Classification of Monofloral Honeys by Measuring Electrical Impedance Based on Neural Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Honeys Used
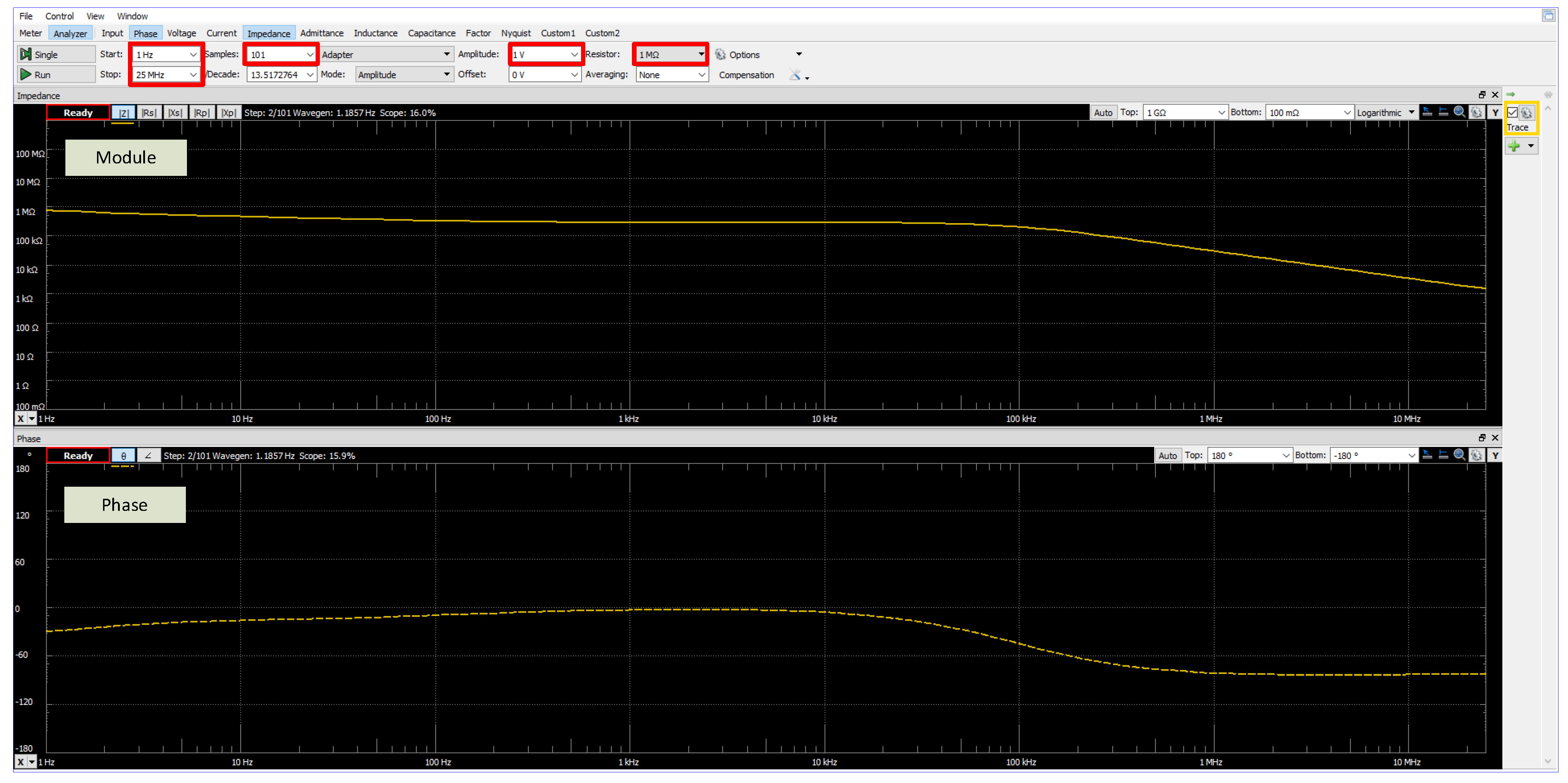
2.2. Equipment Used to Measure Electrical Impedance
2.3. Neural Networks to Sorting
3. Results and Discussion
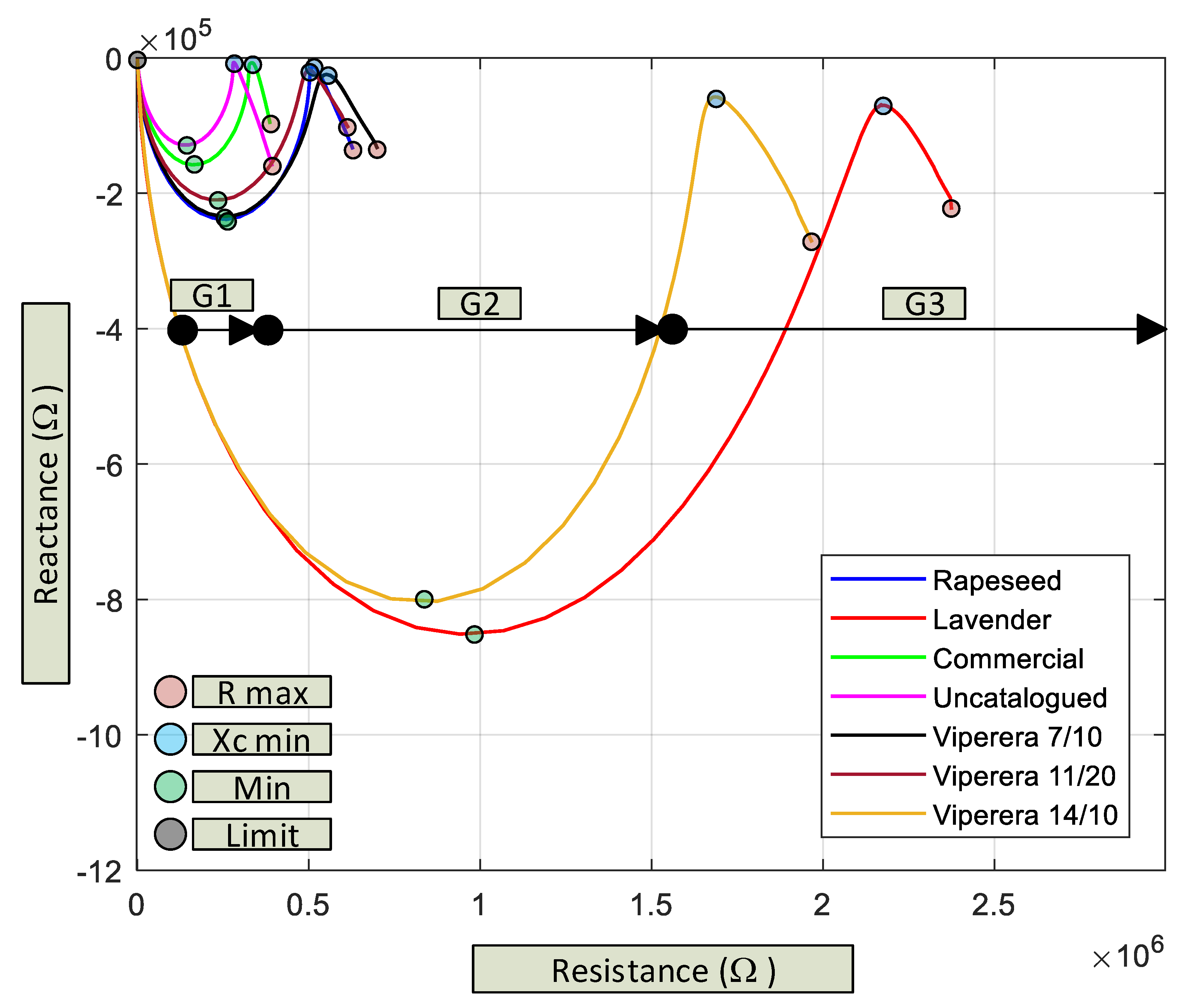
3.1. Evolution of the Impedance Depending on the Varieties of Honey
- Value of the high resistive component at low frequencies (red dot), between 0.35 MΩ and 2.5 MΩ for a frequency of 1 Hz.
- Relative maximum (blue dot) at medium frequencies (between 30 Hz and 1 kHz). In this area, the capacitive behavior is minimized with reactancy values between 6 kΩ and 70 kΩ.
- Relative minimum (green point) (with a negative maximum value of the capacitive component) with frequency values between 10 kHz and 100 kHz and reactance values between 0.1 MΩ and 1 MΩ.
- Common point (grey dot) at high frequency (25 MHz), where all the curve-confluence and the effect of the parasitic components of the measurement system are already evident.
