The Impact of Calcium, Potassium, and Boron Application on the Growth and Yield Characteristics of Durum Wheat under Drought Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Growth Attributes
2.3. Chlorophyll Content
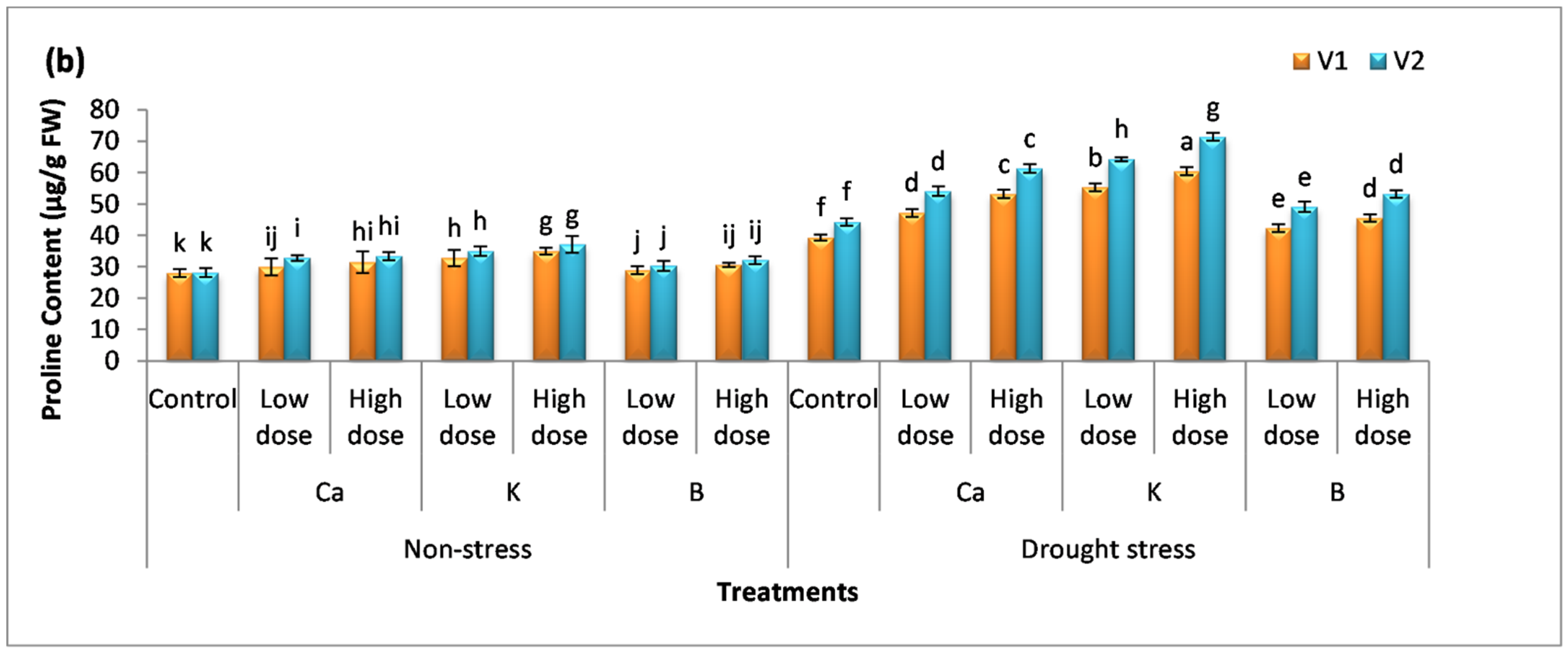
2.4. Proline Content and Sugar Content
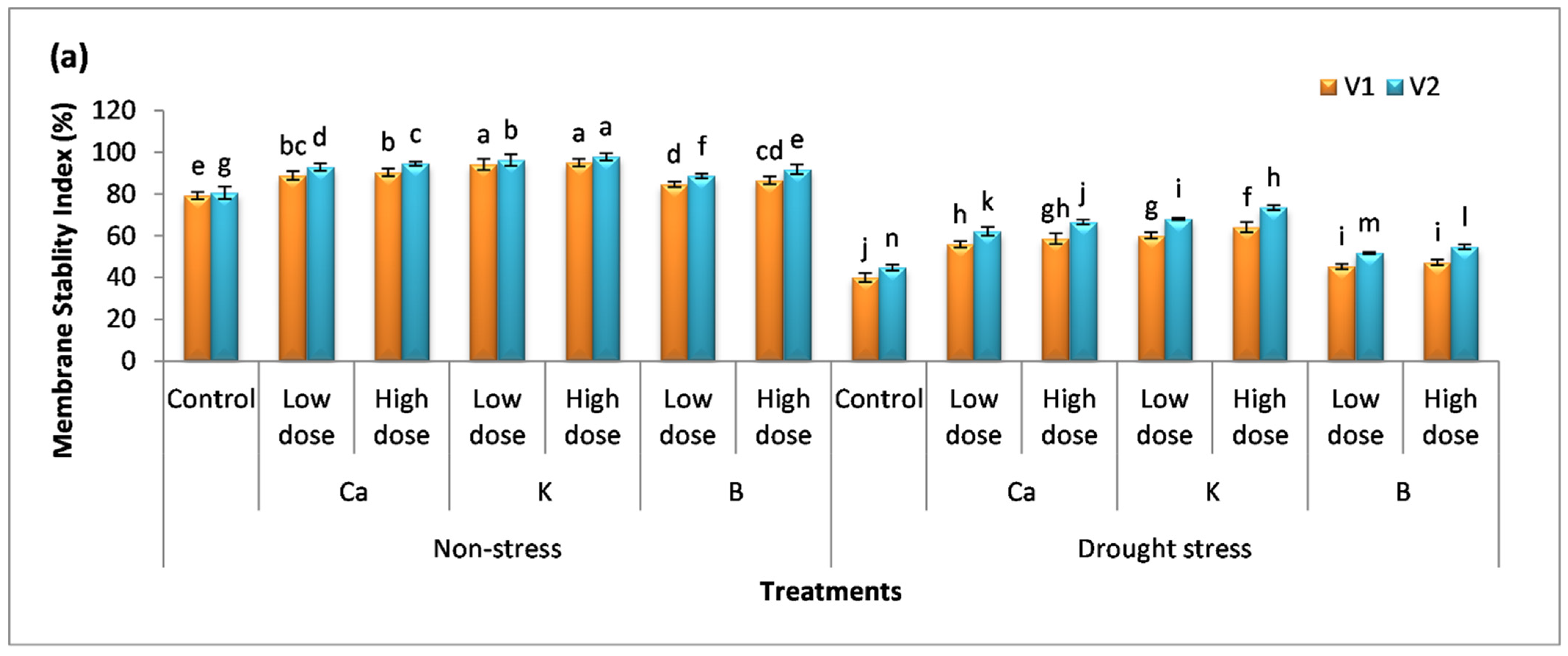
2.5. Membrane Stability Index and Relative Water Content
2.6. Antioxidants Activity
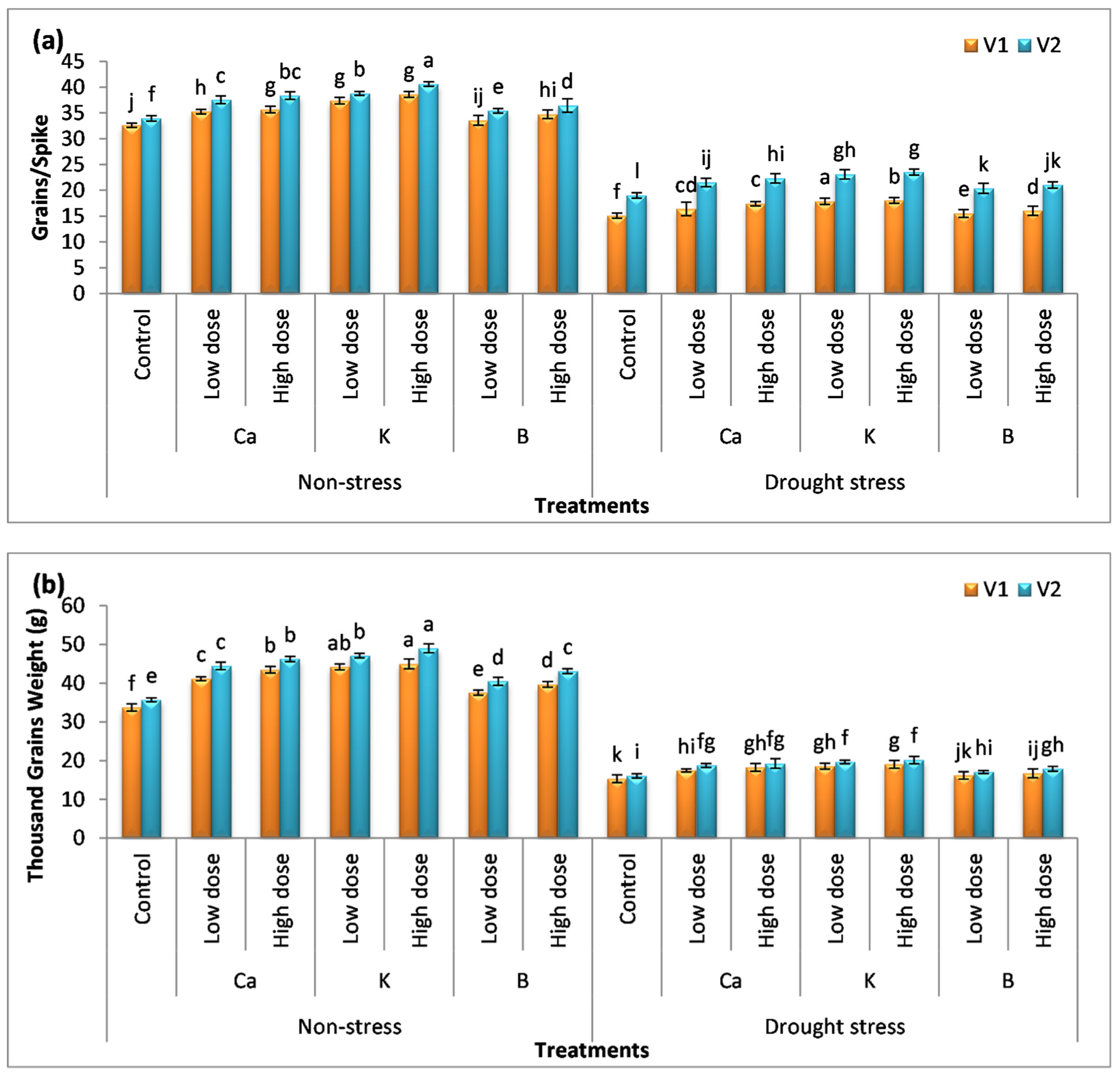
2.7. Yield Attributes
2.8. Analysis of Ca, K, and B in Plant Samples
2.9. Statistical Analysis
3. Results
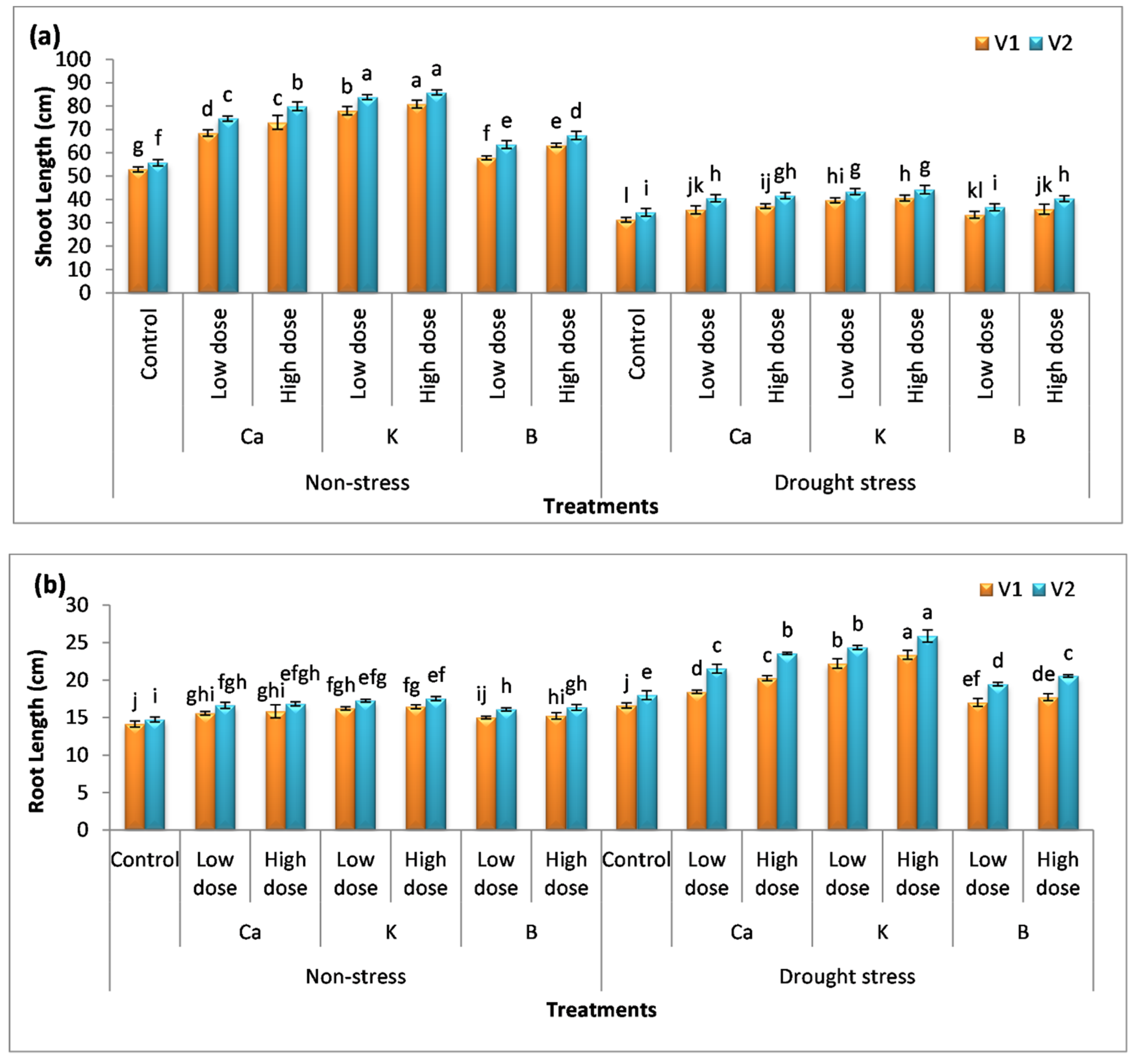
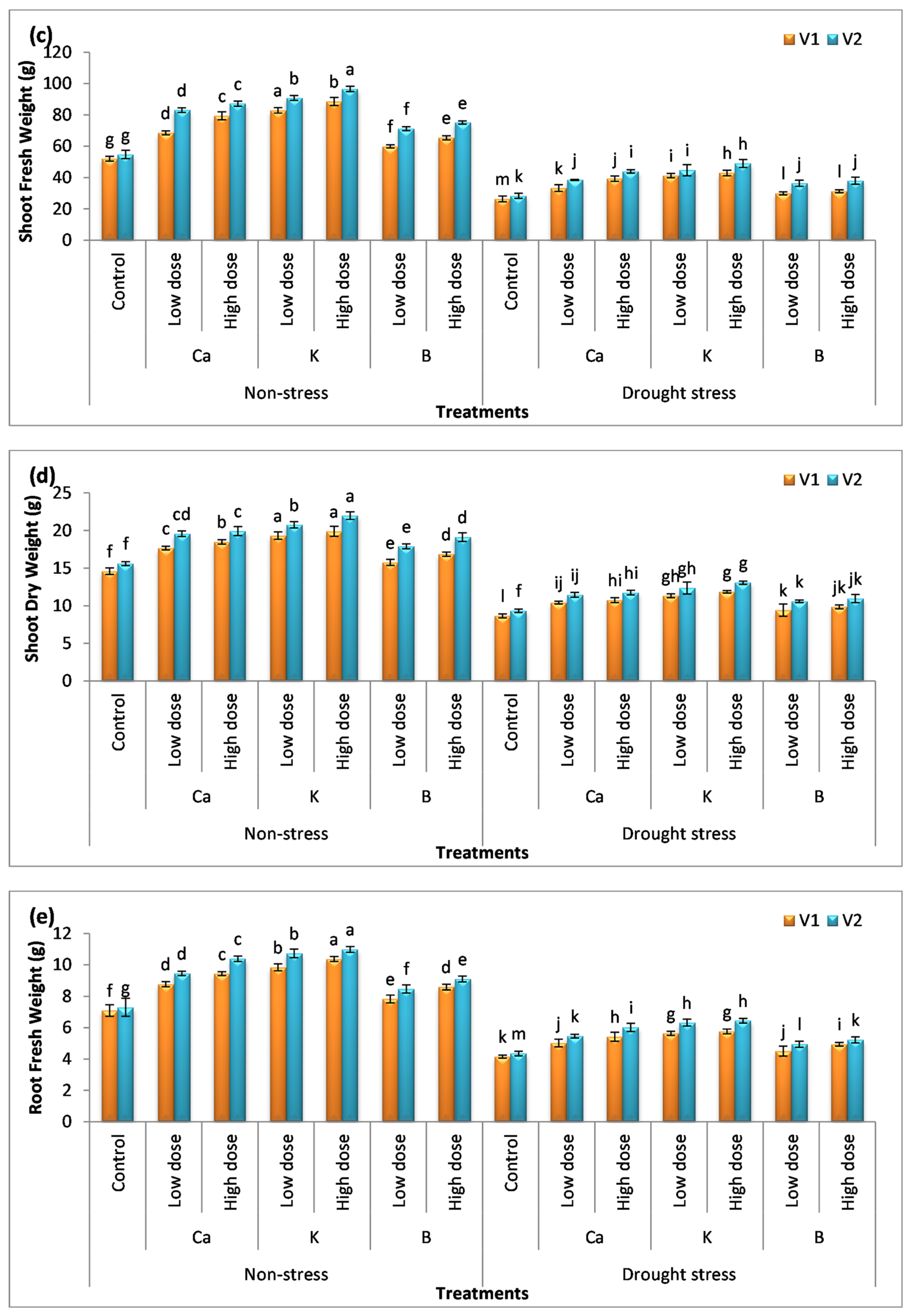
3.1. Effect of Nutrients on Plant Height and Biomass
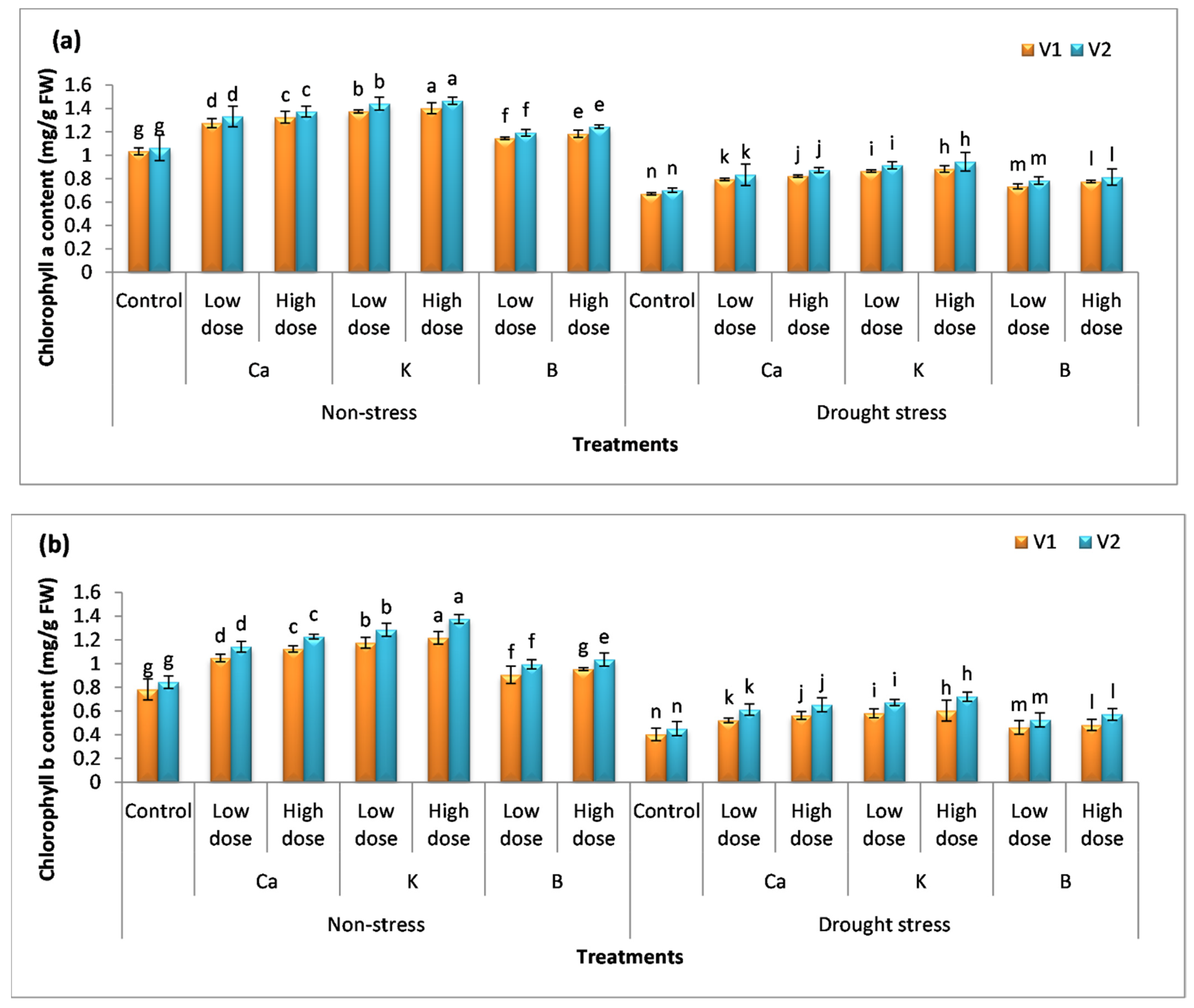
3.2. Effect of Nutrients on Chlorophyll Content
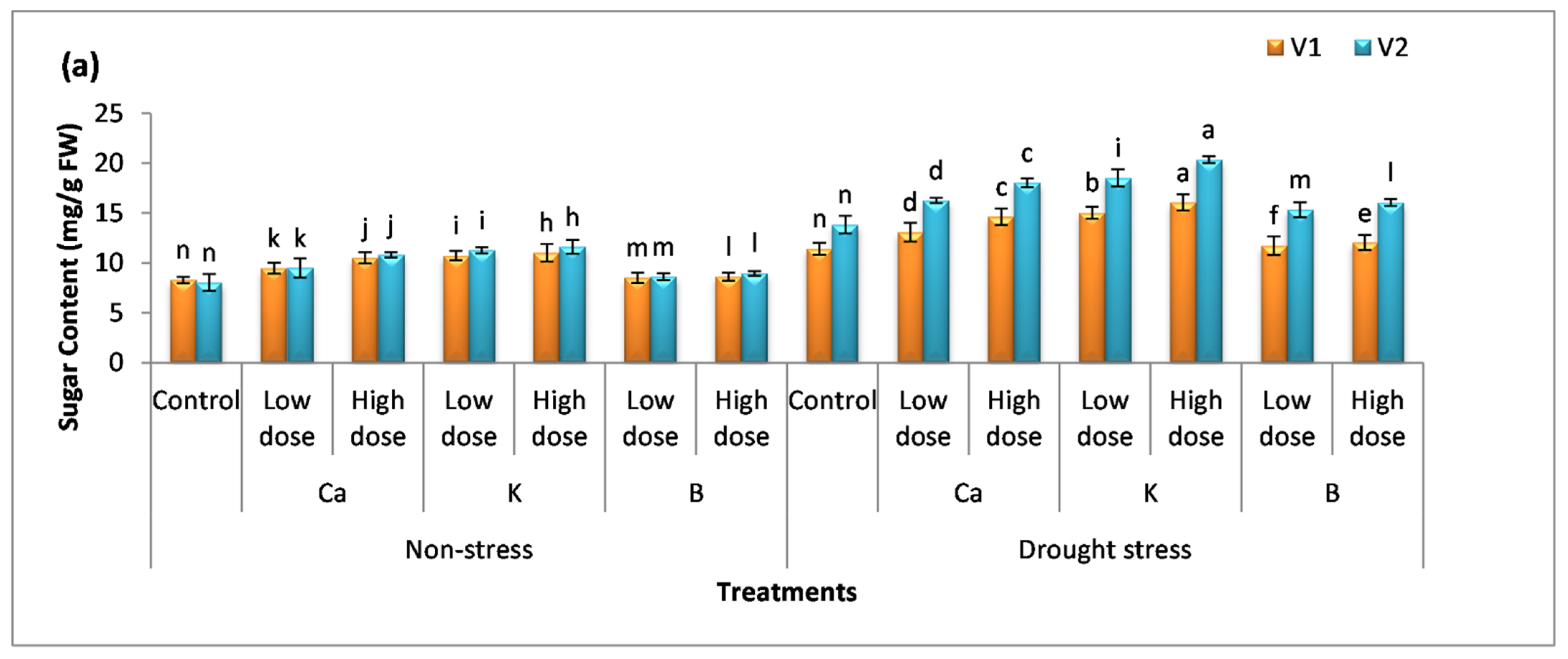
3.3. Effect of Nutrients on Osmolyte Production
3.4. Effect of Nutrients on Membrane Stability Index and Relative Water Content
3.5. Effect of Nutrients on Antioxidant Production
3.6. Effect of Nutrients on Yield Attributes
3.7. Amount of Ca, K, and B in the Root, Shoot, and Seeds of Durum Wheat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseyni Moghaddam, M.S.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Abbas, T.; Yasir, T.A.; Sarwar, N.; Aziz, A.; Javaid, M.M.; Akram, S. Mitigating Drought Stress in Sunflower (Helianthus annuus L.) Through Exogenous Application of β-Aminobutyric Acid. J. Soil Sci. Plant Nutr. 2021, 21, 936–948. [Google Scholar] [CrossRef]
- Pamungkas, S.S.T.; Farid, N. Drought Stress: Responses and Mechanism in Plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef]
- Phakela, K.; van Biljon, A.; Wentzel, B.; Guzman, C.; Labuschagne, M.T. Gluten protein response to heat and drought stress in durum wheat as measured by reverse phase—High performance liquid chromatography. J. Cereal Sci. 2021, 100, 103267. [Google Scholar] [CrossRef]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. The Effect of Drought Stress on the Superoxide Dismutase and Chlorophyll Content in Durum Wheat Genotypes. Adv. Life Sci. 2021, 8, 119–123. [Google Scholar]
- Rea, R.S.; Islam, M.R.; Rahman, M.M.; Nath, B.; Mix, K. Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review. Sustainability 2022, 14, 4525. [Google Scholar] [CrossRef]
- Xi, N.; Chen, D.; Bahn, M.; Wu, H.; Chu, C.; Cadotte, M.W.; Bloor, J.M. Drought soil legacy alters drivers of plant diversity-productivity relationships in oldfield systems. Sci. Adv. 2022, 8, 3368. [Google Scholar] [CrossRef]
