Study on Microbial Community Structure and Soil Nitrogen Accumulation in Greenhouse Vegetable Fields with Different Planting Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Determination of Physicochemical Properties
2.2. DNA Extraction and qPCR Amplification
2.3. Analysis of 16S rRNA High-Throughput Sequencing
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Changes in Soil Physicochemical Properties at Different Planting Years
3.2. Soil microbial Community at Different Planting Years
3.2.1. Changes in the Abundance and Diversity of Soil Bacterial and Archaeal Communities
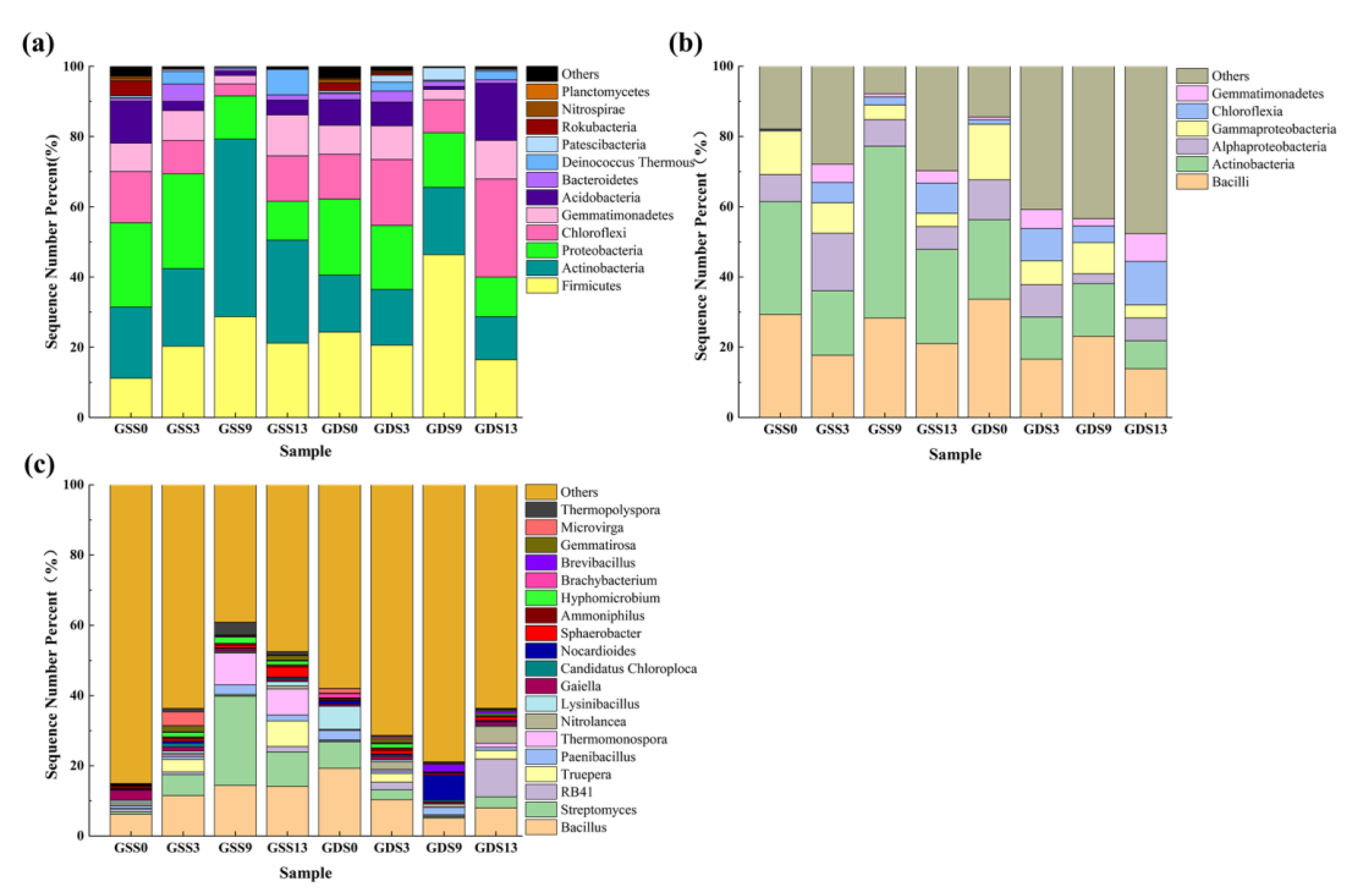
3.2.2. Bacterial Community Structure
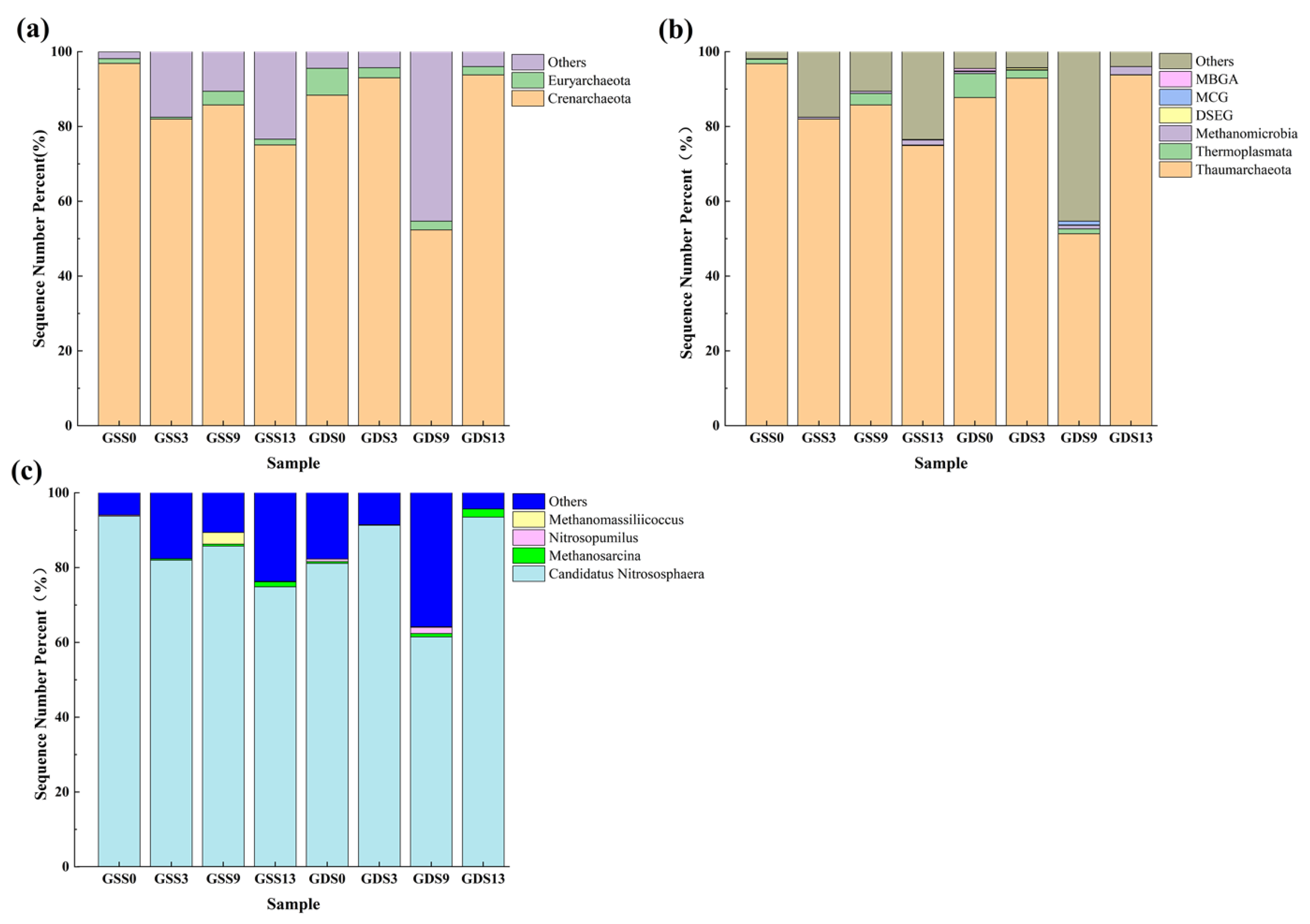
3.2.3. Archaeal Community Structure
3.3. Soil AOA and AOB Communities
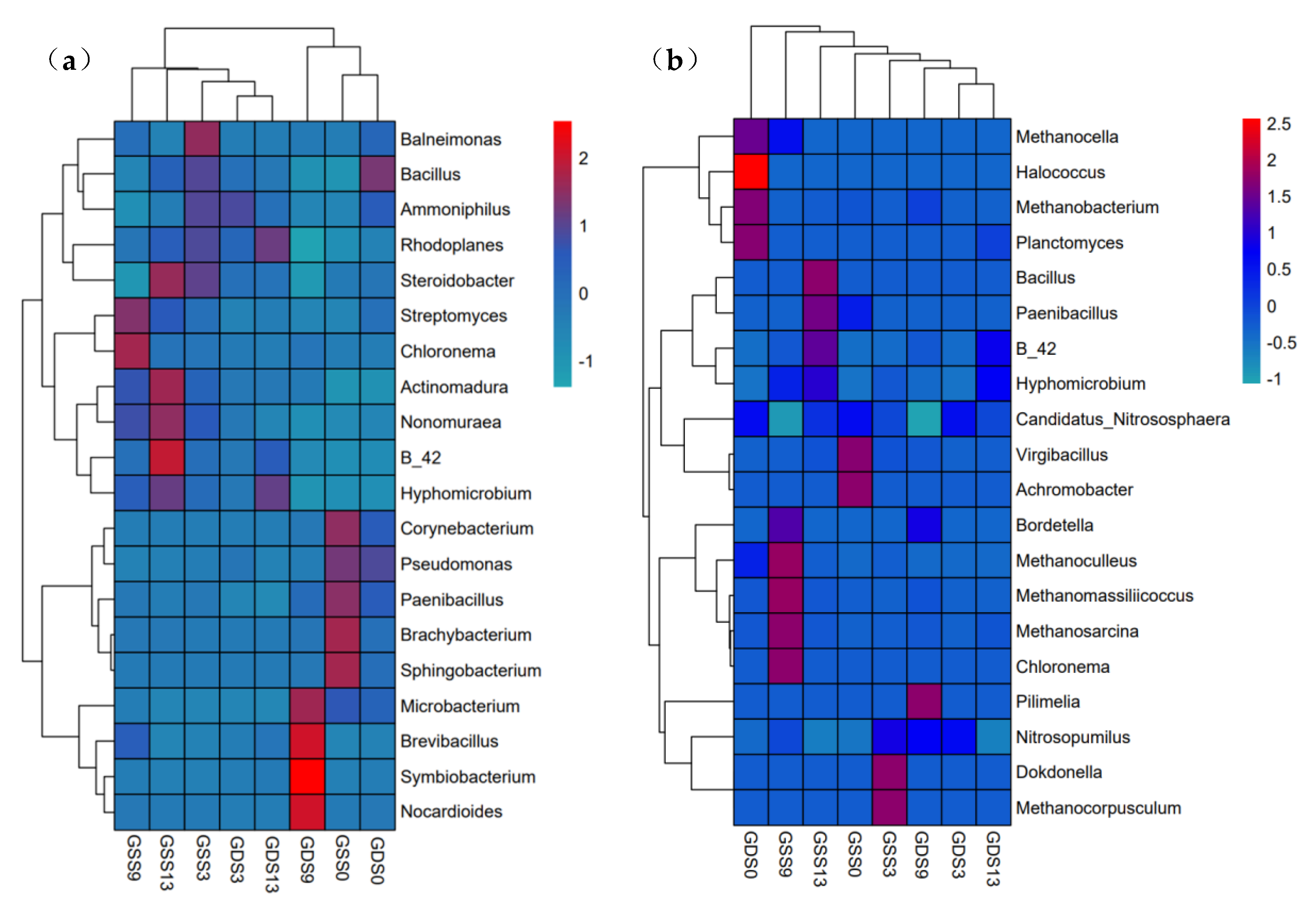
3.4. Clustering Analysis of Species Abundance in Soil Microbial Community
3.5. Correlation Analysis of Soil Environmental Factors and Planting Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.H.; Zhang, Y.P.; Liu, Z.H.; Jiang, L.H.; Lin, H.T.; Shi, J.; Liu, P.; Li, Y. Effects of cultivating years on soil ecological environment in greenhouse of Shouguang City, Shandong Province. Acta Pharmacol. Sin. 2015, 35, 1452–1459. [Google Scholar]
- Ding, W.H.; Lei, H.J.; Xu, C.; Ke, H.D.; Li, H. Characteristics and spatial distribution of apparent nitrogen balance in the greenhouse vegetable cropping system in China. J. Agr. Res. Environ. 2020, 37, 353–360. [Google Scholar]
- Zhang, J.; Li, H.; Deng, J.; Wang, L.G. Assessing impacts of nitrogen management on nitrous oxide emissions and nitrate leaching from greenhouse vegetable systems using a biogeochemical model. Geoderma 2021, 382, 114701. [Google Scholar] [CrossRef]
