Chitosan and Titanium Dioxide Are More Effective in Improving Seed Yield and Quality in Nanoparticle Compared to Non-Structured Form: A Case Study in Five Milk Thistle Ecotypes (Silybum marianum (L.) Gaertn.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Preparation of Spray Solutions (Chitosan) and Suspensions (TiO2)
2.3. Stem Length, Capitulum, and Seed Characteristics
2.4. Leaf Photosynthetic Pigment Content
2.5. Leaf Water Status
2.6. Ratio of Variable to Maximum Chlorophyll Fluorescence
2.7. Leaf Gas-Exchange Traits
2.8. Seed Oil Content
2.9. Seed Mineral Content
2.10. Statistical Analysis
3. Results
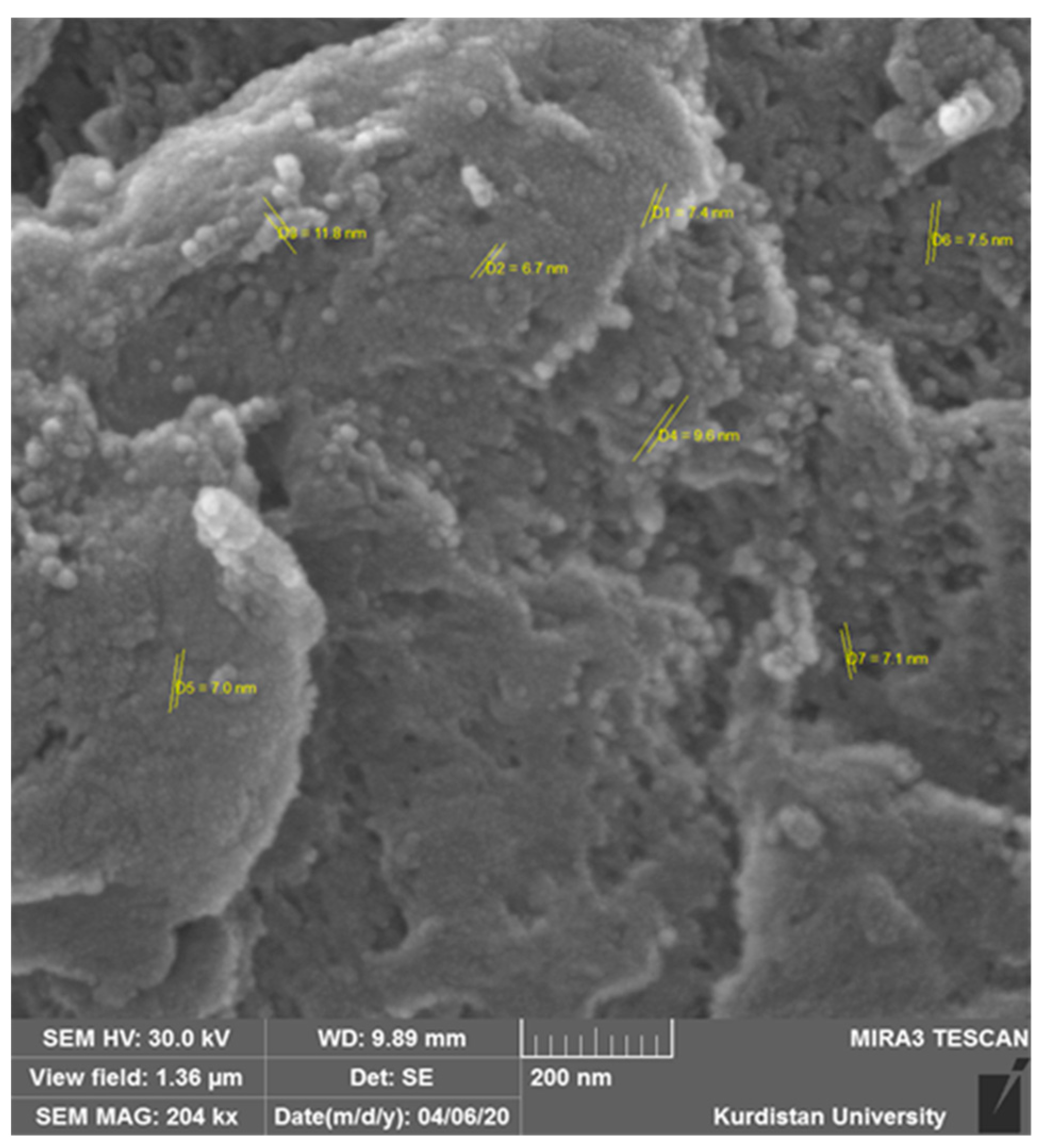
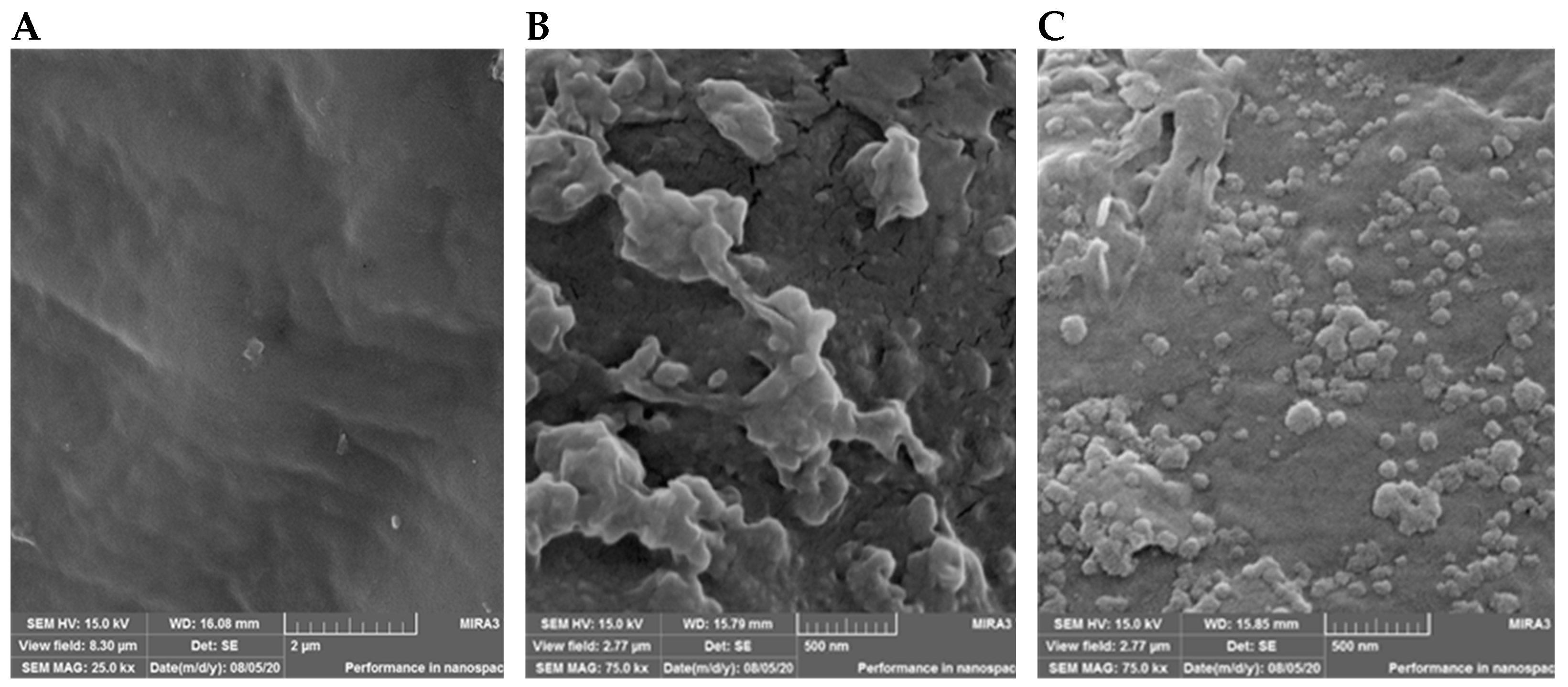
3.1. Validation of the Presence of NPs on the Leaf Surface
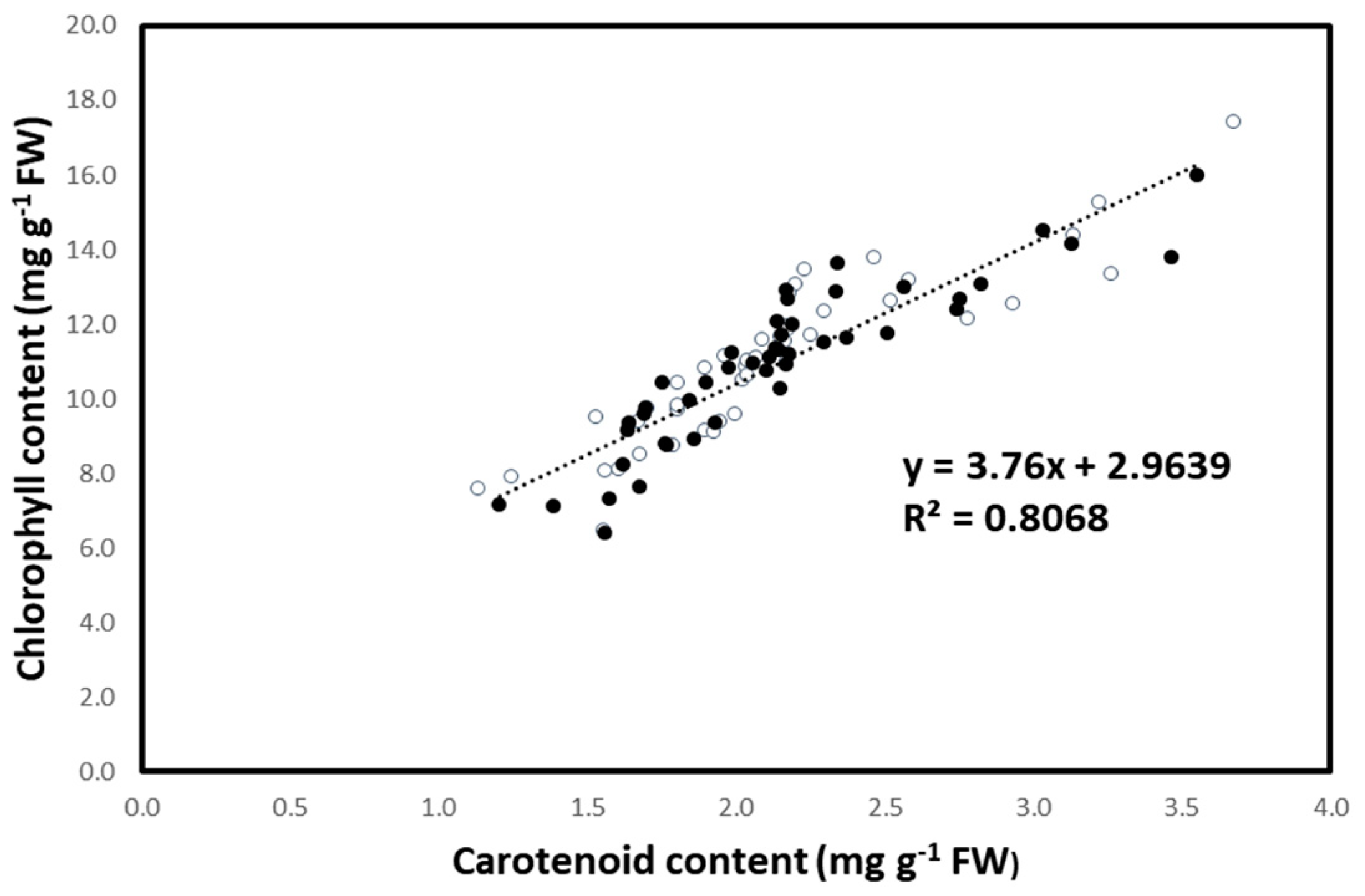
3.2. Stem Length and Leaf Photosynthetic Pigment Content
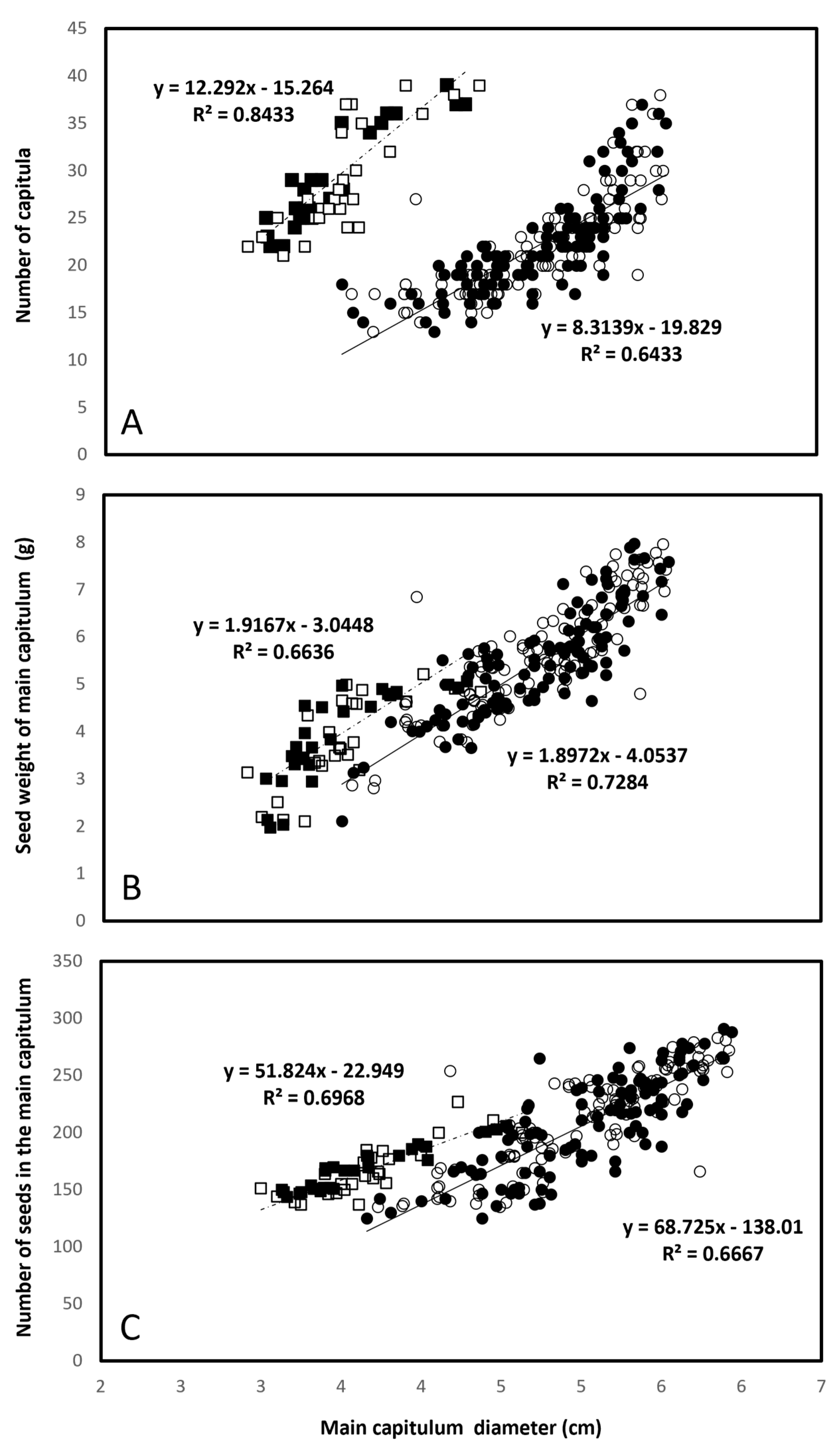
3.3. Traits Underlying Seed Yield
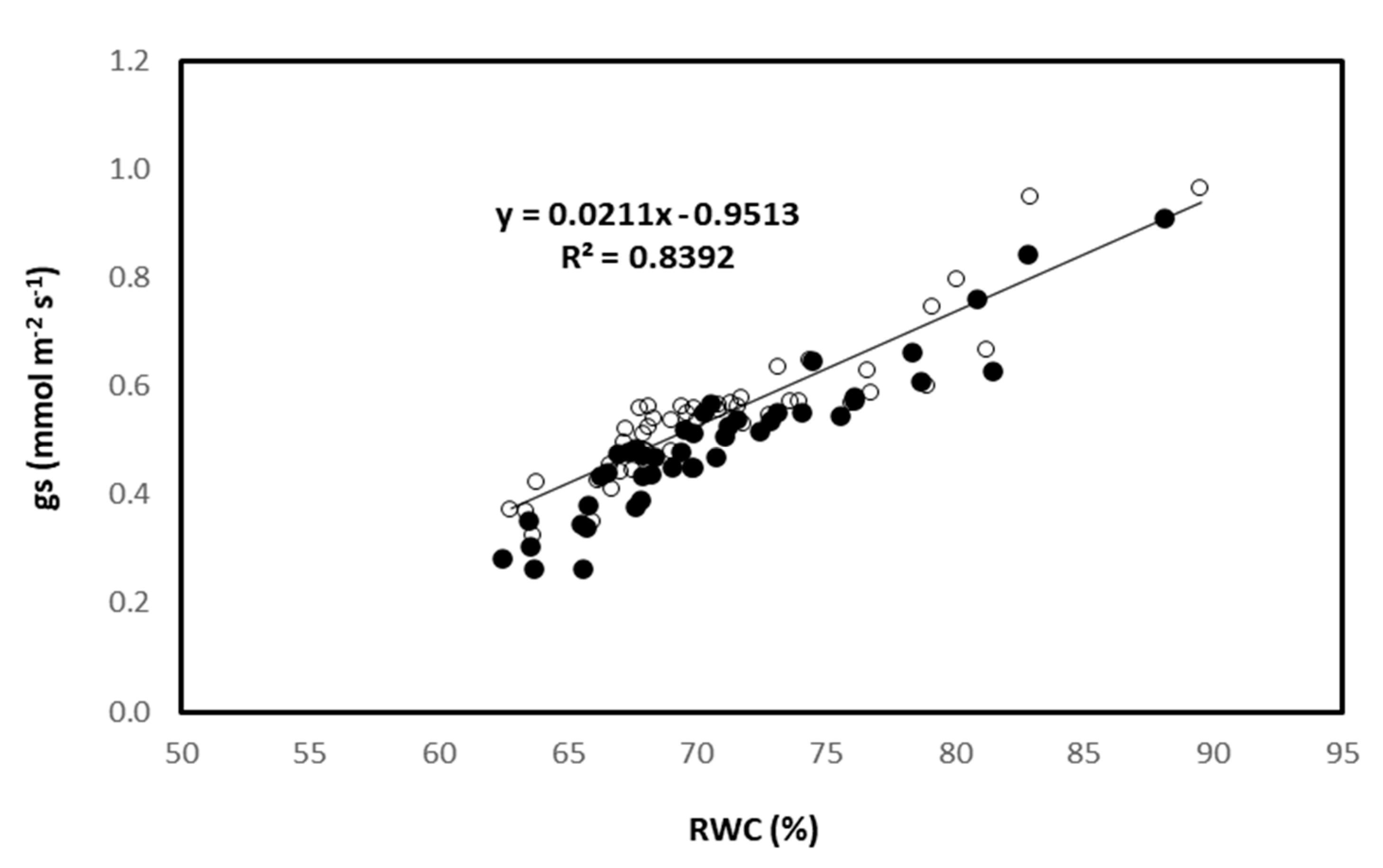
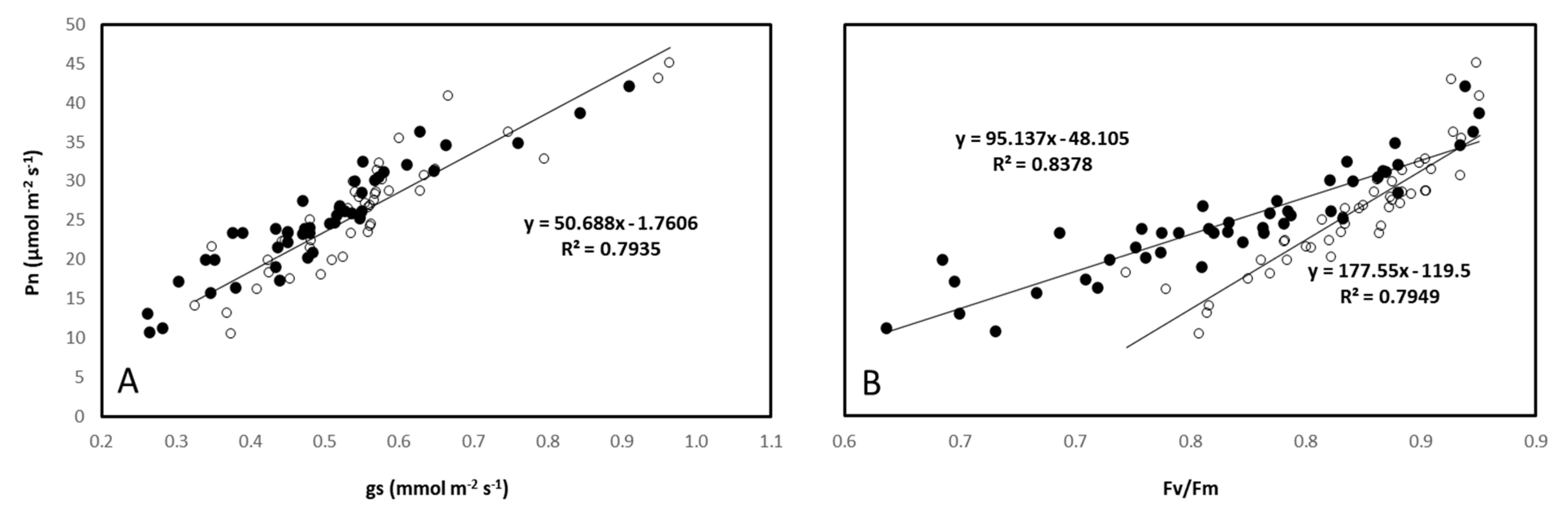
3.4. Leaf Gas–Exchange Traits, Chlorophyll Fluorescence, and Hydration Status
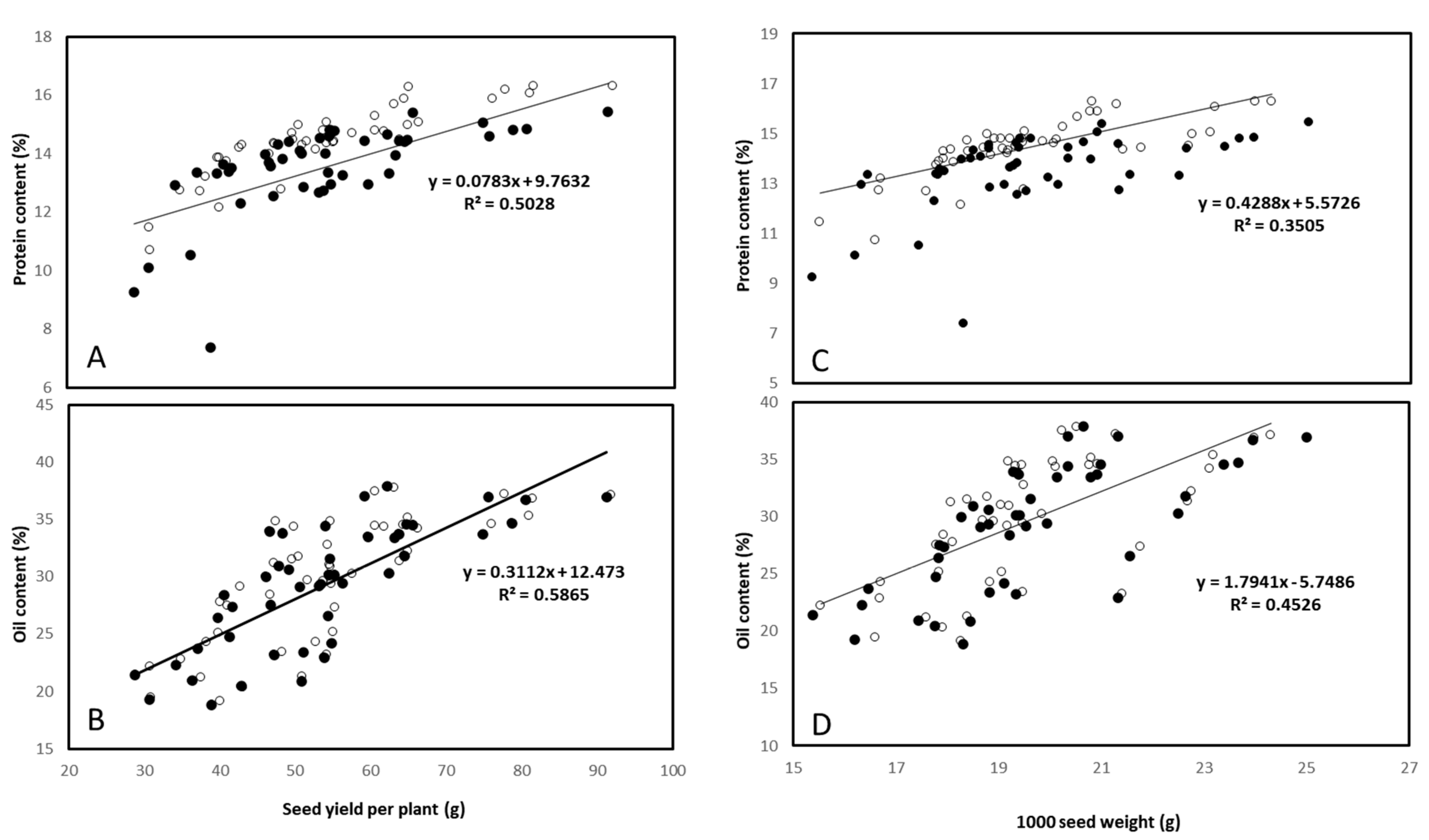
3.5. Seed Mineral, Protein, and Oil Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khan, M.A.; Blackshaw, R.E.; Marwat, K.B. Biology of milk thistle (Silybum marianum) and the management options for growers in north-western Pakistan. Weed Biol. Manag. 2009, 9, 99–105. [Google Scholar] [CrossRef]
- Bielski, S. Milk thistle (Silybum marianum L. Gaertn.) achene yield had a positive response to nitrogen fertilization, row spacing, sowing date, and weed control methods. Ind. Crops Prod. 2021, 160, 113104. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, S.; Liu, H.; Li, B.Z.; Che, L. Constituents and thermal properties of milk thistle seed oils extracted with three methods. LWT 2020, 126, 109282. [Google Scholar] [CrossRef]
- Haban, M.; Otepka, P.; Kobida, L.; Habanova, M. Production and quality of milk thistle (Silybum marianum L. Gaertn.) cultivated in cultural conditions of warm agri–climatic macroregion. Hortic. Sci. 2009, 36, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of milk thistle (Silybum marianum L. Gaertn.), a medicinal weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Alemardan, A.; Karkanis, A.; Salehi, R. Breeding Objectives and Selection Criteria for Milk Thistle [Silybum marianum L. Gaertn.] Improvement. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Lukic, I.; Milovanovic, S.; Pantic, M.; Srbljak, I.; Djuric, A.; Tadic, V.; Tyśkiewicz, K. Separation of high–value extracts from Silybum marianum seeds: Influence of extraction technique and storage on composition and bioactivity. LWT 2022, 160, 113319. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and antioxidant potential of cold–pressed oils—Possible strategies to improve oil stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Rahal, N.B.; Barba, F.J.; Barth, D.; Chevalot, I. Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco–2 cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar] [CrossRef]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- Liava, V.; Karkanis, A.; Tsiropoulos, N. Yield and silymarin content in milk thistle (Silybum marianum L. Gaertn.) fruits affected by the nitrogen fertilizers. Ind. Crops Prod. 2021, 171, 113955. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, H.; Aghaee Dizaj, L.; Aghaee, A.; Ghorbanpour, M. Chitosan–mediated changes in dry matter, total phenol content and essential oil constituents of two origanum species under water deficit stress. Gerund Pflanz. 2021, 73, 181–191. [Google Scholar] [CrossRef]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; Reis, C.O.D.; Karam, D.; Doriguetto, A.C.; Santos, M.H.D.; Santos Filho, P.R.D.S.; Souza, T.C.D. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci. Rep. 2019, 9, 8164. [Google Scholar] [CrossRef] [PubMed]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al–Doss, A.; Ibrahim, E.; Rashwan, E.; El–Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water–stressed barley plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Bakhoum, G.S.; Sadak, M.S.; Badr, E.A.E.M. Mitigation of adverse effects of salinity stress on sunflower plant (Helianthus annuus L.) by exogenous application of chitosan. Bull. Natl. Res. Cent. 2020, 44, 79. [Google Scholar] [CrossRef]