- Akbarimehr, S.; Sayfzadeh, S.; Shahsavari, N.; Valad Abadi, S.A.; Masouleh, E.H. Cycocel, iron and zinc effects on yield and physiological characteristics of wheat under drought stress conditions. J. Plant Nutr. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Anwar, S.; Khalilzadeh, R.; Khan, S.; Bashir, R.; Pirzad, A.; Malik, A. Mitigation of Drought Stress and Yield Improvement in Wheat by Zinc Foliar Spray Relates to Enhanced Water Use Efficiency and Zinc Contents. Int. J. Plant Prod. 2021, 15, 377–389. [Google Scholar] [CrossRef]
- Tadayon, M.S. Effect of foliar nutrition with calcium, boron, and potassium on amelioration of aril browning in pomegranate (Punica granatum cv. ‘Rabab’). J. Hortic. Sci. Biotechnol. 2021, 96, 372–382. [Google Scholar] [CrossRef]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Kandpal, D.G. Review on Impact of Chemical Fertilizers on Environment. Int. J. Mod. Agric. 2021, 10, 758–763. [Google Scholar]
- Sotayo, F.O.; Donli, P.O. A Comparative Study of the Effects of Chemical fertilizer (NPK) and Organic Manure (Cow Dung) on the Growth of Bambara Groundnut in Borno State, Nigeria. DUJOPAS 2021, 7, 182–189. [Google Scholar]
- Kizilgeci, F.; Yildirim, M.; Islam, M.S.; Ratnasekera, D.; Iqbal, M.A.; Sabagh, A.E. Normalized difference vegetation index and chlorophyll content for precision nitrogen management in durum wheat cultivars under semi-arid conditions. Sustainability 2021, 13, 3725. [Google Scholar] [CrossRef]
- Pačuta, V.; Rašovský, M.; Michalska-Klimczak, B.; Wyszyňski, Z. Grain yield and quality traits of durum wheat (Triticum durum Desf.) treated with seaweed-and humic acid-based biostimulants. Agronomy 2021, 11, 1270. [Google Scholar] [CrossRef]
- Hao, B.; Ma, J.; Jiang, L.; Wang, X.; Bai, Y.; Zhou, C.; Ren, S.; Li, C.; Wang, Z. Effects of foliar application of micronutrients on concentration and bioavailability of zinc and iron in wheat landraces and cultivars. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.; Pratt, P. Lithium, sodium, and potassium. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 225–246. [Google Scholar]
- Estefan, G. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Dawar, K.; Khan, H.; Zaman, M.; Muller, C.; Alam, S.S.; Fahad, S.; Alwahibi, M.S.; Alkahtani, J.; Saeed, B.; Saud, S.; et al. The Effect of Biochar and Nitrogen Inhibitor on Ammonia and Nitrous Oxide Emissions and Wheat Productivity. J. Plant Growth Regul. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Weatherley, P.E. Studies in the Water Relations Of The Cotton Plant: I. The Field Measurement of Water Deficits in Leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Ullah, N.; Haq, I.U.; Safdar, N.; Mirza, B. Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter. Toxicol. Ind. Health 2015, 31, 931–937. [Google Scholar] [CrossRef]
- Roy, A.; Kumar, A.; Singh, A.; Mandi, A.; Barman, M. Analysis of genetic diversity and correlation studies on grain yield and its component characters in bread wheat (Triticum aestivum L. em Thell) genotypes. Pharm. Innov. J. 2021, 10, 341–345. [Google Scholar]