- Lei, H.J. Simulation Study on the Effect and Mechanism of Nitrogen Leaching in Greenhouse Vegetable Field under Integration of Water and Fertilizer; Chinese Academy of Agricultural Sciences: Beijing, China, 2021. [Google Scholar]
- Di, H.J.; Cameron, K.C. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol. Biochem. 2014, 73, 59–68. [Google Scholar] [CrossRef]
- Dai, Y.; Di, H.J.; Cameron, K.C.; He, J.Z. Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on ammonia oxidizers and N2O emissions in a grazed pasture soil. Sci. Total Environ. 2013, 465, 125–135. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Ju, C.; Xu, J.; Wu, X.H.; Dong, F.S.; Liu, X.J.; Tian, C.Y.; Zheng, Y.Q. Effects of hexaconazole application on soil microbes community and nitrogen transformations in paddy soils. Sci. Total Environ. 2017, 609, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; He, P.; Zhou, W. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol. Biochem. 2013, 57, 30–42. [Google Scholar] [CrossRef]
- Yin, C.; Fan, F.L.; Song, A.; Fan, X.P.; Ding, H.; Ran, W.; Qiu, H.Z.; Liang, Y.C. The response patterns of community traits of N2O emission-related functional guilds to temperature across different arable soils under inorganic fertilization. Soil Biol Biochem. 2017, 108, 65–77. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Luo, J.; Li, Y.; Wang, L.; Lindsey, S. Effects of 3,4-dimethylpyrazole phosphate (DMPP) on the abundance of ammonia oxidizers and denitrifiers in two different intensive vegetable cultivation soils. J. Soils Sediments 2018, 19, 1250–1259. [Google Scholar] [CrossRef]