3.2. Results of the Classified with Patternnet Neural Network
3.3. Relationship of Physical–Chemical Parameters of the Samples with the Characteristic Values of Electrical Impedance of Each Variety of Unifloral Honey
3.3.1. Humidity
3.3.2. HMF (Hidroximetilfurfural)
3.3.3. Coloration
3.3.4. Conductivity
3.3.5. pH
3.3.6. BRIX Degrees
3.3.7. Pollen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luna, J.M.M.; Luna, A.M.; Fernández, R.E.H. Characterization and Differentiation between Olive Varieties through Electrical Impedance Spectroscopy, Neural Networks and IoT. Sensors 2020, 20, 5932. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Sekino, T. Agilent Impedance Measurement Handbook. In A Guide to Measurement Technology and Techniques; Agilent Technologies: Santa Clara, CA, USA, 2009. [Google Scholar]
- Elbanna, K.; Attalla, K.; Elbadry, M.; Abdeltawab, A.; Gamal-Eldin, H.; Fawzy Ramadan, M. Impact of Floral Sources and Processing on the Antimicrobial Activities of Different Unifloral Honeys. Asian Pac. J. Trop. Dis. 2014, 4, 194–200. [Google Scholar] [CrossRef]
- Wikipedia. Maxwell Bridge. Available online: https://en.wikipedia.org/wiki/Maxwell_bridge (accessed on 10 August 2022).
- A Low Cost RF Impedance Analyzer. Available online: https://www.nutsvolts.com/magazine/article/a_low_cost_rf_impedance_analyzer (accessed on 11 August 2022).
- Analizador Impedancia Valencia. Available online: https://pdfcoffee.com/analizador-impedancia-valencia-pdf-free.html (accessed on 11 August 2022).
- Paszkowski, B.; Wilczek, A.; Szypłowska, A.; Nakonieczna, A.; Skierucha, W. A Low-Frequency Sensor for Determination of Honey Electrical Properties in Varying Temperature Conditions. J. Food Eng. 2014, 138, 17–22. [Google Scholar] [CrossRef]
- Nelson, S. Dielectric Properties of Agricultural Products: Measurements and Applications. IEEE Trans. Electr. Insul. 1991, 26, 845–869. [Google Scholar] [CrossRef]
- Repo, T.; Paine, D.; Taylor, A. Electrical Impedance Spectroscopy in Relation to Seed Viability and Moisture Content in Snap Bean (Phaseolus vulgaris L.). Seed Sci. Res. 2002, 12, 17–29. [Google Scholar] [CrossRef]
- Tegam. The LCR Meter as an Impedance Analyzer. Available online: http://www.tegam.com/wp-content/uploads/2015/10/AN303.pdf (accessed on 11 August 2022).
- El Khaled, D.; Castellano, N.N.; Gazquez, J.A.; García Salvador, R.M.; Manzano-Agugliaro, F. Cleaner Quality Control System Using Bioimpedance Methods: A Review for Fruits and Vegetables. J. Clean. Prod. 2017, 140, 1749–1762. [Google Scholar] [CrossRef]
- Caicedo-Eraso, J.C.; Díaz-Arango, F.O.; Osorio-Alturo, A. Espectroscopia de impedancia eléctrica aplicada al control de la calidad en la industria alimentaria. Cienc. Tecnol. Agropecu. 2019, 21, 100–119. [Google Scholar] [CrossRef]
- Das, C.; Chakraborty, S.; Karmakar, A.; Chattopadhyay, S. Comparative Study for the Impedimetric Detection and Quantification of Adulterants in Different Bio-Consumables. In Proceedings of the 2020 International Symposium on Devices, Circuits and Systems (ISDCS), Howrah, India, 4–6 March 2020; pp. 1–6. [Google Scholar]
- Elamine, Y.; Inácio, P.M.C.; Lyoussi, B.; Anjos, O.; Estevinho, L.M.; da Graça Miguel, M.; Gomes, H.L. Insight into the Sensing Mechanism of an Impedance Based Electronic Tongue for Honey Botanic Origin Discrimination. Sens. Actuators B Chem. 2019, 285, 24–33. [Google Scholar] [CrossRef]
- Karabagias, I.; Maia, M.; Karabagias, V.; Gatzias, I.; Badeka, A. Characterization of Eucalyptus, Chestnut and Heather Honeys from Portugal Using Multi-Parameter Analysis and Chemo-Calculus. Foods 2018, 7, 194. [Google Scholar] [CrossRef]
- Scandurra, G.; Tripodi, G.; Verzera, A. Impedance Spectroscopy for Rapid Determination of Honey Floral Origin. Journal of Food Engineering 2013, 119, 738–743. [Google Scholar] [CrossRef]