- Abdul Majid, S.; Asghar, R.; Murtaza, G. Potassium-calcium Interrelationship Linked to Drought Tolerance in Wheat (Triticum aestivum L.). Pak. J. Bot. 2007, 39, 1609–1621. [Google Scholar]
- Wu, Z.; Wang, X.; Song, B.; Zhao, X.; Du, J.; Huang, W. Responses of Photosynthetic Performance of Sugar Beet Varieties to Foliar Boron Spraying. Sugar Technol. 2021, 23, 1332–1339. [Google Scholar] [CrossRef]
- Abbas, M.; Abdel-Lattif, H.; Shahba, M. Ameliorative Effects of Calcium Sprays on Yield and Grain Nutritional Composition of Maize (Zea mays L.) Cultivars under Drought Stress. Agriculture 2021, 11, 285. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Al-Maliki, S. Interaction Between Calcium Silicate and Pseudomonas Fluorescens and Irrigation Intervals Affecting Soil Microbial Biomass and Yield of Wheat. Nveo-Nat. Volatiles Essent. Oils J. NVEO 2022, 9, 628–645. [Google Scholar]
- Mustafa, H.; Ilyas, N.; Akhtar, N.; Raja, N.I.; Zainab, T.; Shah, T.; Ahmad, A.; Ahmad, P. Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol. Environ. Saf. 2021, 223, 112519. [Google Scholar] [CrossRef]
- Ul-Allah, S.; Ijaz, M.; Nawaz, A.; Sattar, A.; Sher, A.; Naeem, M.; Shahzad, U.; Farooq, U.; Nawaz, F.; Mahmood, K. Potassium Application Improves Grain Yield and Alleviates Drought Susceptibility in Diverse Maize Hybrids. Plants 2020, 9, 75. [Google Scholar] [CrossRef]
- Santos, E.F.; Mateus, N.S.; Rosário, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing potassium content in leaves and stems improves drought tolerance of eucalyptus clones. Physiol. Plant 2021, 172, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Páez, S.E.; Cueto-Niño, Y.A.; Sánchez-Reinoso, A.D.; Garces-Varon, G.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar boron compounds applications mitigate heat stress caused by high daytime temperatures in rice (Oryza sativa L.). Boron mitigates heat stress in rice. J. Plant Nutr. 2021, 44, 2514–2527. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ge, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Mur, L.A.J.; Jiang, D. The different root apex zones contribute to drought priming induced tolerance to a reoccurring drought stress in wheat. Crop J. 2021, 9, 1088–1097. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Naz, M.; Hussain, S.; Javed, T.; Aslam, S.; Raza, A.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.; et al. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE 2021, 16, e0260556. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Maqsood, M.; Nawaz, F.; Abbas, T.; Yasin, S. Boron-induced improvement in physiological, biochemical and growth attributes in sunflower (Helianthus annuus L.) exposed to terminal drought stress. J. Plant Nutr. 2018, 41, 943–955. [Google Scholar] [CrossRef]
