Evaluation of Bacillus velezensis Biocontrol Potential against Fusarium Fungi on Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial and Fungal Strains
2.2. Study of Strain–Pathogen Interaction
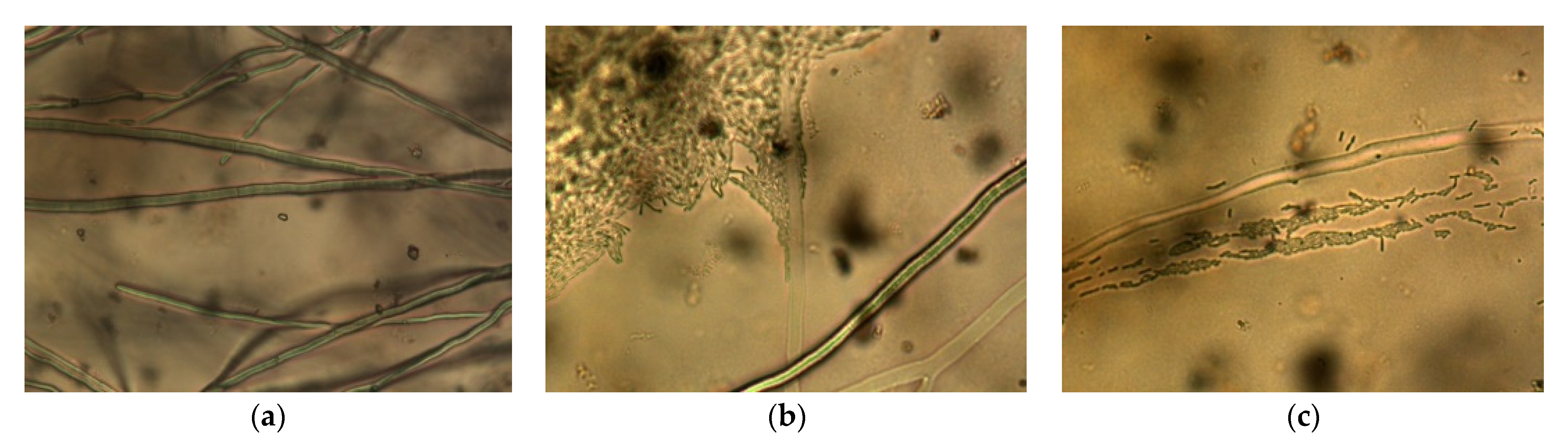
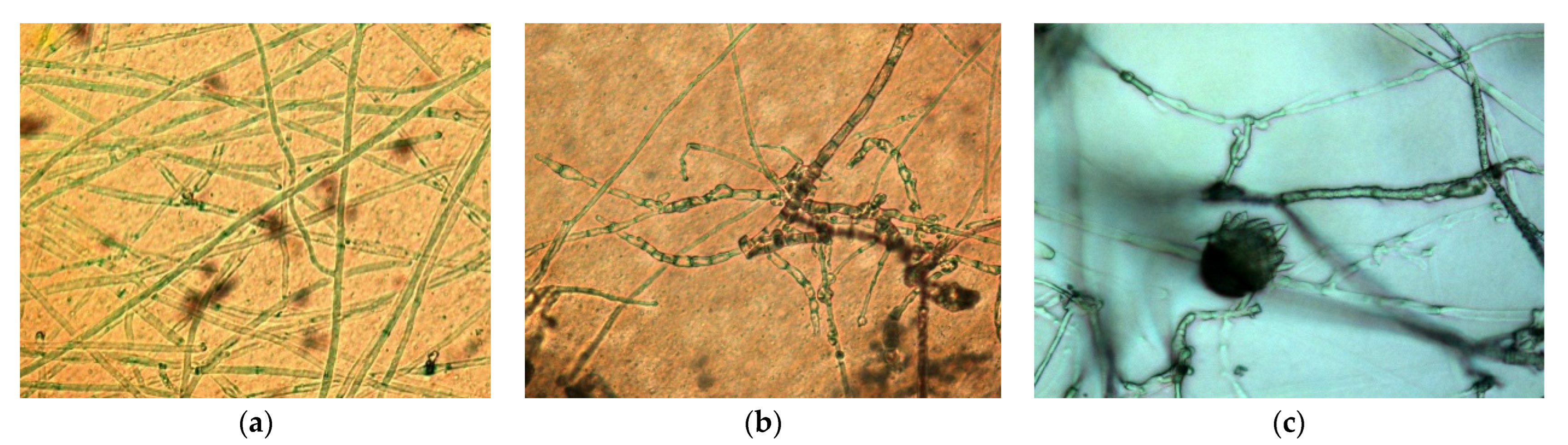
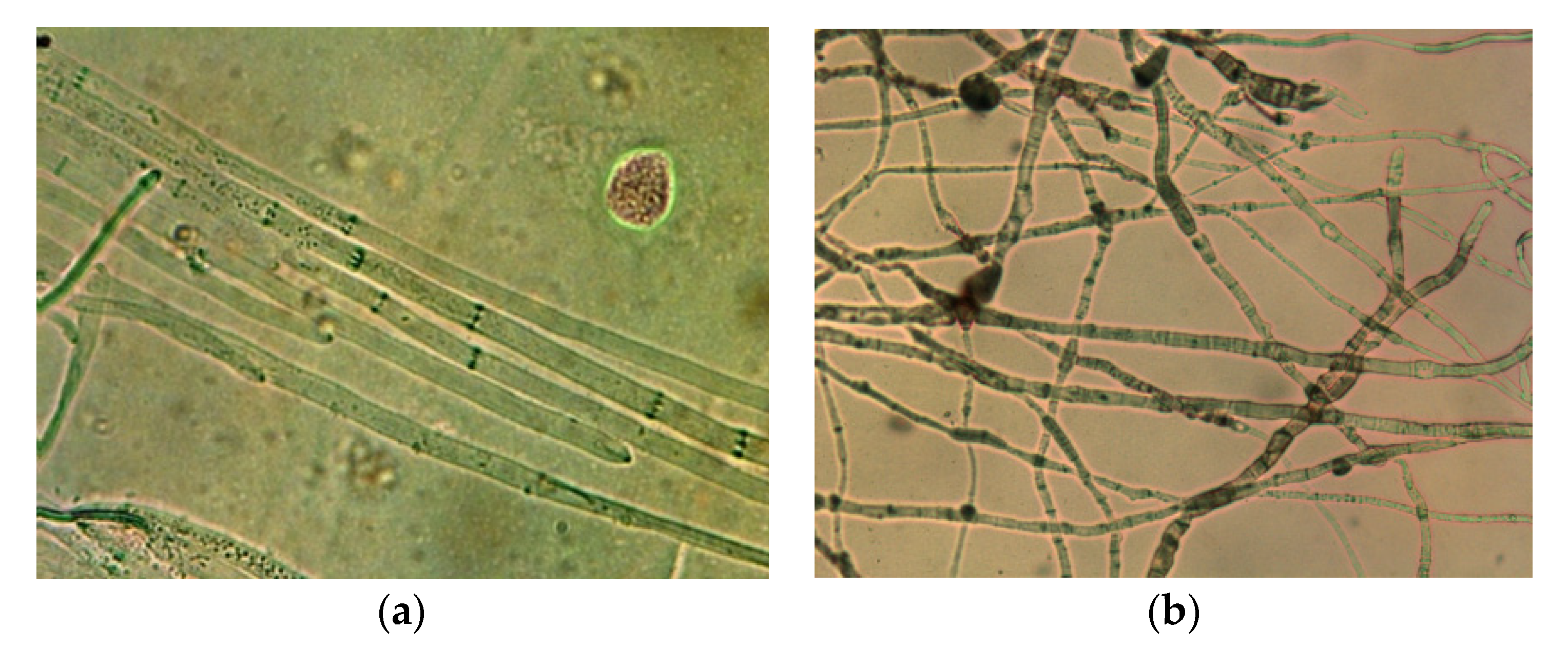
2.2.1. Dual Culture Method with Microscopy
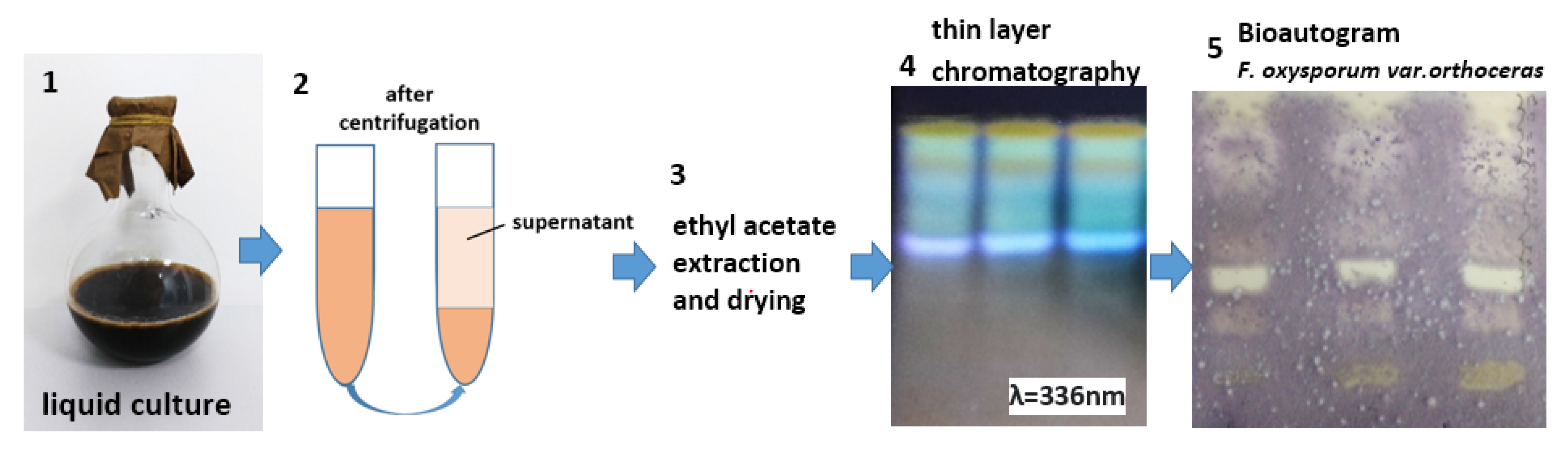
2.2.2. Study of Composition and Quantity of Bacterial Metabolites
2.2.3. Mass Spectrometry and Sample Preparation
2.3. Climate Chamber Experiments Background
2.4. Field Research
3. Results
3.1. Study of Strain–Pathogen Interaction
3.1.1. Modified Dual Culture Method
3.1.2. Analysis of Bacterial Metabolites by the Modified Bioautography Technique
3.1.3. Identification of the Structure of Bacterial Metabolites by Mass Spectrometry
3.2. Climatic Chamber Research
3.3. Field Research
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azizbekyan, R.R. Biological preparations for the protection of agricultural plants (review). Biotechnology 2018, 34, 37–47. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Gimeno, A.; Kagi, A.; Drakopoulos, D.; Banziger, I.; Lehmann, E.; Forrer, H.R.; Keller, B.; Vogelgsang, S. From laboratory to the field: Biological control of Fusarium graminearum on infected maize crop residues. J. Appl. Microbiol. 2020, 129, 680–694. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Effects of planting date and environmental factors on Fusarium ear rot symptoms and fumonisin B1 accumulation in maize grown in six North American locations. Plant Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- Choo, T.M.; Martin, R.A.; Savard, M.E.; Blackwell, B. Effects of planting date and earliness on deoxynivalenol contamination in barley under natural epidemic conditions. Can. J. Plant Sci. 2014, 94, 1363–1371. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Perrone, G.; Mulè, G. Biodiversity of complexes of mycotoxigenic fungal species associated with Fusarium ear rot of maize and Aspergillus rot of grape. Int. J. Food Microbiol. 2007, 119, 11–16. [Google Scholar] [CrossRef]
- Ioos, R.; Belhadj, A.; Menez, M.; Faure, A. The effects of fungicides on Fusarium spp. and Microdochium nivale and their associated trichothecene mycotoxins in French naturally-infected cereal grains. Crop. Prot. 2005, 24, 894–902. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Lozowicka, B.; Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Kuldybayev, N.; Dutbayev, Y. Impact of diversified chemical and biostimulator protection on yield, health status, mycotoxin level, and economic profitability in spring wheat (Triticum aestivum L.) Cultivation. Agronomy 2022, 12, 258. [Google Scholar] [CrossRef]
- Forrer, H.R.; Hecker, A.; Külling, C.; Kessler, P.; Jenny, E.; Krebs, H. Effect of fungicides on fusaria of wheat? AGRAR Forsch. 2000, 7, 258–263. [Google Scholar]
- Devi, S.; Kiesewalter, H.T.; Kovács, R.; Frisvad, J.C.; Weber, T.; Larsen, T.O.; Kovács, Á.T.; Ding, L. Depiction of secondary metabolites and antifungal activity of Bacillus velezensis DTU001. Synth. Syst. Biotechnol. 2019, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Monkhung, S.; Lee, Y.S.; Kim, K.Y. Effects of Lysobacter antibioticus HS124, an effective biocontrol agent against Fusarium graminearum, on crown rot disease and growth promotion of wheat. Can. J. Microbiol. 