- Huang, T.-K.; Chuang, M.-C.; Kung, Y.; Hsieh, B.-C. Impedimetric Sensing of Honey Adulterated with High Fructose Corn Syrup. Food Control 2021, 130, 108326. [Google Scholar] [CrossRef]
- Das, C.; Chakraborty, S.; Acharya, K.; Bera, N.K.; Chattopadhyay, D.; Karmakar, A.; Chattopadhyay, S. FT-MIR Supported Electrical Impedance Spectroscopy Based Study of Sugar Adulterated Honeys from Different Floral Origin. Talanta 2017, 171, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Insuasty-Santacruz, E.; Martínez-Benavides, J.; Jurado-Gámez, H. Determinación Melisopalinológica de Miel de Abejas Apis Mellifera Producida Con Flora de Clima Frío, Principalmente Trifolium repens L. Vet. Zootec. 2017, 11, 74–82. [Google Scholar] [CrossRef]
- Pospiech, M.; Ljasovská, S.; Titěra, D.; Kružík, V.; Javůrková, Z.; Tremlová, B. Pollen Diversity in Honeys of the Czech Republic in the 2019 Season. Potravin. Slovak J. Food Sci. 2020, 14, 1115–1123. [Google Scholar] [CrossRef]
- Karabournioti, S.; Thrasyvoulou, A.; Eleftheriou, E.P. A Model for Predicting Geographic Origin of Honey from the Same Floral Source. J. Apic. Res. 2006, 45, 117–124. [Google Scholar] [CrossRef]
- Pineda Ballesteros, E.; Castellanos Riveros, A.; Téllez Acuña, F.R. Determinantes físicoquímicos de la calidad de la miel: Una revisión bibliográfica. Cuad. Des. Rural 2019, 16, 83. [Google Scholar] [CrossRef]
- Popek, S.; Halagarda, M.; Kursa, K. A New Model to Identify Botanical Origin of Polish Honeys Based on the Physicochemical Parameters and Chemometric Analysis. LWT 2017, 77, 482–487. [Google Scholar] [CrossRef]
- van Gerven, M.; Bohte, S. Editorial: Artificial Neural Networks as Models of Neural Information Processing. Front. Comput. Neurosci. 2017, 11, 114. [Google Scholar] [CrossRef]
- Hara, P.; Piekutowska, M.; Niedbała, G. Selection of Independent Variables for Crop Yield Prediction Using Artificial Neural Network Models with Remote Sensing Data. Land 2021, 10, 609. [Google Scholar] [CrossRef]
- Rodriguez, N.; Reyes Méndez, L.; Salamanca Grosso, G. Aplicación de análisis de cluster y redes neuronales artificiales para la clasificación de mieles colombianas. In Proceedings of the Congreso Iberoamericano de Ingeniería de Alimentos-Cibia9, Valencia, Spain, 13–16 January 2014; Volume 9. [Google Scholar]
- Gavilanes Proaño, D.A. Sistema de Monitoreo Apícola Mediante el uso de Redes Neuronales Artificiales para Identificar la Variación de Población; Universidad Técnica de Ambato: Ambato, Ecuador, 2020. [Google Scholar]
- Martínez, L.; Arguijo, P.; López, J.A.; Melendez-Armenta, R. Configuración de Red Neuronal Para El Análisis de Polen. Res. Comput. Sci. 2019, 148, 91–98. [Google Scholar] [CrossRef]
- Mateo, F.; Tarazona, A.; Mateo, E.M. Comparative Study of Several Machine Learning Algorithms for Classification of Unifloral Honeys. Foods 2021, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Generate Pattern Recognition Network—MATLAB Patternnet—MathWorks España. Available online: https://es.mathworks.com/help/deeplearning/ref/patternnet.html;jsessionid=370562d44f3c46b93a717f92677f (accessed on 12 June 2022).
- Nikolova, K.; Tsankova, D.; Eftimov, T. Fluorescence spectroscopy, colorimetry and neural networks in distinguishing different types of honey. In Proceedings of the V International Conference Industrial Engineering and Environmental Protection 2015 (IIZS 2015), Zrenjanin, Serbia, 15–16 October 2015. [Google Scholar]
- Caston, M.J.P.; González, I.N.; Alaminos, A.B.; Torales, L.I.E.; Alonso, F.J.G. Paramétros de calidad en mieles de diferentes orígenes botánicos producidas en la alpujarra granadina. Anales de Veterinaria de Murcia 2016, 32, 59–71. [Google Scholar]
- DIRECTIVA 2001/110/CE. Available online: https://www.boe.es/doue/2002/010/L00047-00052.pdf. (accessed on 10 June 2022).