- Aydin, M.; Tombuloglu, G.; Sakcali, M.S.; Hakeem, K.R.; Tombuloglu, H. Boron alleviates drought stress by enhancing gene expression and antioxidant enzyme activity. J. Soil Sci. Plant Nutr. 2019, 19, 545–555. [Google Scholar] [CrossRef]
- Zrig, A.; AbdElgawad, H.; Touneckti, T.; Mohamed, H.B.; Hamouda, F.; Khemira, H. Potassium and calcium improve salt tolerance of Thymus vulgaris by activating the antioxidant systems. Sci. Hortic. 2021, 277, 109812. [Google Scholar] [CrossRef]
- Hemon, A.F. The Rhizobium and calcium fertilizer application to peanut plant in dry land. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Virtual, 16–18 August 2021; p. 012011. [Google Scholar]
- Yao, P.; Tun, H.; Fenge, Z.; Hua, L.S. Effects of calcium on physiology and photosynthesis of paris polyphylla sm. under high temperature and strong light stress. Pak. J. Bot. 2022, 54, 809–816. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.N.; Mukherjee, S.; Alamri, S.; Basahi, R.A.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Ali, H.M.; Almohisen, I.A.A. Hydrogen sulfide (H2S) and potassium (K+) synergistically induce drought stress tolerance through regulation of H+-ATPase activity, sugar metabolism, and antioxidative defense in tomato seedlings. Plant Cell Rep. 2021, 1, 1–22. [Google Scholar] [CrossRef]
- Gilani, M.; Danish, S.; Ahmed, N.; Rahi, A.A.; Akrem, A.; Younis, U.; Irshad, I.; Khalid Iqbal, R. Mitigation of Drought Stress In Spinach Using Individual And Combined Applications Of Salicylic Acid And Potassium. Pak. J. Bot. 2020, 52, 1505–1513. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N. Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil 2022, 1–17. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Yasmin, H.; Sayyed, R.Z.; Hasnain, Z.A.; Elsayed, E.; El Enshasy, H.A. Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules 2021, 26, 1569. [Google Scholar] [CrossRef]
- Miranda, M.T.; Silva, D.S.F.; Silveira, N.M.; Pereira, L.; Machado, E.C.; Ribeiro, V.R. Root Osmotic Adjustment and Stomatal Control of Leaf Gas Exchange are Dependent on Citrus Rootstocks Under Water Deficit. J. Plant Growth Regul. 2020, 40, 11–19. [Google Scholar] [CrossRef]
- Aksu, G.; Altay, H. The Effects of Potassium Applications on Drought Stress in Sugar Beet. Sugar Technol. 2020, 22, 1092–1102. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; El-Samad, G.A.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of Agronomic Traits, Nutrient Uptake, Osmolytes and Antioxidants of Maize as Influenced by Exogenous Potassium Silicate under Deficit Irrigation and Semiarid Conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Sohail, M.A.; Nawaz, F.; Aziz, M.; Ahmad, W. Effect of supplemental potassium and chitosan on growth and yield of sunflower (Helianthus annuus L.) under drought stress. Agric. Sci. J. 2021, 3, 56–71. [Google Scholar]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; El-Mageed, T.A.A.; El-Mageed, S.A.