- Kahromi, S.; Khara, J. Chitosan stimulates secondary metabolite production and nutrient uptake in medicinal plant Dracocephalum kotschyi. Sci. Food Agric. 2021, 101, 3898–3907. [Google Scholar] [CrossRef]
- Tantawy, I.A.A.; Soltan, H.H.; Ezzat, A.S. Efficiency of foliar application by chitosan and royal jelly on growth, yield and quality of two garlic cultivars. SVU Int. J. Agric. Sci. 2021, 3, 119–131. [Google Scholar] [CrossRef]
- Saad–Allah, K.M.; Fetouh, M.I.; Elhaak, M.A. Induction of milk thistle (Silybum marianum L. Gaertn) growth and phytochemicals production by natural stimulants. J. Appl. Res. Med. Aromat. Plants 2017, 6, 101–110. [Google Scholar] [CrossRef]
- Ahmad, B.; Shabbir, A.; Jaleel, H.; Khan, M.M.A.; Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant Biol. 2018, 13, 6–15. [Google Scholar] [CrossRef]
- Khater, R.M.; Sabry, R.M.; Pistelli, L.; Abd–ElGawad, A.M.; Soufan, W.; El–Gendy, A.N.G. Effect of Compost and Titanium Dioxide Application on the Vegetative Yield and Essential Oil Composition of Coriander. Sustainability 2021, 14, 322. [Google Scholar] [CrossRef]
- Hussain, S.; Shafiq, I.; Chattha, M.S.; Mumtaz, M.; Brestic, M.; Rastogi, A.; Chen, G.; Allakhverdiev, S.I.; Liu, W.; Yang, W. Effect of Ti treatments on growth, photosynthesis, phosphorus uptake and yield of soybean (Glycine max L.) in maize–soybean relay strip intercropping. Environ. Exp. Bot. 2021, 187, 104476. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Mohamadi, N.; Shekari, F.; Abbasi, A.; Esmailpour, M. The effects of silicon and titanium on safflower (Carthamus tinctorius L.) growth under moisture deficit condition. Acta. Agric. Slov. 2017, 109, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Mohammadhassan, R.; Ferdosi, A.; Seifalian, A.M.; Seifalian, M.; Malmir, S. Nanoelicitors Application Promote Antioxidant Capacity of Asparagus officinalis (In Vitro). J. Trop. Life Sci. 2021, 11, 259–265. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in finger millet plants against blast disease caused by Pyricularia Grisea. Carbohydr. Polym. 2021, 154, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Behnam, H.; Feizi, H.; Alipanah, M. Alleviation the effects of salinity stress using titanium dioxide nano and bulk particles in Echinacea seeds and seedlings. Adv. Hortic. Sci. 2021, 35, 351–360. [Google Scholar] [CrossRef]
- Aghdam, M.T.B.; Mohammadi, H.; Ghorbanpour, M. Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well–watered and drought stress conditions. Rev. Bras. Bot. 2016, 39, 139–146. [Google Scholar] [CrossRef]
- Asgari–Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano–chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef]
- Jafari, L.; Abdollahi, F.; Feizi, H.; Adl, S. Improved Marjoram (Origanum majorana L.) Tolerance to Salinity with Seed Priming Using Titanium Dioxide (TiO2). Iran. J. Sci. Technol. Trans. Sci. 2022, 46, 361–371. [Google Scholar] [CrossRef]
- Lei, C.; Ma, D.; Pu, G.; Qiu, X.; Du, Z.; Wang, H.; Liu, B. Foliar application of chitosan activates artemisinin biosynthesis in Artemisia annua L. Ind. Crops Prod. 2011, 33, 176–182. [Google Scholar] [CrossRef]
- Mumivand, H.; Shayganfar, A.; Tsaniklidis, G.; Emami Bistgani, Z.; Fanourakis, D.; Nicola, S. Pheno–Morphological and Essential Oil Composition Responses to UVA Radiation and Protectants: A Case Study in Three Thymus Species. Horticulturae 2022, 8, 31. [Google Scholar] [CrossRef]
- Yousefzadeh, K.; Houshmand, S.; Shiran, B.; Mousavi–Fard, S.; Zeinali, H.; Nikoloudakis, N.; Gheisari, M.M.; Fanourakis, D. Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy 2022, 12, 1008. [Google Scholar] [CrossRef]