- Caracterización Físico-Química y Evaluación Sanitaria de la miel de Mozambique. 290. Available online: http://hdl.handle.net/10803/5701 (accessed on 5 September 2021).
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Revista Veterinaria Argentina. Comparación de Parámetros Fisicoquímicos En Muestras de Miel Ecológica y Convencional. Available online: https://www.veterinariargentina.com/revista/2022/05/comparacion-de-parametros-fisicoquimicos-en-muestras-de-miel-ecologica-y-convencional/ (accessed on 10 August 2022).
- BOE.Es—DOUE-L-2002-80034 Directiva 2001/110/CE Del Consejo, de 20 de Diciembre de 2001, Relativa a La Miel. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2002-80034 (accessed on 10 August 2022).
- Pérez García, M.R. Universitat Politècnica de València. Ingeniería Del Agua 2014, 18, ix. [Google Scholar] [CrossRef]
- de la Cruz, D.R.; Reyes, E.S.; Durán, S.S.; Sánchez, J.S. Análisis palinológico de mieles comerciales monoflorales. Bot. Complut. 2013, 37, 171–180. [Google Scholar] [CrossRef]
- Montenegro, G.; Gómez, M.; Díaz-Forestier, J.; Pizarro, R. Aplicación de La Norma Chilena Oficial de Denominación de Origen Botánico de La Miel Para La Caracterización de La Producción Apícola. Ciencia e Investigación Agraria 2008, 35, 181–190. [Google Scholar] [CrossRef]
- López Candel, A. Caracterización Fisicoquímica de Mieles de Espliego y Tomillo de la Comunidad Valenciana. 2014. Available online: hdl.handle.net/10251/57240 (accessed on 10 August 2022).
- Analog Discovery 2—Digilent Reference. Available online: https://digilent.com/reference/test-and-measurement/analog-discovery-2/start (accessed on 12 June 2022).
- Impedance Analyzer for Analog Discovery. Available online: https://digilent.com/shop/impedance-analyzer-for-analog-discovery/ (accessed on 12 June 2022).
- Sigmoid Layer—MATLAB—MathWorks España. Available online: https://es.mathworks.com/help/deeplearning/ref/nnet.cnn.layer.sigmoidlayer.html (accessed on 11 August 2022).
- Santos, E.I.; Meerhoff, E.; Rosa, E.G.D.; Ferreira, J.; Raucher, M.; Quintana, W.; Martínez, A.; González, C.; Mancebo, Y. Color and Electrical Conductivity of Honeys Produced by Apis Mellifera in Uruguay. INNOTEC 2018, 16, 51–55. [Google Scholar] [CrossRef]
- Blanco Pérez, M. Parámetros Fisicoquímicos En mieles Monoflorales Españolas de la Campaña 2020. Master’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2021. [Google Scholar]
- Control de Calidad de Las Mieles, ¿qué Medir? Available online: https://www.hannainst.es/blog/1609/Control-de-calidad-de-las-mieles (accessed on 10 August 2022).