- Ward, B.B.; Jensen, M.M. The microbial nitrogen cycle. Front. Microbiol. 2014, 5, 553. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.H. Effects of nitrogen deposition on soil microbial biomass, microbial functional diversity and enzyme activities in fir plantations of subtropical China. Adv. Mat. Res. 2013, 610–613, 323–330. [Google Scholar] [CrossRef]
- Xin, C.; Zhang, L.M.; Shen, J.P.; Wei, W.X.; He, J.Z. Abundance and community structure of ammonia-oxidizing archaea and bacteria in an acid paddy soil. Biol. Fertil. Soils 2011, 47, 323–331. [Google Scholar]
- Gesche, B.; Julia, S.; Ralf, C. Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiol. Lett. 2010, 73, 134–148. [Google Scholar]
- Lin, Y.X.; Ding, W.X.; Liu, D.Y.; He, T.H.; Yoo, G.; Yuan, J.J.; Chen, Z.M.; Fan, J.L. Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Rafique, R.; Hennessy, D.; Kiely, G. Nitrous oxide emission from grazed grassland under different management systems. Ecosystems 2011, 14, 563–582. [Google Scholar] [CrossRef]
- Tao, R.; Zhao, X.R.; Wu, X.L.; Hu, B.W.; Vanyanbah, K.B.; Li, J.; Chu, G.X. Nitrapyrin coupled with organic amendment mitigates N2O emissions by inhibiting different ammonia oxidizers in alkaline and acidic soils. Appl. Soil. Ecol. 2021, 166, 104062. [Google Scholar] [CrossRef]
- Senbayram, M.; Chen, R.; Budai, A.; Bakken, L.; Dittert, K. N2O emission and the N2O/(N2O+N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric. Ecosyst. Environ. 2012, 147, 4–12. [Google Scholar] [CrossRef]