- Safari, M.; Mousavi–Fard, S.; Rezaei Nejad, A.; Sorkheh, K.; Sofo, A. Exogenous salicylic acid positively affects morpho–physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 2021, 19, 969–984. [Google Scholar] [CrossRef]
- Anitha, A.; Rani, V.D.; Krishna, R.; Sreeja, V.; Selvamurugan, N.; Nair, S.V.; Tamura, h.; Jayakumar, R. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O–carboxymethyl and N, O–carboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2009, 78, 672–677. [Google Scholar] [CrossRef]
- Zomorrodi, N.; Rezaei Nejad, A.; Mousavi–Fard, S.; Feizi, H.; Tsaniklidis, G.; Fanourakis, D. Potency of titanium dioxide nanoparticles, sodium hydrogen sulfide and salicylic acid in ameliorating the depressive effects of water deficit on periwinkle ornamental quality. Horticulturae 2022, 8, 675. [Google Scholar] [CrossRef]
- Ivani, R.; Sanaei Nejad, S.H.; Ghahraman, B.; Astaraei, A.R.; Feizi, H. Role of bulk and Nanosized SiO2 to overcome salt stress during Fenugreek germination (Trigonella foenum–graceum L.). Plant Signal. Behav. 2018, 13, e1044190. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi–Majd, M.; Mousavi–Fard, S.; Rezaei Nejad, A.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022, 9, 15. [Google Scholar] [CrossRef]
- Ahmadi–Majd, M.; Rezaei Nejad, A.; Mousavi–Fard, S.; Fanourakis, D. Postharvest application of single, multi–walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2022, 97, 346–360. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Taheri–Garavand, A.; Rezaei Nejad, A.; Fanourakis, D.; Fatahi, S.; Ahmadi–Majd, M. Employment of artificial neural networks for non–invasive estimation of leaf water status using color features: A case study in Spathiphyllum wallisii. Acta. Physiol. Plant. 2021, 43, 78. [Google Scholar] [CrossRef]
- Sørensen, H.K.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Nejad, A.R.; Ottosen, C.O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. 2020, 267, 109354. [Google Scholar] [CrossRef]
- Javadi Asayesh, E.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae 2021, 7, 485. [Google Scholar] [CrossRef]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Estaji, A.; Souri, M.K.; Omidbaigi, R. Evaluation of different levels of nitrogen and flower pruning on milk thistle (Silybum marianum L.) yield and fatty acids. J. Med. Spice Plants 2011, 16, 170–175. [Google Scholar]
- Chatzistathis, T.; Fanourakis, D.; Aliniaeifard, S.; Kotsiras, A.; Delis, C.; Tsaniklidis, G. Leaf age–dependent effects of boron toxicity in two cucumis melo varieties. Agronomy 2021, 11, 759. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil. Sci. 1962, 93, 68. [Google Scholar] [CrossRef] [Green Version]
- Waling, I.; Van Vark, W.; Houba, V.J.G.; Van der Lee, J.J. Soil and plant analysis, a series of syllabi: Part 7. In Plant Analysis Procedures; Wageningen Agriculture University: Wageningen, The Netherlands, 1989. [Google Scholar]
- Bishni, U.R.; Hughes, I.L. Agronomic performance and protein content of fall planted triticale, wheat and Rye. Agronomy 1979, 71, 359–360. [Google Scholar] [CrossRef]
- Qavami, N.; Labbafi, M.R.; Dehghani–Meshkani, M.R.; Mehrafarin, A. Determination of seed and oil yield and yield components in two variety of milk thistle (Silybum marianum Gaetrn.) based on path analysis and regression. J. Med. Plant 2012, 11, 78–85. [Google Scholar]
- Yaghoubian, I.; Antar, M.; Ghassemi, S.; Modarres–Sanavy, S.A.M.; Smith, D.L. The Effects of Hydro–Priming and Colonization with Piriformospora indica and Azotobacter chroococcum on Physio–Biochemical Traits, Flavonolignans and Fatty Acids Composition of Milk Thistle (Silybum marianum) under Saline Conditions. Plants 2022, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari Moheb Seraj, R.; Behnamian, M.; Ahmadikhah, A.; Shariati, V.; Dezhsetan, S. Chitosan and salicylic acid regulate morpho–physiological and phytochemical parameters and improve water–deficit tolerance in milk thistle (Silybum marianum L.). Acta. Physiol. Plant 2021, 43, 101. [Google Scholar] [CrossRef]
