L.) Schott) in South Eastern Nigeria. Int. J. Pathog. Res. 2022, 9, 20–30. [Google Scholar] [CrossRef]
- Cai, G.; Scofield, S.R. Mitochondrial genome sequence of Phytophthora sansomeana and comparative analysis of Phytophthora mitochondrial genomes. PLoS ONE 2020, 15, e0231296. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, W.; McCoy, A.G.; Gao, X.; Collins, P.J.; Zhang, N.; Wen, Z.; Cao, S.; Wani, S.H.; Gu, C.; et al. Molecular mapping of quantitative disease resistance loci for soybean partial resistance to Phytophthora sansomeana. Theor. Appl. Genet. 2021, 134, 1977–1987. [Google Scholar] [CrossRef]
| Host | Common Name | Family | Isolation Locations | Koch’s Criteria a | Publications | Designation |
|---|---|---|---|---|---|---|
| Abies amabilis | Pacific silver fir | Pinaceae | P | [29] | Phytophthora megasperma Group 1 | |
| Abies concolor | White fir | Pinaceae | P | [29] | Phytophthora megasperma Group 1 | |
| Abies fraseri | Fraser fir | Pinaceae | North Carolina, New York, Wisconsin, Michigan (USA) | K | [30,31] | Phytophthora sansomeana |
| Abies magnifica var. shastensis | Shast red fir | Pinaceae | P | [29] | Phytophthora megasperma Group 1 | |
| Abies procera | Noble fir | Pinaceae | P | [29] | Phytophthora megasperma Group 1 | |
| Atractylodes macrocephala | Bai Zhu | Asteraceae | Korea | I + P | [32] | Phytophthora sansomeana |
| Chamaecyparis lawsonia | Lawson cypress | Cupressaceae | Europe | I | [33] | Phytophthora sansomeana |
| Daucus carota | Wild carrot | Apiaceae | New York (USA) | I | [6] | Phytophthora megasperma DF protein group |
| Gerbera spp. | African daisy | Asteraceae | Japan | R | [34] | Phytophthora sansomeana |
| Glycine max | Soybean | Fabaceae | Ontario (CA), China, Iowa, Illinois, Indiana, Kansas, Michigan, Minnesota, Nebraska, Ohio, South Dakota, Wisconsin (USA) | K | [2,23,24,25,26,27] | Phytophthora sansomeana |
| Malus domestica | Common apple | Rosaceae | Czech Republic | K | [35] | Phytophthora sansomeana |
| Medicago sativa | Alfalfa | Fabaceae | P | [9] | Phytophthora megasperma Douglas-fir Group 1 | |
| Pisum sativa | Field pea | Fabaceae | Alberta (CA) | K | [36] | Phytophthora sansomeana |
| Prunus domestica | European plum | Rosaceae | Europe | I | [33] | Phytophthora sansomeana |
| Prunus insitia | St. Julien plum | Rosaceae | P | [35] | Phytophthora sansomeana | |
| Prunus mahaleb | Mahaleb cherry | Rosaceae | P | [11] | Phytophthora megasperma DF1 | |
| Pseudotsuga menziesii | Douglas-fir | Pinaceae | Oregon (USA) | K | [7,9] | Phytophthora megasperma Douglas-fir Group 1; Phytophthora megasperma DF1 |
| Pyrus communis | Common pear | Rosaceae | Czech Republic | I | [35] | Phytophthora sansomeana |
| Silene latifolia subsp. Alba | White cockle | Caryophyllaceae | New York (USA) | I | [6,8] b | Phytophthora megasperma DF protein group; Phytophthora megasperma mtDNA Group F1 |
| Wasabia japonica | Wasabi | Brassicaceae | British Columbia (CA) | K | [37] | Phytophthora sansomeana |
| Zea mays | Maize | Poaceae | Michigan, Ohio (USA) | K | [38,39] | Phytophthora sansomeana |
| Species | Designation | Isolates | Publication |
|---|---|---|---|
| Phytophthora megasperma | Douglas-fir Group 1 | Hamm 284; Hamm 304 *; Hamm 306; Hamm 385; Hamm B2-17 *; Hamm B3A * | [9] |
| Phytophthora megasperma | Douglas-fir D1 | Hamm 345 *; Hamm B2-17 *; Hamm B3A * | [7] |
| Phytophthora megasperma | DF | Hamm 304 *; Hamm 345 *; Hamm B2-17 *; Hamm B3A *; Stack Car2(86) *; Stack WCl(75) * | [6] |
| Phytophthora megasperma | Group 1 | [10] | |
| Phytophthora megasperma | DF1 | Hamm 304 *; Hamm 306 | [11] |
| Phytophthora megasperma f. sp. glycinea | Race non-classifiable | 20 isolates including OSU 1819B *, 2323 *, 2516A *, 9284 * | [2] |
| Phytophthora megasperma | mtDNA group F1 | Hamm 304 *; Hamm 306; Hamm B3A *; IMI280906; Stack Car2(86) *; Stack WCl(75) * | [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detranaltes, C.E.; Ma, J.; Cai, G. Phytophthora sansomeana, an Emerging Threat to Soybean Production. Agronomy 2022, 12, 1769. https://doi.org/10.3390/agronomy12081769
Detranaltes CE, Ma J, Cai G. Phytophthora sansomeana, an Emerging Threat to Soybean Production. Agronomy. 2022; 12(8):1769. https://doi.org/10.3390/agronomy12081769
Chicago/Turabian StyleDetranaltes, Christopher Evan, Jianxin Ma, and Guohong Cai. 2022. "Phytophthora sansomeana, an Emerging Threat to Soybean Production" Agronomy 12, no. 8: 1769. https://doi.org/10.3390/agronomy12081769
APA StyleDetranaltes, C. E., Ma, J., & Cai, G. (2022). Phytophthora sansomeana, an Emerging Threat to Soybean Production. Agronomy, 12(8), 1769. https://doi.org/10.3390/agronomy12081769