- Szukics, U.; Abell, G.J.; Hodl, V.; Mitter, B.; Sessitsch, A.; Hackl, E.; Boltenstern, Z. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Lett. 2010, 72, 395–406. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, X.B.; Wang, Y.Z.; Bai, L.Y.; Shu, S.M.; Wu, C.X.; Li, L.F.; Duan, R. Effects of vegetable cultivation years on microbial biodiversity and abundance of nitrogen cycling in greenhouse soils. Chinese J. Appl. Ecol. 2014, 25, 1115–1124. [Google Scholar]
- Chen, H.; Yin, C.; Fan, X.; Ye, M.; Peng, H.; Li, T.; Zhao, Y.; Wakelin, S.A.; Chu, G.; Liang, Y. Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci. Total Environ. 2019, 694, 133658. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.S.; Ni, Y.Y.; Gao, N.; Bian, B.Y.; Zheng, S.N.; Lin, X.G.; Chu, H.Y. Bacterial community composition is shaped by soil secondary salinization and acidification brought on by high nitrogen fertilization rates. Appl. Soil. Ecol. 2016, 108, 76–83. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, Y.Y.; Si, D.D.; Chen, Q.W. Evolution of microbial community along with increasing solid concentration during high-solids anaerobic digestion of sewage sludge. Bioresour. Technol. 2016, 216, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Jobard, M.; Pessiot, J.; Nouaille, R.; Telesphore, S.N. Microbial diversity supporting dark fermentation of waste. Trends Biotechnol. 2014, 32, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; Peterjohn, W.T.; Schlesinger, W.H. N fertilization effects on denitrification and N cycling in an aggrading forest. Ecol. Appl. 2006, 16, 2168–2176. [Google Scholar] [CrossRef]
- He, J.Z.; Zhang, L.M. Key processes and microbial mechanisms of soil nitrogen transformation. Microbiol. China 2013, 40, 98–108. [Google Scholar]