- Zangani, E.; Zehtab–Salmasi, S.; Andalibi, B.; Zamani, A.A.; Hashemi, M. Exogenous nitric oxide improved production and content of flavonolignans in milk thistle seeds under water deficit system. Acta. Physiol. Plant. 2021, 43, 87. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Sukweenadhi, J.; Sunderraj, S.; Shanmugam, R.; Thangavelu, L.; Mijakovic, I.; Perumalsamy, H. Chitosan, chitosan nanoparticles and modified chitosan biomaterials, a potential tool to combat salinity stress in plants. Carbohydr. Polym. 2022, 284, 119189. [Google Scholar] [CrossRef]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi–Sarvestani, Z.; Kassaee, M.Z.; Modarres–Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi–Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of drought damages by exogenous chitosan and yeast extract with modulating the photosynthetic pigments, antioxidant defense system and improving the productivity of garlic plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchawan, S.; Lotrakul, P.; Bunjongrat, R.; Chaidee, A.; Bangyeekhun, T. Chitosan effects on floral production, gene expression, and anatomical changes in the Dendrobium orchid. Sci. Hortic. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Van, S.N.; Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar]
- Ramadan, T.; Sayed, S.A.; Abd–Elaal, A.K.; Amro, A. The combined effect of water deficit stress and TiO2 nanoparticles on cell membrane and antioxidant enzymes in Helianthus annuus L. Physiol. Mol. Biol Plants 2022, 28, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiONPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum Moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Shabbir, A.; Khan, M.M.A.; Ahmad, B.; Sadiq, Y.; Jaleel, H.; Uddin, M. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynth. 2019, 57, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Hidangmayum, A.; Dwivedi, P. Chitosan Based Nanoformulation for Sustainable Agriculture with Special Reference to Abiotic Stress: A Review. J. Polym. Environ. 2022, 30, 1264–1283. [Google Scholar] [CrossRef]
- Kolesnikov, L.E.; Popova, E.V.; Novikova, I.I.; Kolesnikova, Y.R.; Balagurova, E.D. Application of Chitosan to Protect Wheat from Diseases and Boost Yields. Appl. Biochem. Microbiol. 2022, 58, 329–335. [Google Scholar] [CrossRef]


| Parameter | Year | |
|---|---|---|
| 2019/2020 | 2020/2021 | |
| Sand (%) | 22.5 | 22.1 |
| Slit (%) | 32.2 | 32.5 |
| Clay (%) | 45.3 | 45.4 |
| Electrical conductivity (dS m−1) | 2.4 | 2.3 |
| Organic carbon (%) | 0.4 | 0.43 |
| N (%) | 0.06 | 0.058 |
| P (ppm) | 11.8 | 12.04 |
| K (ppm) | 275 | 268 |
| Mean Square | ||||
|---|---|---|---|---|
| S.O.V | df | Stem Length | Chlorophyll Content | Carotenoid Content |
| Year (Y) | 1 | 12.03 ns | 1.16 ns | 0.014 ns |
| Replication (Year) (Ea) | 4 | 1.67 | 0.43 | 0.03 |
| Cultivar (Cul) | 4 | 3357.06 ** | 24.13 ** | 3.34 ** |
| Compound (Com) | 8 | 547.87 ** | 134.93 ** | 6.09 ** |
| Cul × Y | 4 | 0.20 ns | 0.09 ns | 0.006 ** |
| Com × Y | 8 | 1.17 ns | 0.53 ns | 0.02 ns |
| Cul × Com | 32 | 27.16 ** | 1.20 ** | 0.22 ** |
| Cul × Com × Y | 32 | 1.08 ns | 0.16 ns | 0.01 ns |
| Error (Eb) | 176 | 2.46 | 0.29 | 0.02 |
| CV (%) | 1.01 | 4.94 | 6.65 | |
| Ecotype | Compound/Concentration (mg L−1) | Stem Length (cm) | Chlorophyll Content (mg g−1 FW) | Carotenoid Content (mg g−1 FW) |
|---|---|---|---|---|
| ‘Sari’ | Control | 140.66 b’ | 7.69 v | 1.56 t |
| Ti 50 | 143 a’ | 8.91 r-t | 1.84 o-q | |
| Ti 100 | 145.83 xy | 10.62 mn | 1.89 n-q | |
| Ti NP 50 | 146.33 w–y | 11.67 i–k | 2.31 gh | |
| Ti NP 100 | 147.83 vw | 12.05 h–j | 2.40 g | |
| Ch 50 | 145.5 yz | 10.72 mn | 2 l–o | |
| Ch 100 | 146.83 v–y | 11.21 k–m | 2.10 j–l | |
| Ch NP 50 | 151.83 p–s | 14.89 b | 3.12 c | |
| Ch NP 100 | 150.66 r–t | 12.84 fg | 2.67 ef | |
| ‘Khomin’ | Control | 147.5 v–x | 7.37 v | 1.16 u |
| Ti 50 | 149.83 tu | 9.33 p–s | 1.58 t | |
| Ti 100 | 150.33 st | 9.89 op | 1.82 p–r | |
| Ti NP 50 | 152.16 o–r | 11.83 ij | 2.16 h–l | |
| Ti NP 100 | 153.33 n–q | 12.76 fg | 2.17 h–k | |
| Ch 50 | 150.33 st | 10.43 no | 1.77 p–s | |
| Ch 100 | 153 n–q | 11.47 j–l | 2.10 j–l | |