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Anokye, E.; Lowor, S.T.; Dogbatse, J.A.; Padi, F.K. Potassium Application Positively Modulates Physiological Responses of Cocoa Seedlings to Drought Stress. Agronomy 2021, 11, 563. [Google Scholar] [CrossRef]
- Azad, N.; Rezayian, M.; Hassanpour, H.; Niknam, V.; Ebrahimzadeh, H. Physiological Mechanism of Salicylic Acid in Mentha pulegium L. under salinity and drought stress. Braz. J. Bot. 2021, 44, 359–369. [Google Scholar] [CrossRef]
- Wasaya, A.; Affan, M.; Yasir, T.A.; Atiqueur, R.; Mubeen, K.; Rehman, H.U.; Ali, M.; Nawaz, F.; Galal, A.; Iqbal, M.A.; et al. Foliar Potassium Sulfate Application Improved Photosynthetic Characteristics, Water Relations and Seedling Growth of Drought-Stressed Maize. Atmosphere 2021, 12, 663. [Google Scholar] [CrossRef]
- Raina, M.; Kumar, A.; Yadav, N.; Kumari, S.; Yusuf, M.A.; Mustafiz, A.; Kumar, D. StCaM2, a calcium binding protein, alleviates negative effects of salinity and drought stress in tobacco. Plant Mol. Biol. 2021, 106, 85–108. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Yan, J.; Di, P.; Li, J.; Sun, X.; Han, G.; Ni, L.; Jiang, M.; Yuan, J.; et al. Brassinosteroid-signaling Kinase 1 Phosphorylating Calcium/calmodulin-dependent Protein Kinase Functions in Drought Tolerance in Maize. New Phytol. 2021, 231, 695–712. [Google Scholar] [CrossRef]
- Nurwahyuni, E.; Putra, E.T.S. The role of calcium in drought stress response induced through antioxidant activity in oil palm (Elaeis guineensis Jacq.) seedlings. E-J. Menara Perkeb. 2021, 89, 1. [Google Scholar] [CrossRef]
- Ali, L.G.; Nulit, R.; Ibrahim, M.H.; Yien, C.Y.S. Efficacy of KNO3, SiO2 and SA priming for improving emergence, seedling growth and antioxidant enzymes of rice (Oryza sativa), under drought. Sci. Rep. 2021, 11, 3864. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Yang, G.; Rizwan, M.; Ali, S.; Zhou, Y.; Wang, Q.; Deng, L.; Wang, Y.; et al. Boron supply alleviates cadmium toxicity in rice (Oryza sativa L.) by enhancing cadmium adsorption on cell wall and triggering antioxidant defense system in roots. Chemosphere 2021, 266, 128938. [Google Scholar] [CrossRef]
- Hussain, S.; Irfan, M.; Sattar, A.; Hussain, S.; Ullah, S.; Abbas, T.; Ur-Rehman, H.; Nawaz, F.; Al-Hashimi, A.; Elshikh, M.S. Alleviation of cadmium stress in wheat through the combined application of boron and biochar via regulating morpho-physiological and antioxidant defense mechanisms. Agronomy 2022, 12, 434. [Google Scholar] [CrossRef]