- Staniškienė, B.; Matusevičius, P.; Budreckienė, R. Honey as an Indicator of Environmental Pollution. Medus—Aplinkos Taršos Indikatorius 2006, 36, 53–58. [Google Scholar]
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality Characterization of Artisanal and Retail Turkish Blossom Honeys: Determination of Physicochemical, Microbiological, Bioactive Properties and Aroma Profile. Ind. Crops Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]


| Number of Sample | Pollen Analysis | Humidity | Hmf (mg/kg) | Coloration | Mm Pfund | Conductivity (mS/cm) | pH | Brix (%) |
|---|---|---|---|---|---|---|---|---|
| 1 (14/10) | Viperera | 14.8 | 5.89 | Extra-light amber | 37 | 0.27 | 4.05 | 82.3 |
| 2 (07/10) | Viperera | 17 | 4.23 | Extra-light amber | 37.3 | 0.26 | 3.84 | 80.5 |
| 3 (11/20) | Viperera | 17.4 | 3.96 | Extra-light amber | 43 | 0.30 | 4.4 | 80.5 |
| 4 | Rapeseed | 18 | 8.16 | White | 28 | 0.32 | 4.2 | 81.1 |
| 5 | Lavender | 15.1 | 3.88 | Extra-light amber | 40.3 | 0.16 | 3.6 | 82 |
| 6 (commercial) | Honey of a “thousand flowers” | 18 ** | 4.0 * | Extra-light amber | 48 ** | 0.3 ** | - | - |
| 7 (uncatalogued) | Uncatalogued | 18 ** | 4.0 * | Dark amber | 90 ** | 0.9 ** | - | - |
| Number of Sample | Pollen Analysis | Dominant Pollens >45% | Accompanying Pollens >16–45% | Minority Pollens >3–15% | Residual or Isolated Pollens <3% |
|---|---|---|---|---|---|
| 1 (14/10) | Viperera | Echium 50% | - | Cistus 11% Vicia 20% Prunus 9% Lavandula stoechas 5% | Cytisus 3% Trifolium 2% |
| 2 (07/10) | Viperera | Echium 62% | - | Cistus 14% Type “Rubus” 6% Rosmarius 5% Cruciferous 5% Cytisus 4% | Lavender 2% Anchusa 2% |
| 3 (11/20) | Viperera | Echium 74% | - | Rosmarius 9% Cruciferous 8% Cistus 6% Cytisus 3% | - |
| 4 | Rapeseed | Brassica 57% | - | Prunus 10% Cytisus 9% Diplotaxis 8% Rosmarius 7% Helianthus 6% | Anchusa 3% |
| 5 | Lavender | Lavender 50% | Prunus 20% | Citisus 9% Rubus 6% | Cirisium 3% |
| 6 ** (commercial) | Honey of a “thousand flowers” | - | - | - | - |
| 7 ** (uncatalogued) | Uncatalogued | - | - | - | - |
| F_min | Uncatalogued | Commercial | Vip11/20 | Rapeseed | Vip 07/10 | Vip14/10 | Lavender |
|---|---|---|---|---|---|---|---|
| R (Ω) | 3.94 × 105 | 3.87 × 105 | 6.16 × 105 | 6.29 × 105 | 7.01 × 105 | 1.97 × 106 | 2.38 × 106 |
| X (Ω) | −1.60 × 105 | −9.76 × 104 | −1.04 × 105 | −1.35 × 105 | −1.34 × 105 | −2.72 × 105 | −2.24 × 105 |
| F (Hz) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Group | G1 | G1 | G2 | G2 | G2 | G3 | G3 |
| X_min | Uncatalogued | Commercial | Vip 11/20 | Rapeseed | Vip 07/10 | Vip 14/10 | Lavender |
|---|---|---|---|---|---|---|---|
| R (Ω) | 1.29 × 105 | 1.59 × 105 | 2.18 × 105 | 2.60 × 105 | 2.44 × 105 | 8.75 × 105 | 9.41 × 105 |
| X (Ω) | −1.29 × 105 | −1.58 × 105 | −2.10 × 105 | −2.39 × 105 | −2.34 × 105 | −8.03 × 105 | −8.51 × 105 |