| Ch NP 50 | 156.83 jk | 14.25 c | 3.13 c | |
| Ch NP 100 | 154.66 l–n | 13.08 ef | 2.57 f | |
| ‘Khorramabad’ | Control | 158.33 ij | 7.51 v | 1.31 u |
| Ti 50 | 160.5 gh | 8.37 tu | 1.64 st | |
| Ti 100 | 161.66 fg | 954 qr | 1.67 r–t | |
| Ti NP 50 | 163.83 de | 10.63 mn | 2.06 k–m | |
| Ti NP 100 | 168.66 c | 11.44 j–l | 2.16 h–l | |
| Ch 50 | 163 ef | 9.03 q–s | 1.87 n–q | |
| Ch 100 | 163.83 de | 9.93 pq | 2.07 j–m | |
| Ch NP 50 | 179.66 a | 13.16 d–f | 2.28 g–i | |
| Ch NP 100 | 177.66 b | 11.92 ij | 2.18 h–l | |
| ‘Ahvaz’ | Control | 144 za’ | 6.42 w | 1.55 t |
| Ti 50 | 145.16 yz | 7.86 uv | 1.64 st | |
| Ti 100 | 145.66 yz | 9.49 p–r | 1.68 r–t | |
| Ti NP 50 | 150.83 r–t | 11 l–n | 2.07 j–m | |
| Ti NP 100 | 151.66 q–s | 12.01 h–j | 2.15 i–l | |
| Ch 50 | 146.16 w–y | 9.74 q | 1.74 q–s | |
| Ch 100 | 148.16 uv | 11.04 l–n | 2 l–n | |
| Ch NP 50 | 155.5 k–m | 13.70 cd | 2.40 g | |
| Ch NP 100 | 152.66 o-q | 12.98 ef | 2.18 h–k | |
| ‘Budakalazi’ | Control | 153.16 n–q | 8.78 st | 1.77 q–s |
| Ti 50 | 153.5 n–p | 9.37 p–s | 1.93 m-p | |
| Ti 100 | 154.5 mn | 11.14 k–m | 2.01 l–n | |
| Ti NP 50 | 156.66 jk | 12.26 g–i | 2.76 de | |
| Ti NP 100 | 159.83 hi | 12.60 f–h | 2.84 d | |
| Ch 50 | 153.66 no | 10.94 l–n | 2.17 h–l | |
| Ch 100 | 156.33 kl | 11.51 j–l | 2.23 h–j | |
| Ch NP 50 | 165.00 d | 16.72 a | 3.61 a | |
| Ch NP 100 | 161.33 f–h | 13.56 de | 3.36 b |
| Mean Square | |||||||
|---|---|---|---|---|---|---|---|
| S.O.V | df | Number Seed (Main Capitulum) | Weight Seed (Main Capitulum) | 1000 Seed Weight | Capitulum Number | Main Capitulum Diameter | Seed Yield per Plant |
| Year (Y) | 1 | 113.42 ns | 0.35 * | 0.038 ns | 1.20 ns | 0.07 ns | 37.72 ns |
| Replication (Year) (Ea) | 4 | 61.25 | 0.04 | 0.16 | 9.36 | 0.05 | 6.82 |
| Cultivar (Cul) | 4 | 47847.83 ** | 55.75 ** | 176.5 ** | 889.16 ** | 25.54 ** | 4807.31** |
| Compound (Com) | 8 | 31727.12 ** | 26.37 ** | 45.25 ** | 602.26 ** | 4.55 ** | 3248.63 ** |
| Cul × Y | 4 | 74.32 ns | 0.10 * | 0.03 ns | 0.55 ns | 0.01 ns | 4.46 ns |
| Com × Y | 8 | 33.6 ns | 0.007 ns | 0.175 ** | 0.95 ns | 0.02 ns | 1.80 ns |
| Cul × Com | 32 | 838.40 ** | 0.45 * | 1.15 ** | 25.78 ** | 0.11 ** | 74.5 ** |
| Cul × Com × Y | 32 | 26.41 ns | 0.006 ns | 0.03 ns | 0.9 ns | 0.02 ns | 1.33 ns |
| Error (Eb) | 176 | 63.94 | 0.03 | 0.10 | 1.84 | 0.03 | 12.2 |
| CV (%) | 3.97 | 3.74 | 1.67 | 5.79 | 4.12 | 2.7 | |
| Ecotype | Compound/Concentration (mg L−1) | Number Seed (Main Capitulum) | Weight Seed (Main Capitulum; g) | 1000 Seed Weight (g) | Capitulum Number | Main Capitulum Diameter (cm) | Seed Yield per Plant (g) |
|---|---|---|---|---|---|---|---|
| ‘Sari’ | Control | 167.5 st | 4.13 v | 18.28 rs | 14.5 u | 4.19 n | 39.52 t |
| Ti 50 | 177.16 p–r | 4.69 p-s | 18.83 m-o | 18.16 p–s | 4.61 m | 51.92 lm | |
| Ti 100 | 195.5 mn | 5.34 mn | 19.09 l–n | 19.33 n–p | 4.97 jk | 54.89 k | |
| Ti NP 50 | 231.67 g–i | 5.69 kl | 20.12 hi | 24.33 hi | 5.63 e–g | 60.71 hi | |
| Ti NP 100 | 239 fg | 5.94 ij | 20.78 f | 25.33 gh | 5.42 d–g | 63.78 e–g | |
| Ch 50 | 216 jk | 5.47 lm | 19.5 k | 20.16 mn | 5.28 f–i | 54 k | |
| Ch 100 | 226.33 i | 5.82 jk | 19.89 ij | 24.66 g–i | 5.31 f–h | 56.93 j | |
| Ch NP 50 | 270 b | 7.6 ab | 21.29 e | 32.66 c | 5.70 a–c | 76.69 c | |
| Ch NP 100 | 253.33 de | 4.94 e | 20.91 f | 28 de | 5.37 e–g | 75.41 c | |
| ‘Khomin’ | Control | 143 uv | 4.16 uv | 17.51 u | 17 r–t | 4.18 n | 36.94 u |
| Ti 50 | 150.83 u | 4.55 r–t | 17.83 tu | 18.5 o–r | 4.49 m | 42.93 r | |
| Ti 100 | 162.5 t | 4.61 q–s | 18.42 qr | 18.83 n–q | 4.90 jk | 50.90 mn | |
| Ti NP 50 | 200.16 lm | 5.22 n | 19.18 k–m | 22.66 j–k | 5.29 f–i | 54.51 k | |
| Ti NP 100 | 228.8 hi | 5.36 mn | 19.3 kl | 23.16 j–k | 5.31 f–h | 54.96 k | |
| Ch 50 | 182.83 o–q | 4.72 pr | 18.67 o–q | 18 p–s | 4.9 jk | 51.10 mn | |
| Ch 100 | 185.16 op | 4.91 op | 19.85 m–o | 21.33 lm | 5.09 h–j | 53.53 kl | |
| Ch NP 50 | 255.56 cd | 6.97 de | 20.89 f | 25.16 gh | 5.59 b–d | 65.28 de | |
| Ch NP 100 | 253.5 de | 6.54 f | 19.41 kl | 23.83 h–j | 5.49 c–f | 62.13 gh | |