- Domingos, C.d.S.; Besen, M.R.; Neto, M.E.; Costa, E.J.O. Can calcium and boron leaf application increase soybean yield and seed quality? Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 171–181. [Google Scholar] [CrossRef]
- Sharafi, Y.; Letters, M.R.N.A.S. Effect of Boron on Pollen Attributes in Different Cultivars of Malus domestica L. Natl. Acad. Sci. Lett. 2021, 44, 189–194. [Google Scholar] [CrossRef]
- Nawaz, F.; Shehzad, M.A.; Majeed, S.; Ahmad, K.S.; Aqib, M.; Usmani, M.M.; Shabbir, R.N. Role of mineral nutrition in improving drought and salinity tolerance in field crops. In Agronomic Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 129–147. [Google Scholar]
- Sofi, K.A.; Gulzar, A.; Islam, T.; Gulzar, R. Response of Boron Nutrition on Growth and Yield of Rice Grown under Temperate Conditions of Kashmir Valley. Asian J. Adv. Res. 2021, 6, 7–11. [Google Scholar]
- Ali, S.; Shah, S.; Arif, M. Agronomic Biofortification with Zinc and Iron for the Improvement of Wheat Phenology and Yield. Sarhad J. Agric. 2021, 37, 714–1097. [Google Scholar] [CrossRef]
- Aslam, M.M.; Farhat, F.; Siddiqui, M.A.; Yasmeen, S.; Khan, M.T.; Sial, M.A.; Khan, I.A. Exploration of physiological and biochemical processes of canola with exogenously applied fertilizers and plant growth regulators under drought stress. PLoS ONE 2021, 16, e0260960. [Google Scholar] [CrossRef]
- Salman, M.; Ullah, S.; Razzaq, K.; Rajwana, I.A.; Akhtar, G.; Faried, H.N.; Hussain, A.; Amin, M.; Khalid, S. Combined foliar application of calcium, zinc, boron and time influence leaf nutrient status, vegetative growth, fruit yield, fruit biochemical and anti-oxidative attributes of “Chandler” strawberry. J. Plant Nutr. 2022, 45, 1–12. [Google Scholar] [CrossRef]




| Parameters | |
|---|---|
| Texture | Sandy loam |
| pH | 8.83 |
| K | 154 ppm |
| Ca | 68 ppm |
| B | 13 ppm |
| Organic matter | 1.26 |
| Treatments | Root | Shoot | Grains | |||||
|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V1 | V2 | V1 | V2 | |||
| Non-stress | Control | 327.0 ± 17.0 f | 353.3 ± 14.8 f | 440.5 ± 24.3 f | 470.6 ± 12.8 fg | 511.7 ± 18.0 g | 528.5 ± 35.7 g | |
| Ca | Low dose | 492.0 ± 21.3 b | 540.4 ± 19.4 b | 613.4 ± 17.8 b | 666.4 ± 17.3 b | 654.0 ± 11.2 b | 689.4 ± 30.8 b | |
| High dose | 516.8 ± 31.4 a | 566.6 ± 12.7 a | 643.3 ± 21.7 a | 698.1 ± 14.3 a | 675.2 ± 10.4 a | 705.4 ± 26.1 a | ||
| K | Low dose | 399.7 ± 12.6 c | 454.1 ± 21.7 cd | 525.6 ± 32.4 cd | 573.0 ± 16.3 d | 583.4 ± 19.0 d | 613.8 ± 14.0 d | |
| High dose | 408.1 ± 20.1 c | 473.3 ± 16.5 c | 536.5 ± 38.7 c | 602.3 ± 27.0 c | 601.3 ± 12.3 c | 628.0 ± 12.0 c | ||
| B | Low dose | 346.5 ± 24.7 e | 420.8 ± 12.6 e | 503.7 ± 26.2 e | 547.0 ± 31.8 e | 537.6 ± 21.4 f | 576.7 ± 17.8 f | |