2019, 65, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Palazzini, J.M.; Ramirez, M.L.; Torres, A.M.; Chulze, S.N. Potential biocontrol agents for Fusarium head blight and deoxynivalenol production in wheat. Crop. Prot. 2007, 11, 1702–1710. [Google Scholar] [CrossRef]
- Mahmoud, A.F. Genetic variation and biological control of Fusarium graminearum isolated from wheat in Assiut-Egypt. Plant Pathol. J. 2016, 32, 145–156. [Google Scholar] [CrossRef]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.; et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef]
- Chae, G.P.; Shoda, M.; Kubota, H. Suppressive effect of Bacillus subtilis and its products on phytopathogenic microorganisms. J. Ferment. Bioeng. 1990, 69, 1–7. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Peypoux, F. Iturins, a special class of pore-forming lipopeptides: Biological and physicochemical properties. Toxicology 1994, 87, 151–174. [Google Scholar] [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Dunlap, C.A.; Bowman, M.J.; Chulze, S.N. Bacillus velezensis R.C.218 as biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: Genome sequencing and secondary metabolite cluster profiles. Microbiol. Res. 2016, 192, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, V.V.; Vasilyev, I.Y.; Ilnitskaya, E.V.; Garkovenko, A.V.; Asaturova, A.M.; Tomashevich, N.S.; Kozitsyn, A.E.; Milovanov, A.V.; Grigoreva, T.V.; Shternshis, M.V. Draft genome sequence of the plant growth-promoting bacterium Bacillus subtilis strain BZR 517, isolated from winter wheat, now reclassified as Bacillus velezensis strain BZR 517. Microbiol. Resour. Announc. 2020, 9, e00853-20. [Google Scholar] [CrossRef] [PubMed]
- Garkovenko, A.V.; Vasilyev, I.Y.; Ilnitskaya, E.V.; Radchenko, V.V.; Asaturova, A.M.; Kozitsyn, A.E.; Tomashevich, N.S.; Milovanov, A.V.; Grigoreva, T.V.; Shternshis, M.V. Draft Genome Sequence of Bacillus velezensis BZR 336 g, a Plant Growth-Promoting Antifungal Biocontrol Agent Isolated from Winter Wheat. Microbiol. Resour. Announc. 2020, 23, e00450-20. [Google Scholar] [CrossRef]