| ‘Khorramabad’ | Control | 147.5 u | 2.10 z | 16.39 v | 22.16 kl | 3.21 t | 30.81 w |
| Ti 50 | 149 u | 2.96 y | 16.5 v | 28.83 h–j | 3.30 st | 34.55 v | |
| Ti 100 | 151.83 u | 3.38 x | 16.57 v | 25.33 gh | 3.46 rs | 37.67 u | |
| Ti NP 50 | 168.5 r–t | 4.47 st | 18.17 r–t | 28.5 de | 3.57 p–r | 46.66 q | |
| Ti NP 100 | 177.33 p–r | 4.74 p–r | 18.44 p–r | 35.16 b | 3.79 op | 48.73 op | |
| Ch 50 | 151.5 u | 3.5 x | 17.80 tu | 28 gf | 3.47 rs | 40.41 st | |
| Ch 100 | 166.5 st | 3.81 w | 17.88 tu | 27.16 ef | 3.26 qr | 46.74 q | |
| Ch NP 50 | 208 kl | 4.99 o | 19.55 jk | 37.66 a | 4.45 m | 54.43 k | |
| Ch NP 100 | 187 no | 4.81 oq | 18.79 n–p | 35.83 b | 3.95 o | 49.82 no | |
| ‘Ahvaz’ | Control | 134.33 v | 2.58 y | 15.44 v | 15.66 tu | 3.78 o–q | 29.80 w |
| Ti 50 | 136.66 v | 3.76 w | 17.80 tu | 16.66 st | 4.46 m | 40.57 st | |
| Ti 100 | 150.33 u | 4.2 uv | 18.02 tu | 17.66 q–s | 4.52 m | 40.89 st | |
| Ti NP 50 | 195 mn | 5.31 mn | 19.34 kl | 18.33 p–r | 4.63 lm | 49.07 op | |
| Ti NP 100 | 201.67 lm | 5.61 kl | 20.20 hi | 20 m–o | 4.66 lm | 54.37 k | |
| Ch 50 | 151.5 u | 4.37 tu | 19.18 k–m | 17.16 r–t | 4.57 m | 41.61 rs | |
| Ch 100 | 175 q–s | 4.68 q–s | 19.21 kl | 18.16 p–s | 4.61 m | 46.97 q | |
| Ch NP 50 | 245.16 ef | 5.91 ij | 20.58 fg | 22.5 j–l | 5.06 ij | 62.64 g | |
| Ch NP 100 | 241.83 f | 5.77 jk | 20.29 gh | 21.33 mL | 4.84 kl | 59.89 i | |
| ‘Budakalazi’ | Control | 205.66 l | 5.33 mn | 19.41 kl | 15.83 tu | 4.63 lm | 47.70 pq |
| Ti 50 | 215.83 jk | 5.5 lm | 21.37 e | 19.5 n–p | 5.099 ij | 53.98 k | |
| Ti 100 | 224.83 ij | 6.06 hi | 21.66 e | 22.33 j–l | 5.22 g–i | 54.79 k | |
| Ti NP 50 | 255.83 d | 7.12 de | 23.24 c | 28.16 de | 5.55 b–e | 65.53 d | |
| Ti NP 100 | 261 b–d | 7.17 cd | 23.43 c | 28.83 d | 5.61 b–d | 79.83 b | |
| Ch 50 | 226.83 i | 6.28 gh | 22.59 d | 22.5 j–l | 5.36 e–g | 63.14 fg | |
| Ch 100 | 237.16 f–h | 6.49 gf | 22.70 d | 24.5 g–i | 5.42 d–g | 64.68 d–f | |
| Ch NP 50 | 283.32 a | 7.62 a | 24.65 a | 36.5 ab | 5.84 a | 91.55 a | |
| Ch NP 100 | 265 bc | 7.39 bc | 23.96 b | 31.66 c | 5.76 ab | 80.99 b |
| Mean Square | ||||||
|---|---|---|---|---|---|---|
| S.O.V | df | Pn | gs | Tr | Fv/Fm | RWC |
| Year (Y) | 1 | 91.83 * | 0.19 ** | 117.16 ** | 0.18 ** | 4.82 ns |
| Replication (Year) (Ea) | 4 | 8.45 | 0.002 | 0.21 | 0.0002 | 11.92 |
| Cultivar (Cul) | 4 | 290.56 ** | 0.03 ** | 5.66 ** | 0.008 ** | 207.12 ** |
| Compound (Com) | 8 | 1395.41 ** | 0.45 ** | 51.48 ** | 0.07 ** | 827.06 ** |
| Cul × Y | 4 | 10.11 ** | 0.004 ** | 2.08 ** | 0.0006 * | 0.95 ns |
| Com × Y | 8 | 7.68 ** | 0.003 ** | 0.23 ns | 0.007 ** | 0.48 ns |
| Cul × Com | 32 | 22.51 ** | 0.02 ** | 1.02 ** | 0.0003 * | 23.36 ** |
| Cul × Com × Y | 32 | 6.17 ** | 0.001 ** | 0.40 ** | 0.0005 ** | 0.39 ns |
| Error (Eb) | 176 | 2.39 | 0.0006 | 0.17 | 0.0002 | 6.12 |
| CV (%) | 6.05 | 4.69 | 6.10 | 1.80 | 3.48 | |
| Ecotype | Compound/Concentration (mg L−1) | Pn (µmol m−2 s−1) | gs (mmol m−2 s−1) | Tr (mmol m−2 s−1) | Fv/Fm | RWC (%) |
|---|---|---|---|---|---|---|
| ‘Sari’ | Control | 15.58 u | 0. 31 v | 5.39 st | 0.70 t | 63.63 st |
| Ti 50 | 20.74 s | 0.34 u | 6.07 qr | 0.75 op | 65.85 rs | |
| Ti 100 | 22.86 qr | 0.43 rs | 6.23 p–r | 0.77 mn | 67.92 n–r | |
| Ti NP 50 | 27.32 jk | 0.51 l–n | 7.53 f–i | 0.81 g–i | 70.80 i–m | |
| Ti NP 100 | 32.45 ef | 0.56 hi | 7.82 d–g | 0.83 d–f | 74.03 e–h | |
| Ch 50 | 23.40 pq | 0.49 n–p | 6.73 l–o | 0.79 j–l | 69.04 k–q | |
| Ch 100 | 25.06 m–p | 0.50 l–n | 7.26 i–k | 0.80 i–k | 69.85 j–o | |
| Ch NP 50 | 40.93 b | 0.89 b | 8.96 a | 0.86 aba | 82.92 b | |
| Ch NP 100 | 33.84 de | 0.77 c | 8.26 sd | 0.84 cd | 80.49 bc | |
| ‘Khomin’ | Control | 15.69 u | 0.34 u | 3.41 v | 0.68 u | 65.87 rs |
| Ti 50 | 22.82 qr | 0.40 st | 5.51 s | 0.74 pq | 67.35 o–r | |
| Ti 100 | 23.7 p–q | 0.49 n | 6.15 qr | 0.77 m–o | 67.86 n–r | |
| Ti NP 50 | 26.83 kl | 0.55 h–j | 7.58 f–i | 0.81 g–i | 70.58 i–n | |
| Ti NP 100 | 29.36 hi | 0.56 g–i | 7.79 e–h | 0.82 e–g | 70.97 i–l | |
| Ch 50 | 24.22 o–q | 0.51 k–n | 6.49 n–q | 0.78 k–m | 68.02 m–r | |