| F (Hz) | 1.07 × 105 | 9.05 × 104 | 5.43 × 104 | 5.43 × 104 | 4.58 × 104 | 1.65 × 104 | 1.39 × 104 |
| Group | G1 | G1 | G2 | G2 | G2 | G3 | G3 |
| X_max | Uncatalogued | Commercial | Vip 11/20 | Rapeseed | Vip 07/10 | Vip 14/10 | Lavender |
|---|---|---|---|---|---|---|---|
| R (Ω) | 2.81 × 105 | 3.35 × 105 | 4.99 × 105 | 5.13 × 105 | 5.52 × 105 | 1.69 × 106 | 2.17 × 106 |
| X (Ω) | −6.86 × 103 | −7.32 × 103 | −1.80 × 104 | −1.23 × 104 | −2.46 × 104 | −5.77 × 104 | −6.93 × 104 |
| F (Hz) | 7.68 × 102 | 2.33 × 102 | 2.76 × 102 | 3.28 × 102 | 2.33 × 102 | 1.40 × 102 | 5.03 × 101 |
| Group | G1 | G1 | G2 | G2 | G2 | G3 | G3 |
| Challenges | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Honeys | Rapeseed | 1 | 1 | 1 | 1 | 1 | 1.09 × 10−8 | 7.88 × 10−7 | 7.45 × 10−7 | 6.99 × 10−7 | 6.42 × 10−7 |
| Lavender | 5.81 × 10−7 | 5.78 × 10−7 | 5.75 × 10−7 | 5.67 × 10−7 | 5.65 × 10−7 | 1 | 1 | 1 | 1 | 1 | |
| Commercial | 1.79 × 10−8 | 1.79 × 10−8 | 1.79 × 10−8 | 1.78 × 10−8 | 1.77 × 10−8 | 4.21 × 10−4 | 1.43 × 10−4 | 1.19 × 10−4 | 9.61 × 10−3 | 7.25 × 10−3 | |
| Uncatalogued | 3.15 × 10−4 | 3.15 × 10−4 | 3.15 × 10−4 | 3.15 × 10−4 | 3.14 × 10−4 | 2.83× 10−1 | 2.10 × 10−2 | 2.98 × 10−2 | 4.45 × 10−2 | 7.55 × 10−2 | |
| Viperera 7/10 | 1.20 × 10−7 | 1.20 × 10−7 | 1.20 × 10−7 | 1.21 × 10−7 | 1.20 × 10−7 | 1.69 × 10−7 | 5.87 × 10−7 | 7.28 × 10−7 | 9.34 × 10−7 | 1.30 × 10−8 | |
| Viperera 11/20 | 1.19 × 10−7 | 1.19 × 10−7 | 1.18 × 10−7 | 1.16 × 10−7 | 1.16 × 10−7 | 9.39 × 10−7 | 3.06 × 10−8 | 3.76 × 10−8 | 4.77 × 10−8 | 6.52 × 10−8 | |
| Viperera 14/10 | 6.56 × 10−7 | 6.56 × 10−7 | 6.56 × 10−7 | 6.56 × 10−7 | 6.57 × 10−7 | 1.27 × 10−8 | 1.32 × 10−8 | 1.33 × 10−8 | 1.34 × 10−8 | 1.35 × 10−8 | |
| Challenges | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
| Honeys | Rapeseed | 1.95 × 10−8 | 1.95 × 10−8 | 1.95 × 10−8 | 1.95 × 10−8 | 1.95 × 10−8 | 4.01 × 10−5 | 4.01 × 10−5 | 4.01 × 10−5 | 4.01 × 10−5 | 4.02 × 10−5 |
| Lavender | 3.40 × 10−2 | 3.40 × 10−2 | 3.40 × 10−2 | 3.40 × 10−2 | 3.40 × 10−2 | 9.18 × 10−9 | 9.18 × 10−9 | 9.19 × 10−9 | 9.19 × 10−9 | 9.19 × 10−9 | |
| Commercial | 1 | 1 | 1 | 1 | 1 | 5.06 × 10−8 | 5.06 × 10−8 | 5.07 × 10−8 | 5.07 × 10−8 | 5.07 × 10−8 | |
| Uncatalogued | 4.24 × 10−8 | 4.24 × 10−8 | 4.24 × 10−8 | 4.24 × 10−8 | 4.24 × 10−8 | 1 | 1 | 1 | 1 | 1 | |
| Viperera 7/10 | 1.60 × 10−5 | 1.60 × 10−5 | 1.60 × 10−5 | 1.60 × 10−5 | 1.60 × 10−5 | 8.65 × 10−7 | 8.65 × 10−7 | 8.65 × 10−7 | 8.66 × 10−7 | 8.66 × 10−7 | |
| Viperera 11/20 | 1.36 × 10−5 | 1.36 × 10−5 | 1.36 × 10−5 | 1.36 × 10−5 | 1.36 × 10−5 | 4.59 × 10−7 | 4.59 × 10−7 | 4.59 × 10−7 | 4.59 × 10−7 | 4.59 × 10−7 | |
| Viperera 14/10 | 1.30 × 10−8 | 1.30 × 10−8 | 1.30 × 10−8 | 1.30 × 10−8 | 1.30 × 10−8 | 4.65 × 10−6 | 4.65 × 10−6 | 4.65 × 10−6 | 4.65 × 10−6 | 4.66 × 10−6 | |