- Asaturova, A.M.; Khomyak, A.I.; Tomashevich, N.S.; Pavlova, M.D.; Zhevnova, N.A.; Dubyaga, V.M.; Kozitsin, A.Y.; Sidorova, T.M.; Nadykta, V.D.; Ismailov, V.Y.A. Conditions for the cultivation of new bacillus bacteria being micro bioproduct producers. J. Pur. Appl. Microbiol. 2015, 4, 2797–2804. [Google Scholar]
- Huang, H.C.; Hoes, J.A. Penetration and infection of Sclerotinia sclerotiorum by Coniothyrium minitans. Can. J. Bot. 1976, 54, 406. [Google Scholar] [CrossRef]
- Kirchner, J. Thin Layer Chromatography; Science-Thought: Moscow, Russia, 1981; pp. 221–285. [Google Scholar]
- Sidorova, T.M.; Asaturova, A.M.; Homyak, A.I.; Tomashevich, N.S. Isolation and characterization of antifungal metabolites of Bacillus subtilis strains BZR 336 g and BZR 517 using the modified bioauthography method. Agric. Boil. 2019, 1, 178–185. [Google Scholar] [CrossRef]
- Dolzhenko, V.I. Guidelines for Registration Testing of Fungicides in Agriculture; Rosinformagrotech: Saint Petersburg, Russia, 2009; pp. 250–261. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 2, 265–267. [Google Scholar] [CrossRef]