| High dose | 366.6 ± 25.1 d | 433.3 ± 17.4 de | 512.3 ± 31.2 de | 562.2 ± 26.8 de | 563.3 ± 35.2 e | 600.8 ± 14.0 e | ||
| Stress | Control | 219.3 ± 11.9 kl | 225.0 ± 6.3 j | 343.6 ± 25.4 k | 355.1 ± 17.5 j | 385.0 ± 10.2 k | 396.3 ± 10.7 k | |
| Ca | Low dose | 270.6 ± 8.5 gh | 285.6 ± 14.6 gh | 425.0 ± 20.0 g | 457.0 ± 21.1 g | 484.3 ± 23.0 h | 515.3 ± 28.h | |
| High dose | 284.5 ± 16.4 g | 291.2 ± 8.3 g | 445.3 ± 29.4 f | 474.3 ± 24.0 f | 494.6 ± 16.5 h | 534.66 ± 17.9 g | ||
| K | Low dose | 255.7 ± 21.3 ij | 274.4 ± 15.7 ghi | 395.6 ± 34.1 hi | 421.6 ± 18.2 h | 432.0 ± 29.11 i | 452.0 ± 14.6 i | |
| High dose | 262.1 ± 15.0 hi | 277.7 ± 12.0 ghi | 403.3 ± 19.7 h | 432.3 ± 13.7 h | 438.6 ± 15.9 i | 463.0 ± 17.3 i | ||
| B | Low dose | 229.0 ± 22.9 l | 257.1 ± 18.4 ij | 365.7 ± 14.6 j | 391.0 ± 15.8 i | 405.0 ± 14.2 j | 425.3 ± 16.0 j | |
| High dose | 242.6 ± 7.6 jk | 264.5 ± 24.5 hi | 382.3 ± 16.2 i | 403.3 ± 30.2 i | 416.0 ± 27.8 j | 434.6 ± 10.2 j | ||
| Treatments | Root | Shoot | Grains | |||||
|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V1 | V2 | V1 | V2 | |||
| Non-stress | Control | 10.0 ± 0.1 de | 10.3 ± 0.2 ef | 23.4 ± 0.4 g | 27.0 ± 0.5 f | 33.0 ± 0.0 f | 34.9 ± 0.6 g | |
| Ca | Low dose | 12.2 ± 0.2 bc | 12.6 ± 0.2 bc | 27.2 ± 0.2 d | 32.4 ± 0.2 d | 38.2 ± 0.3 d | 43.4 ± 0.4 d | |
| High dose | 12.4 ± 0.2 bc | 13.5 ± 0.3 ab | 28.2 ± 0.3 c | 33.5 ± 0.5 c | 39.4 ± 0.5 c | 45.6 ± 0.8 c | ||
| K | Low dose | 13.6 ± 0.0 ab | 14.5 ± 0.1 a | 33.3 ± 0.4 b | 41.1 ± 0.3 b | 53.2 ± 0.3 b | 62.8 ± 1.8 b | |
| High dose | 14.3 ± 0.2 a | 14.9 ± 0.0 a | 37.4 ± 0.3 a | 45.4 ± 0.6 a | 60.5 ± 1.1 a | 65.8 ± 2.0 a | ||
| B | Low dose | 11.5 ± 0.1 cd | 11.8 ± 0.1 cd | 25.5 ± 0.2 f | 29.7 ± 0.4 e | 35.8 ± 0.6 d | 38.8 ± 0.7 f | |
| High dose | 11.8 ± 0.1 c | 12.5 ± 0.1 bc | 26.2 ± 0.2 e | 30.0 ± 0.4 e | 38.1 ± 0.6 c | 40.7 ± 0.8 e | ||
| Drought stress | Control | 6.9 ± 0.1 de | 8.0 ± 0.2 h | 12.0 ± 0.1 l | 13.0 ± 0.0 l | 15.1 ± 0.3 k | 17.0 ± 0.1 k | |
| Ca | Low dose | 7.1 ± 0.9 gh | 8.7 ± 1.0 gh | 13.4 ± 0.2 j | 14.6 ± 0.0 ij | 17.0 ± 0.3 ij | 20.1 ± 0.4 j | |
| High dose | 8.1 ± 0.8 fg | 9.9 ± 0.9 fg | 13.7 ± 0.1 j | 15.1 ± 0.0 i | 17.4 ± 0.1 i | 20.7 ± 0.7 j | ||
| K | Low dose | 9.0 ± 0.1 ef | 10.6 ± 0.2 def | 16.0 ± 0.0 i | 17.3 ± 0.1 g | 23.4 ± 0.8 h | 28.2 ± 0.7 i | |
| High dose | 9.5 ± 0.1 ef | 11.4 ± 0.1 cde | 18.2 ± 0.1 h | 21.0 ± 0.0 g | 25.0 ± 0.0 g | 30.7 ± 0.8 h | ||
| B | Low dose | 6.1 ± 1.8 h | 7.9 ± 1.1 h | 12.6 ± 0.2 k | 13.8 ± 0.0 k | 16.1 ± 0.1 jk | 19.2 ± 0.2 j | |
| High dose | 7.3 ± 1.2 gh | 8.5 ± 0.6 gh | 12.8 ± 0.0 k | 14.2 ± 0.0 jk | 16.5 ± 0.3 ij | 19.6 ± 0.3 dj | ||
| Treatments | Root | Shoot | Grains | |||||
|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V1 | V2 | V1 | V2 | |||