| Ch 100 | 24.13 o–q | 0.52 k–n | 7.16 i–l | 0.80 h–j | 69.43 k–p | |
| Ch NP 50 | 38.64 c | 0.64 e | 8.80 ab | 0.87 a | 81.37 bc | |
| Ch NP 100 | 29.99 gh | 0.60 f | 8.23 c–e | 0.84 cd | 76.38 de | |
| ‘Khorramabad’ | Control | 10.85 v | 0.32 uv | 3.07 v | 0.68 u | 62.63 t |
| Ti 50 | 16.61 tu | 0.40 t | 5.83 rs | 0.72 qr | 66.09 rs | |
| Ti 100 | 17.74 t | 0.46 o–q | 6.40 o–q | 0.74 pq | 66.88 p–r | |
| Ti NP 50 | 24.38 o–q | 0.50 l–n | 7.23 i–k | 0.79 i–k | 69.02 k–q | |
| Ti NP 100 | 26.48 k–n | 0.54 i–k | 7.33 h–k | 0.81 f–h | 71.43 h–k | |
| Ch 50 | 20.01 s | 0.49 no | 6.90 k–n | 0.76 no | 67.45 o–r | |
| Ch 100 | 20.58 s | 0.50 l–m | 7.01 j–m | 0.77 mn | 67.91 n–r | |
| Ch NP 50 | 29.61 h | 0.59 fg | 7.95 c–f | 0.85 bc | 73.14 f–i | |
| Ch NP 100 | 30.10 gh | 0.55 h–j | 7.50 f–i | 0.82 fg | 71.66 g–k | |
| ‘Ahvaz’ | Control | 11.92 v | 0.31 uv | 4.65 u | 0.71 st | 63.54 st |
| Ti 50 | 16.24 tu | 0.39 t | 4.91 u | 0.72 rs | 66.27 q–s | |
| Ti 100 | 21.47 rs | 0.45 qr | 5.42 st | 0.76 no | 68.18 l–r | |
| Ti NP 50 | 27.29 jk | 0.52 k–m | 7.03 j–m | 0.80 h–k | 69.96 j–o | |
| Ti NP 100 | 28.63 h–j | 0.55 h–j | 7.41 g–j | 0.81 g–i | 73.25 f–i | |
| Ch 50 | 24.96 n–p | 0.50 mn | 5.78 rs | 0.78 l–m | 67.32 o–r | |
| Ch 100 | 27.71 i–k | 0.53 j–l | 6.68 m–p | 0.79 j–l | 68.93 k–q | |
| Ch NP 50 | 35.46 d | 0.70 d | 7.79 e–h | 0.86 ab | 78.73 cd | |
| Ch NP 100 | 31.42 fg | 0.64 e | 7.45 g–j | 0.84 cd | 74.46 e–g | |
| ‘Budakalazi’ | Control | 19.89 s | 0.38 t | 5 tu | 0.71 st | 63.64 st |
| Ti 50 | 20.70 s | 0.43 qr | 5.80 rs | 0.77 m–o | 66.89 p–r | |
| Ti 100 | 23.58 pq | 0.46 pq | 6.10 qr | 0.79 kl | 69.46 k–p | |
| Ti NP 50 | 26.77 k–m | 0.55 h–j | 7.41 g–j | 0.83 d–f | 75.77 ef | |
| Ti NP 100 | 29.60 h | 0.57 f–h | 7.80 d–g | 0.84 c–e | 76.45 de | |
| Ch 50 | 25.49 l–o | 0.52 k–n | 6.35 o–q | 0.80 h–j | 71.46 h–k | |
| Ch 100 | 26.77 k–n | 0.53 j–l | 7.26 i–k | 0.81 g–i | 72.64 g–j | |
| Ch NP 50 | 43.62 a | 0.93 a | 9.91 a | 0.87 a | 88.82 a | |
| Ch NP 100 | 33.83 de | 0.60 f | 8.38 bc | 0.85 bc | 78.79 cd |
| Mean Square | |||||||
|---|---|---|---|---|---|---|---|
| S.O.V | df | Mg | Fe | N | Ti | Protein | Oil |
| Year (Y) | 1 | 7.21 ns | 0.98 ns | 1.57 ** | 1.004 ns | 61.01 ** | 24.19 ns |
| Replication (Year) (Ea) | 4 | 32875.36 | 3.06 | 0.01 | 0.60 | 0.40 | 21.91 |
| Cultivar (Cul) | 4 | 43008.02 ** | 4247.36 ** | 0.18 ** | 52.78 ** | 7.27 ** | 169.48 ** |
| Compound (Com) | 8 | 63107.58 ** | 1946.56 ** | 1.31 ** | 803.69 ** | 51.30 ** | 859.9 ** |
| Cul × Y | 4 | 596.37 ns | 0.33 ns | 0.11 ** | 0.19 ns | 4.62 ** | 0.44 ns |
| Com × Y | 8 | 189.53 ns | 0.39 ns | 0.033 ** | 0.061 ns | 1.30 ** | 0.16 ns |
| Cul × Com | 32 | 3473.95 ** | 51.42 ** | 0.024 ** | 20.97 ** | 0.97 ** | 8.99 ** |
| Cul × Com × Y | 32 | 471.86 ns | 0.35 ns | 0.016 ** | 0.065 ns | 0.63 ** | 0.18 ns |
| Error (Eb) | 176 | 1892.86 | 5.02 | 0.007 | 0.74 | 0.27 | 0.47 |
| CV (%) | 2.52 | 4.02 | 3.76 | 4.49 | 3.76 | 2.33 | |
| Ecotype | Compound/Concentration (mg L−1) | Mg (μg/g) | Fe (μg/g) | N (%) | Ti (μg/g) | Protein (%) | Oil (%) |
|---|---|---|---|---|---|---|---|
| ‘Sari’ | control | 1705 j–s | 37.78 vw | 1.56 x | 5.01 z | 9.76 x | 18.99 u |
| Ti 50 | 1704.6 j–s | 43.34 tu | 2.16 q–s | 21.24 f–j | 13.50 q–s | 23.85 op | |
| Ti 100 | 1726.6 h–o | 51.45 no | 2.19 o–s | 21.40 f–i | 13.68 o–s | 24.67 mn | |
| Ti NP 50 | 1745.5 e–l | 65.31 g | 2.21 n–s | 22.02 f | 13.85 n–s | 33.91e | |
| Ti NP 100 | 1769.5 e–h | 66.02 fg | 2.38 c–h | 25.62 c | 14.91 c–h | 33.96 e | |
| Ch 50 | 1719.1 i–q | 46.57 q–s | 2.18 o–s | 15.34 v | 13.67 o–s | 29.29 ij | |
| Ch 100 | 1734.2 g–n | 55.38 j–m | 2.23 m–q | 18.73 o–q | 13.97 m–q | 29.85 hi | |
| Ch NP 50 | 1881.1 a | 67.11 e–g | 2.46 a–e | 21.11 f–j | 15.38 a–e | 37.08 ab | |
| Ch NP 100 | 1832.5 a–c | 68 ef | 2.47 a–c | 20.69 h–l | 15.47 a–c | 34.13 de | |
| ‘Khomin’ | Control | 1669.6 q–u | 36.25 vw | 1.85 v | 6.40 xy | 11.61 v | 21.06 st |
| Ti 50 | 1678.2 o– u | 44.43 st | 2.12 r–t | 20.34 j–m | 13.30 r–t | 20.42 t | |