- Asaturova, A.; Zhevnova, N.; Tomashevich, N.; Pavlova, M.; Kremneva, O.; Volkova, G.; Sidorov, N. Efficacy of new local bacterial agents against Pyrenophora tritici-repentis in Kuban Region, Russia. Agronomy 2022, 12, 373. [Google Scholar] [CrossRef]
- Shternshis, M.; Asaturova, A.; Shpatova, T.; Zhevnova, N.; Homyak, A. Promising Bacillus subtilis strains BZR 336 g and BZR 517 for biocontrol of blackcurrant against Septoria leaf spot under unfavorable climate conditions. J. Plant Pathol. 2021, 103, 295–298. [Google Scholar] [CrossRef]
- Asaturova, A.; Shternshis, M.; Tsvetkova, V.; Shpatova, T.; Maslennikova, V.; Zhevnova, N.; Homyak, A. Biological control of important fungal diseases of potato and raspberry by two Bacillus velezensis strains. PeerJ 2021, 9, e11578. [Google Scholar] [CrossRef]




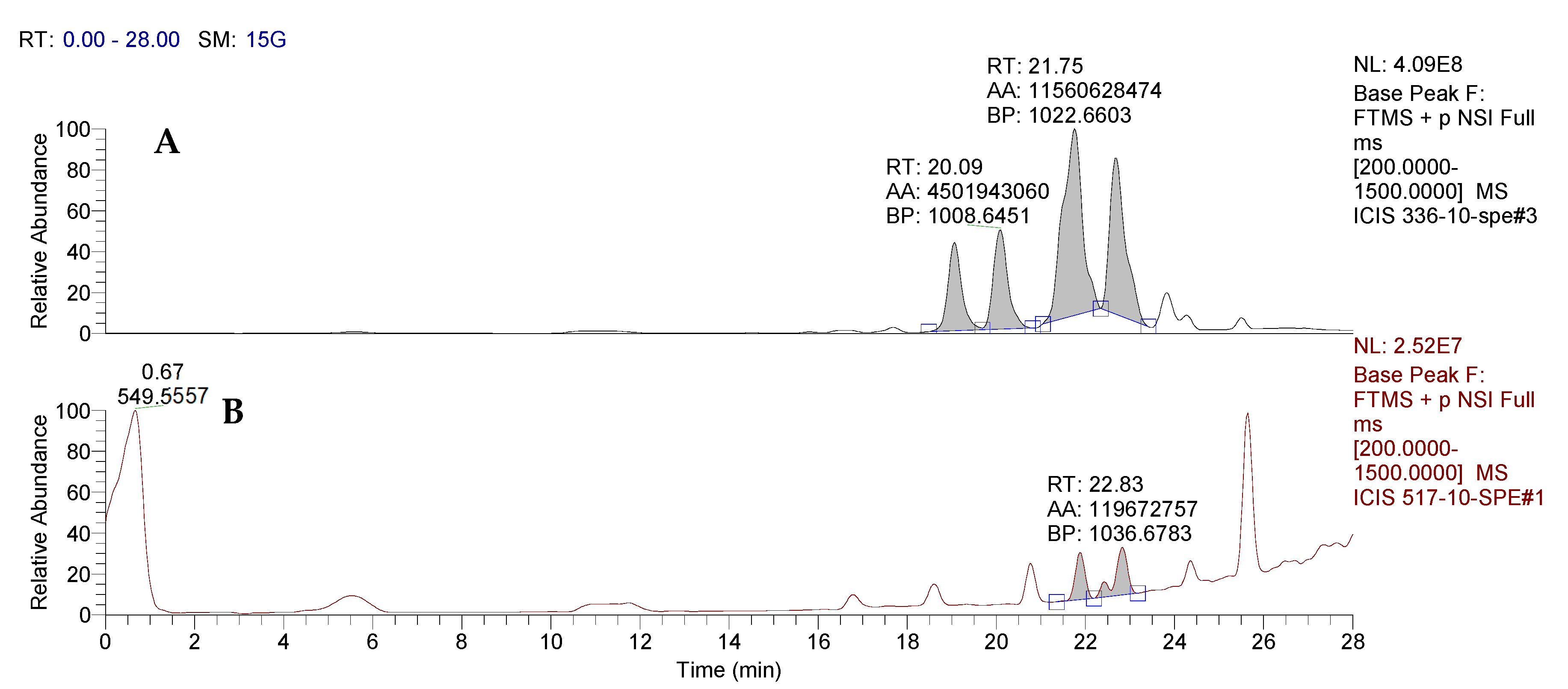
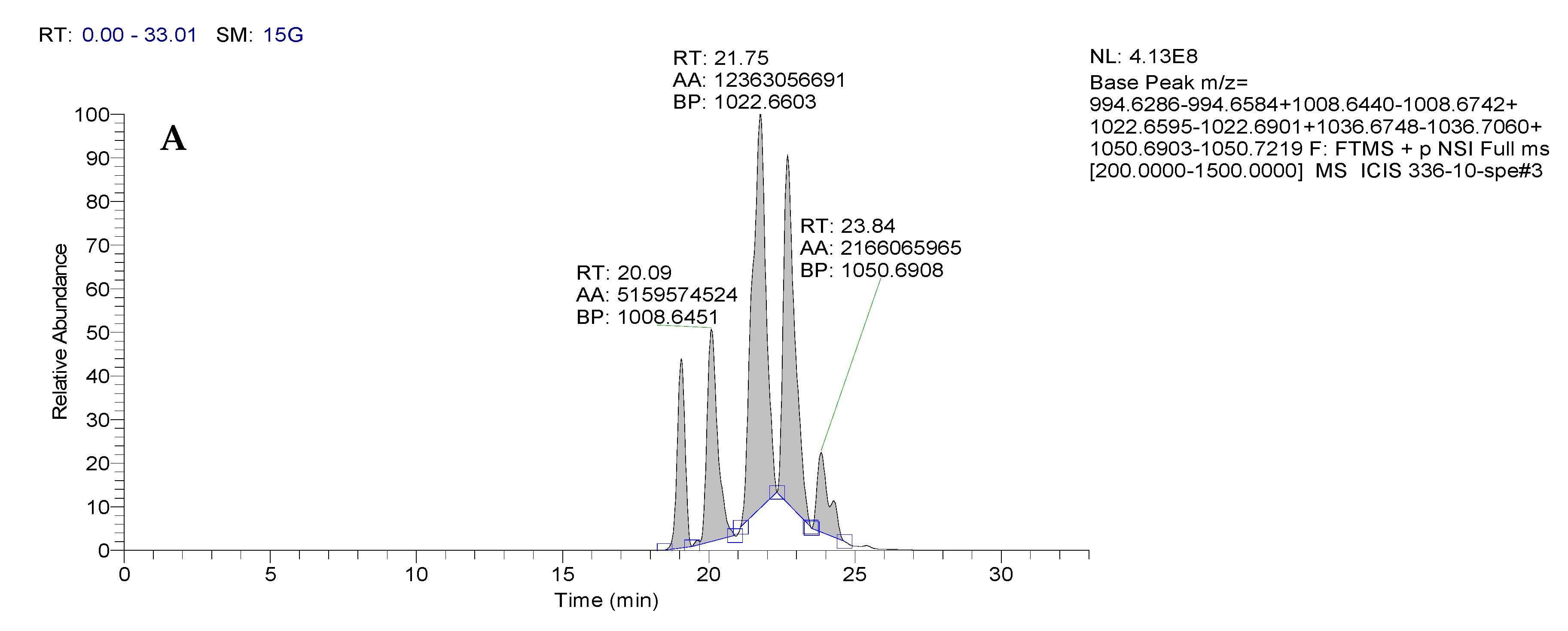
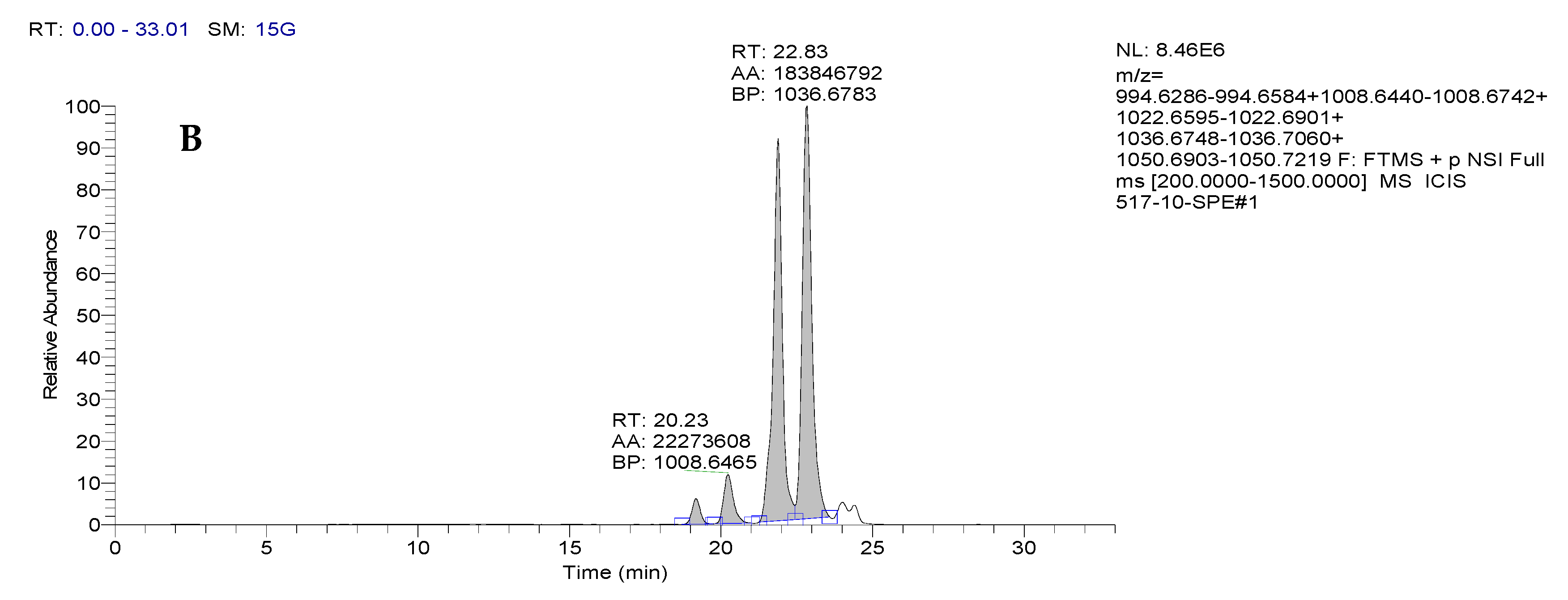
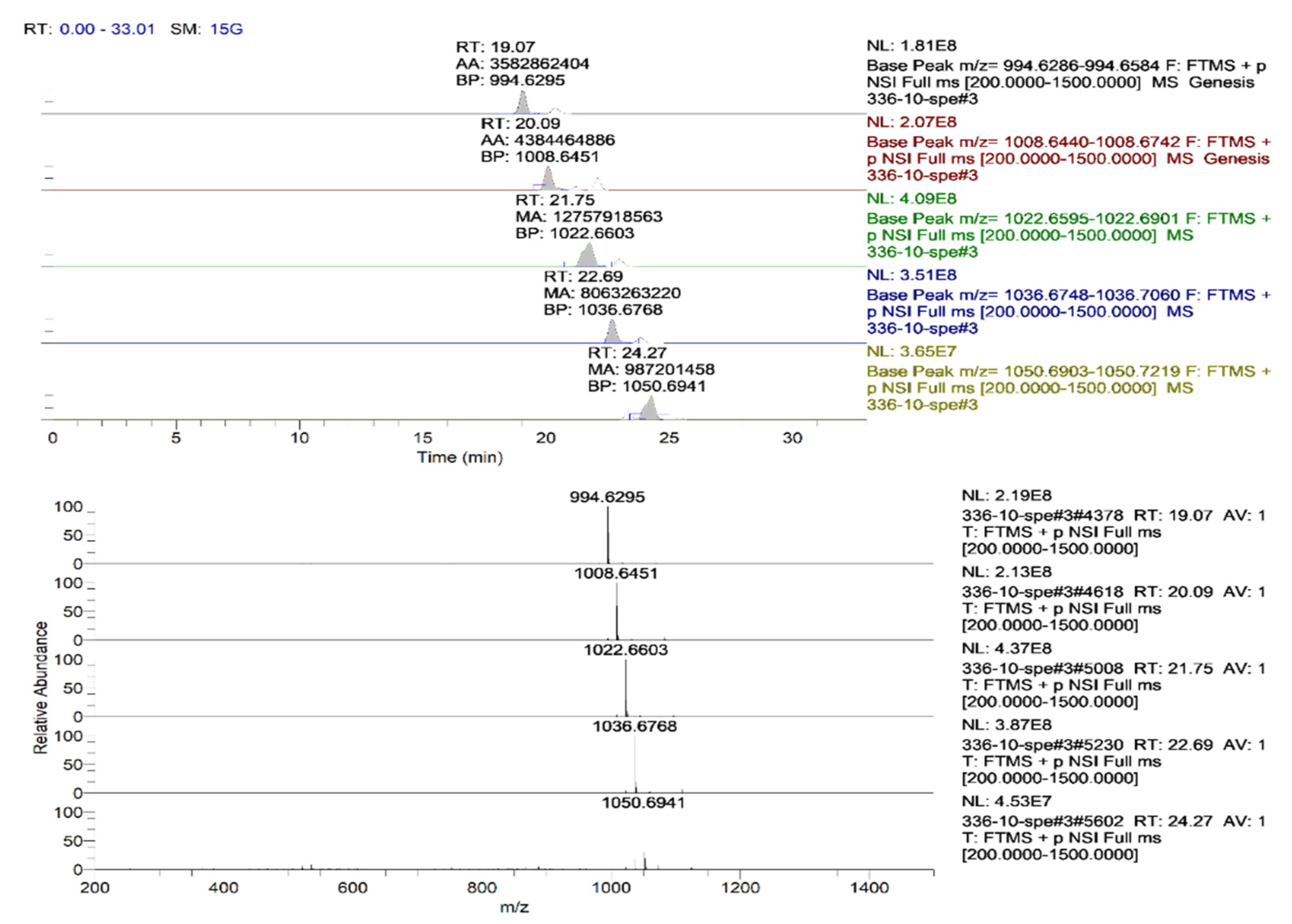

| Retention Time, RT | Sample No. | m/zob | m/zth | Ionization Mode | Molecular Formula | Error (ppm) | Assignment |
|---|---|---|---|---|---|---|---|