- Dai, S.Y.; Liu, Q.; Zhao, J.; Zhang, J.B. Ecological niche differentiation of ammonia-oxidising archaea and bacteria in acidic soils due to land use change. Soil Res. 2018, 56, 71–79. [Google Scholar] [CrossRef]
- Huang, X.R.; Zhao, J.; Su, J.; Jia, Z.J.; Shi, X.L.; Wright, A.L.; Barker, X.Z.; Jiang, X.J. Neutrophilic bacteria are responsible for autotrophic ammonia oxidation in an acidic forest soil. Soil Biol. Biochem. 2018, 119, 83–89. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chapman, S.J.; Nicol, G.W.; Yao, H.Y. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Luo, J.; Di, H.J.; Liu, D.; Fan, J.; Ding, W. Nitrosospira cluster 8a play a predominant role in the nitrification process of a subtropical Ultisol under long-term inorganic and organic fertilization. Appl. Environ. Microbiol. 2018, 84, 18. [Google Scholar] [CrossRef]
- Meng, D.L.; Yang, Y.; Wu, Y.Z.; Wu, M.N.; Qin, H.L.; Zhu, Y.J.; Wei, W.X. Effects of Continuous Cropping of Vegetables on Ammonia Oxidizers Community Structure. Environ. Sci. 2012, 33, 1331–1338. [Google Scholar]
- Wang, Y.; Wang, Q.Z. Effect of planting years on microbial community and functional diversity of greenhouse vegetable soils. Acta Agr. Zhejiangensis 2013, 25, 567–576. [Google Scholar]
- Liu, S.T.; Li, H.; Li, Q. Research advances in effect of continuous cropping obstacles on facility vegetable. Anhui Agr. Sci. Bul. 2013, 19, 56–58. [Google Scholar]
- Li, C.H. Effects of Green Manure Returning to Field on Nitrogen and Microbial Characteristics in Saline-Alkali Soil, Shihezi University: Shihezi, China, 2021.