| Non-stress | Control | 2.3 ± 0.1 i | 2.3 ± 0.0 i | 11.4 ± 0.2 ef | 11.5 ± 0.1 g | 13.0 ± 0.3 h | 14.5 ± 0.0 jk | |
| Ca | Low dose | 2.4 ± 0.0 m | 2.6 ± 0.0 f | 11.9 ± 0.2 def | 12.4 ± 0.9 f | 14.5 ± 0.2 g | 16.2 ± 0.0 h | |
| High dose | 2.4 ± 0.1 l | 2.7 ± 0.0 e | 12.8 ± 0.8 cd | 13.4 ± 0.4 ef | 15.6 ± 0.2 f | 18.3 ± 0.2 g | ||
| K | Low dose | 2.6 ± 0.0 d | 2.7 ± 0.1 d | 13.5 ± 0.5 c | 13.9 ± 0.8 e | 16.2 ± 0.1 e | 10.0 ± 0.9 f | |
| High dose | 2.6 ± 0.0 c | 2.8 ± 0.0 c | 14.6 ± 0.8 b | 14.8 ± 0.1 cd | 18.0 ± 0.4 d | 20.6 ± 0.3 e | ||
| B | Low dose | 2.8 ± 0.0 b | 3.1 ± 0.1 b | 16.6 ± 1.2 a | 18.5 ± 0.3 b | 21.2 ± 0.8 b | 25.8 ± 0.3 b | |
| High dose | 3.0 ± 0.0 a | 3.2 ± 0.0 a | 17.2 ± 0.0 a | 19.7 ± 0.3 a | 24.2 ± 0.2 a | 27.4 ± 1.0 a | ||
| Drought stress | Control | 1.9 ± 0.0 n | 1.9 ± 0.0 n | 8.3 ± 0.0 j | 9.2 ± 0.1 j | 11.1 ± 0.0 j | 13.1 ± 0.9 m | |
| Ca | Low dose | 2.0 ± 0.0 m | 2.1 ± 0.0 m | 8.6 ± 0.0 ij | 9.8 ± 0.1 ij | 11.3 ± 0.0 j | 13.8 ± 0.5 l | |
| High dose | 2.0 ± 0.0 l | 2.1 ± 0.0 l | 9.3 ± 0.0 hij | 10.3 ± 0.3 hi | 11.5 ± 0.0 j | 14.0 ± 0.2 kl | ||
| K | Low dose | 2.1 ± 0.0 k | 2.2 ± 0.0 k | 9.6 ± 0.0 hi | 10.7 ± 0.7 gh | 12.0 ± 0.0 i | 14.6 ± 0.8 l | |
| High dose | 2.1 ± 0.0 j | 2.2 ± 0.0 j | 10.1 ± 0.0 gh | 11.3 ± 0.1 g | 12.3 ± 0.0 i | 15.5 ± 0.3 kl | ||
| B | Low dose | 2.3 ± 0.0 h | 2.5 ± 0.0 h | 11.0 ± 0.0 fg | 14.0 ± 0.0 de | 17.6 ± 0.1 d | 21.5 ± 0.1 d | |
| High dose | 2.5 ± 0.0 e | 2.6 ± 0.0 g | 12.1 ± 0.1 de | 15.4 ± 0.2 c | 19.7 ± 0.6 c | 24.4 ± 0.3 c | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, N.; Ilyas, N.; Arshad, M.; Meraj, T.A.; Hefft, D.I.; Jan, B.L.; Ahmad, P. The Impact of Calcium, Potassium, and Boron Application on the Growth and Yield Characteristics of Durum Wheat under Drought Conditions. Agronomy 2022, 12, 1917. https://doi.org/10.3390/agronomy12081917
Akhtar N, Ilyas N, Arshad M, Meraj TA, Hefft DI, Jan BL, Ahmad P. The Impact of Calcium, Potassium, and Boron Application on the Growth and Yield Characteristics of Durum Wheat under Drought Conditions. Agronomy. 2022; 12(8):1917. https://doi.org/10.3390/agronomy12081917
Chicago/Turabian StyleAkhtar, Nosheen, Noshin Ilyas, Muhammad Arshad, Tehseen Ahmad Meraj, Daniel Ingo Hefft, Basit Latief Jan, and Parvaiz Ahmad. 2022. "The Impact of Calcium, Potassium, and Boron Application on the Growth and Yield Characteristics of Durum Wheat under Drought Conditions" Agronomy 12, no. 8: 1917. https://doi.org/10.3390/agronomy12081917
APA StyleAkhtar, N., Ilyas, N., Arshad, M., Meraj, T. A., Hefft, D. I., Jan, B. L., & Ahmad, P. (2022). The Impact of Calcium, Potassium, and Boron Application on the Growth and Yield Characteristics of Durum Wheat under Drought Conditions. Agronomy, 12(8), 1917. https://doi.org/10.3390/agronomy12081917