| Challenges | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | ||
| Honeys | Rapeseed | 1.03 × 10−7 | 9.49 × 10−6 | 1.10 × 10−7 | 1.51 × 10−7 | 1.34 × 10−7 | 1.62 × 10−7 | 1.64 × 10−7 | 1.70 × 10−7 | 1.73 × 10−7 | 1.88 × 10−7 |
| Lavender | 2.70 × 10−7 | 2.43 × 10−7 | 2.72 × 10−7 | 5.49 × 10−7 | 4.47 × 10−7 | 3.81 × 10−7 | 3.84 × 10−7 | 3.97 × 10−7 | 4.02 × 10−7 | 4.30 × 10−7 | |
| Commercial | 5.48 × 10−3 | 4.86 × 10−3 | 5.91 × 10−3 | 1.08 × 10−4 | 8.80 × 10−3 | 3.13 × 10−6 | 3.16 × 10−6 | 3.31 × 10−6 | 3.39 × 10−6 | 3.83 × 10−6 | |
| Uncatalogued | 1.97 × 10−8 | 1.82 × 10−8 | 2.09 × 10−8 | 2.92 × 10−8 | 2.59 × 10−8 | 1.44 × 10−8 | 1.44 × 10−8 | 1.41 × 10−8 | 1.40 × 10−8 | 1.34 × 10−8 | |
| Viperera 7/10 | 1 | 1 | 1 | 1 | 1 | 7.12 × 10−7 | 7.12 × 10−7 | 7.10 × 10−7 | 7.10 × 10−7 | 7.07 × 10−7 | |
| Viperera 11/20 | 1.59 × 10−8 | 1.34 × 10−8 | 1.73 × 10−8 | 4.39 × 10−8 | 3.25 × 10−8 | 1 | 1 | 1 | 1 | 1 | |
| Viperera 14/10 | 1.33 × 10−7 | 1.32 × 10−7 | 1.33 × 10−7 | 1.41 × 10−7 | 1.39 × 10−7 | 2.45 × 10−1 | 2.48 × 10−1 | 2.55 × 10−1 | 2.59 × 10−1 | 2.76 × 10−1 | |
| Challenges | ||||||
|---|---|---|---|---|---|---|
| 31 | 32 | 33 | 34 | 35 | ||
| Honeys | Rapeseed | 2.01 × 10−7 | 2.01 × 10−7 | 2.01 × 10−7 | 2.01 × 10−7 | 2.01 × 10−7 |
| Lavender | 1.06 × 10−8 | 1.06 × 10−8 | 1.06 × 10−8 | 1.06 × 10−8 | 1.06 × 10−8 | |
| Commercial | 1.75 × 10−8 | 1.75 × 10−8 | 1.75 × 10−8 | 1.75 × 10−8 | 1.75 × 10−8 | |
| Uncatalogued | 3.04 × 10−6 | 3.04 × 10−6 | 3.04 × 10−6 | 3.04 × 10−6 | 3.04 × 10−6 | |
| Viperera 7/10 | 1.13 × 10−7 | 1.13 × 10−7 | 1.13 × 10−7 | 1.13 × 10−7 | 1.13 × 10−7 | |
| Viperera 11/20 | 2.60 × 10−1 | 2.60 × 10−1 | 2.60 × 10−1 | 2.60 × 10−1 | 2.60 × 10−1 | |
| Viperera 14/10 | 1 | 1 | 1 | 1 | 1 | |
| Pollen Analysis | Mm Pfund | Coloration | Conductivity (mS/cm) |
|---|---|---|---|
| Lavender | 40.3 | Extra-light amber | 0.16 |
| Viperera 7/20 | 37.3 | Extra-light amber | 0.26 |
| Viperera 14/20 | 37 | Extra-light amber | 0.27 |
| Viperera 11/20 | 43 | Extra-light amber | 0.30 |
| Rapeseed | 28 | White | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
María, E.G.; Madueño-Luna, A.; Ruiz-Canales, A.; Luna, J.M.M. Classification of Monofloral Honeys by Measuring Electrical Impedance Based on Neural Networks. Agronomy 2022, 12, 1929. https://doi.org/10.3390/agronomy12081929
María EG, Madueño-Luna A, Ruiz-Canales A, Luna JMM. Classification of Monofloral Honeys by Measuring Electrical Impedance Based on Neural Networks. Agronomy. 2022; 12(8):1929. https://doi.org/10.3390/agronomy12081929
Chicago/Turabian StyleMaría, Eduardo González, Antonio Madueño-Luna, Antonio Ruiz-Canales, and José Miguel Madueño Luna. 2022. "Classification of Monofloral Honeys by Measuring Electrical Impedance Based on Neural Networks" Agronomy 12, no. 8: 1929. https://doi.org/10.3390/agronomy12081929