- He, J.Z.; Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zheng, Y.M.; Xu, M.G.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2010, 9, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Valerie, D.A.; Seitz, K.W.; Nina, D.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Zhang, M.M.; Alves, R.J.E.; Zhang, D.D.; Han, L.L.; He, J.Z.; Zhang, L.M. Time-dependent shifts in populations and activity of bacterial and archaeal ammonia oxidizers in response to liming in acidic soils. Soil Boil. Biochem. 2017, 112, 77–89. [Google Scholar] [CrossRef]
- Li, W.X.; Zheng, M.M.; Wang, C.; Shen, R.F. Nitrososphaera may be a major driver of nitrification in acidic soils. Soils 2021, 53, 13–20. [Google Scholar]
- Chang, E.H.; Chung, R.S.; Tsai, Y.H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2010, 53, 134–140. [Google Scholar] [CrossRef]


| Target Group | Primer Name | Sequence (5′–3′) | Length of Amplicon (bp) | References |
|---|---|---|---|---|
| Archaeal amoA 1 | 524F10extF Arch958RmodR | TGYCAGCCGCCGCGGTAA YCCGGCGTTGAVTCCAATT | 434 | [24] |
| Bacterial amoA 2 | 338F 806R | ACTCCTACGGGAGGCAGCAG GGACTACHVGGGTWTCTAAT | 468 | [25] |
| Soil Layer (cm) | Treatment | pH | EC (mS/m) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | NO2−-N (mg/kg) | Avail P (mg/kg) | Avail K (mg/kg) | SOM (g/kg) |
|---|---|---|---|---|---|---|---|---|---|
| 0–20 | GSS0 | 5.9 c | 24.2 d | 2.1 c | 104.0 c | 20.4 c | 77.5 d | 282.0 c | 10.5 c |
| GSS3 | 6.2 b | 37.3 c | 28.1 b | 207.0 b | 56.1 b | 255.0 c | 309.0 bc | 46.3 b | |
| GSS9 | 7.1 a | 70.1 b | 53.3 a | 281.0 b | 92.3 a | 446.0 b | 342.0 b | 58.6 a | |
| GSS13 | 7.2 a | 99.9 a | 53.5 a | 1340.0 a | 91.9 a | 468.0 a | 972.0 a | 64.4 a | |
| 20–40 | GDS0 | 6.1 b | 20.1 c | 4.7 d | 73.8 b | 10.2 d | 76.5 d | 392.0 d | 14.4 c |
| GDS3 | 6.4 b | 20.7 c | 16.8 c | 83.7 b | 25.2 c | 123.0 c | 309.0 c | 18.8 b | |
| GDS9 | 7.1 a | 23.3 b | 11.7 b | 88.3 b | 40.7 b | 167.0 b | 352.0 b | 24.3 b | |
| GDS13 | 7.3 a | 35.2 a | 29.1 a | 366.0 a | 50.9 a | 238.0 a | 604.0 a | 20.7 a |
| Treatment | OTUs | Chao1 Index | Shannon Index | Simpson Index | |
|---|---|---|---|---|---|
| Bacteria | GSS0 | 1129 | 1606.43 | 10.00 | 1.00 |
| GSS3 | 1182 | 1461.16 | 9.23 | 1.00 | |
| GSS9 | 681 | 847.08 | 7.89 | 0.98 | |
| GSS13 | 790 | 841.00 | 8.15 | 0.99 | |
| GDS0 | 1430 | 1824.08 | 9.91 | 1.00 | |
| GDS3 | 1425 | 1694.23 | 9.76 | 1.00 | |
| GDS9 | 786 | 896.00 | 8.22 | 0.99 | |
| GDS13 | 930 | 932.27 | 8.30 | 0.99 | |
| Archaea | GSS0 | 134 | 169.00 | 5.61 | 0.96 |
| GSS3 | 89 | 123.00 | 3.83 | 0.76 | |
| GSS9 | 86 | 147.00 | 4.17 | 0.87 | |
| GSS13 | 64 | 93.00 | 1.83 | 0.48 | |
| GDS0 | 131 | 149.00 | 5.74 | 0.96 | |
| GDS3 | 113 | 163.00 | 4.53 | 0.89 | |
| GDS9 | 226 | 257.00 | 5.90 | 0.97 | |
| GDS13 | 52 | 68.00 | 3.61 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhao, C.; Chen, Q.; Liu, T.; Li, L.; Liu, X.; Wang, X. Study on Microbial Community Structure and Soil Nitrogen Accumulation in Greenhouse Vegetable Fields with Different Planting Years. Agronomy 2022, 12, 1911. https://doi.org/10.3390/agronomy12081911
Li L, Zhao C, Chen Q, Liu T, Li L, Liu X, Wang X. Study on Microbial Community Structure and Soil Nitrogen Accumulation in Greenhouse Vegetable Fields with Different Planting Years. Agronomy. 2022; 12(8):1911. https://doi.org/10.3390/agronomy12081911
Chicago/Turabian StyleLi, Luzhen, Changsheng Zhao, Qingfeng Chen, Ting Liu, Lei Li, Xuzhen Liu, and Xiaokai Wang. 2022. "Study on Microbial Community Structure and Soil Nitrogen Accumulation in Greenhouse Vegetable Fields with Different Planting Years" Agronomy 12, no. 8: 1911. https://doi.org/10.3390/agronomy12081911
APA StyleLi, L., Zhao, C., Chen, Q., Liu, T., Li, L., Liu, X., & Wang, X. (2022). Study on Microbial Community Structure and Soil Nitrogen Accumulation in Greenhouse Vegetable Fields with Different Planting Years. Agronomy, 12(8), 1911. https://doi.org/10.3390/agronomy12081911