| Ti 100 | 1693.2 m–t | 45.17 r–t | 2.26 k–p | 20.82 g–k | 14.15 k–p | 21.07 st | |
| Ti NP 50 | 1717.2 j–r | 56.02 i–l | 2.35 f–k | 24.22 e | 14.71 f–k | 30.59 gh | |
| Ti NP 100 | 1725.4 h–o | 58.17 hi | 2.36 e–i | 25.44 cd | 14.80 e–i | 30.53 gh | |
| Ch 50 | 1699.1 k–t | 49.52 op | 2.28 i–o | 17.34 tu | 14.26 i–o | 29.37 ij | |
| Ch 100 | 1701.8 j–s | 54.72 lm | 2.34 f–k | 17.65 st | 14.67 f–k | 29.47 ij | |
| Ch NP 50 | 1737.7 g–m | 59.56 h | 2.53 a | 20.43 i–m | 15.83 a | 34.84 cd | |
| Ch NP 100 | 1764 e–i | 58.89 h | 2.34 f–l | 17.69 r–t | 14.62 f–l | 34.09 de | |
| ‘Khorramabad’ | Control | 1631.1 u | 35.55 w | 1.66 w | 7.09 x | 10.41 w | 19.37 u |
| Ti 50 | 1675 p–u | 41.74 u | 2.05 t–u | 21.20 f–j | 12.84 tu | 22.56 qr | |
| Ti 100 | 1686.1 n–t | 44.53 st | 2.12 r–t | 21.23 f–j | 13.28 st | 23.99 no | |
| Ti NP 50 | 1706.8 j–s | 54.89 k–m | 2.26 j–p | 21.70 fg | 14.18 j–p | 30.61 gh | |
| Ti NP 100 | 1727.3 h–o | 55.85 i–l | 2.32 g–m | 29.07 b | 14.51 g–m | 31.21 g | |
| Ch 50 | 1694.8 m–t | 54.44 lm | 2.16 q–s | 19.53 m–o | 13.54 q–s | 26.96l | |
| Ch 100 | 1697.1 l–t | 53.23 mn | 2.20 n–s | 19.80 l–n | 13.79 n–s | 27.97 k | |
| Ch NP 50 | 1727.9 h–n | 55.96 i–l | 2.39 c–g | 20.74 g–l | 14.95 c–g | 32.13 f | |
| Ch NP 100 | 1748.1 e–k | 57.51 h–j | 2.35 f–k | 20.56 i–l | 14.71 f–k | 31.16 g | |
| ‘Ahvaz’ | Control | 1634.9 u | 38.37 v | 1.65 wx | 5.50 yz | 10.36 wx | 21.82 rs |
| Ti 50 | 1650.8 tu | 44.06 s–u | 2.18 p–s | 20.43 i–m | 13.63 p–s | 24.93 m | |
| Ti 100 | 1684.9 n–t | 44.44 st | 2.19 o–s | 20.56 i–l | 13.69 o–s | 27.56 kl | |
| Ti NP 50 | 1724.4 h–p | 53.61 l–n | 2.26 k–p | 21.62 f–h | 14.16 k–p | 34.11 de | |
| Ti NP 100 | 1738.9 f–m | 57.3 h–k | 2.29 h–n | 21.82 f | 14.32 h–n | 34.62 c–e | |
| Ch 50 | 1699 k–t | 47.27 p–r | 2.23 m–q | 16.50 u | 13.93 m–q | 28.76 j | |
| Ch 100 | 1714.6 i–s | 48.06 pq | 2.24 l–q | 17.98 q–t | 14.02 l–q | 34.38 c–e | |
| Ch NP 50 | 1750.3 e–j | 59.70 h | 2.42 b–f | 18.58 o–s | 15.17 b–f | 37.84 a | |
| Ch NP 100 | 1780.4 e–g | 58.25 hi | 2.37 d–h | 18.04 q–t | 14.86 d–i | 37.25 ab | |
| ‘Budakalazi’ | Control | 1669.2 r–u | 59.28 h | 2.02 u | 13.75 w | 12.67 u | 23.30 o–q |
| Ti 50 | 1665.2 s–t | 66.19 fg | 2.16 q–s | 19.30 n–p | 13.55 q–s | 23.09 pq | |
| Ti 100 | 1675.2 p–u | 67.24 e–g | 2.22 n–r | 20.08 k–n | 13.89 n–s | 26.94 l | |
| Ti NP 50 | 1788 c–f | 72.11 bc | 2.36 f–j | 24.51 de | 14.77 f–j | 34.39 cde | |
| Ti NP 100 | 1792.7 c–e | 74.62 b | 2.47 a–d | 31.76 a | 15.43 a–d | 35.02 c | |
| Ch 50 | 1756.7 e–i | 69.04 de | 2.22 n–q | 18.06 q–t | 13.90 n–q | 30.79 g | |
| Ch 100 | 1783.5 c–g | 70.89 cd | 2.35 f–k | 18.33 p–s | 14.71 f–k | 32.02 f | |
| Ch NP 50 | 1830 b–d | 79.36 a | 2.54 a | 18.55 o–s | 15.87 a | 37.04 b | |
| Ch NP 100 | 1845.2 ab | 81.16 a | 2.49 ab | 18.65 o–r | 15.57 ab | 36.78 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, S.; Mousavi-Fard, S.; Rezaei Nejad, A.; Mumivand, H.; Sorkheh, K.; Nikoloudakis, N.; Fanourakis, D. Chitosan and Titanium Dioxide Are More Effective in Improving Seed Yield and Quality in Nanoparticle Compared to Non-Structured Form: A Case Study in Five Milk Thistle Ecotypes (Silybum marianum (L.) Gaertn.). Agronomy 2022, 12, 1827. https://doi.org/10.3390/agronomy12081827
Jafari S, Mousavi-Fard S, Rezaei Nejad A, Mumivand H, Sorkheh K, Nikoloudakis N, Fanourakis D. Chitosan and Titanium Dioxide Are More Effective in Improving Seed Yield and Quality in Nanoparticle Compared to Non-Structured Form: A Case Study in Five Milk Thistle Ecotypes (Silybum marianum (L.) Gaertn.). Agronomy. 2022; 12(8):1827. https://doi.org/10.3390/agronomy12081827
Chicago/Turabian StyleJafari, Samira, Sadegh Mousavi-Fard, Abdolhossein Rezaei Nejad, Hasan Mumivand, Karim Sorkheh, Nikolaos Nikoloudakis, and Dimitrios Fanourakis. 2022. "Chitosan and Titanium Dioxide Are More Effective in Improving Seed Yield and Quality in Nanoparticle Compared to Non-Structured Form: A Case Study in Five Milk Thistle Ecotypes (Silybum marianum (L.) Gaertn.)" Agronomy 12, no. 8: 1827. https://doi.org/10.3390/agronomy12081827