| 19.07 | BZR 336 g | 994.6301 | 994.6435 | [M + H]+ | C50H87N7O13 | 13.5 | Surfactin A-CH2 |
| 20.09 | BZR 336 g | 1008.6451 | 1008.6591 | [M + H]+ | C51H89N7O13 | 13.9 | Surfactin A |
| 21.75 | BZR 336 g | 1022.6611 | 1022.6748 | [M + H]+ | C52H91N7O13 | 13.4 | Surfactin B |
| 22.69 | BZR 336 g | 1036.6768 | 1036.6904 | [M + H]+ | C53H92N7O13 | 13.1 | Surfactin C |
| 23.84 | BZR 336 g | 1050.6950 | 1050.7061 | [M + H]+ | C54H94N7O13 | 10.6 | Surfactin C + CH2 |
| 19.18 | BZR 517 | 994.6307 | 994.6435 | [M + H]+ | C50H87N7O13 | 12.9 | Surfactin A-CH2 |
| 20.23 | BZR 517 | 1008.6465 | 1008.6591 | [M + H]+ | C51H89N7O13 | 12.5 | Surfactin A |
| 21.88 | BZR 517 | 1022.6619 | 1022.6748 | [M + H]+ | C52H91N7O13 | 12.6 | Surfactin B |
| 22.83 | BZR 517 | 1036.6783 | 1036.6904 | [M + H]+ | C53H92N7O13 | 11.7 | Surfactin C |
| Treatment | Germination, % | Development, % | BE, % |
|---|---|---|---|
| Control | 87.6 a | 48.2 b | - |
| Chemical standard Kinto Duo, SC | 92.3 a | 35.9 ab | 25.5 |
| Biological standard Fitosporin-M, L | 91 a | 29.1 ab | 39.6 |
| B. velezensis BZR 336 g | 94.3 a | 37.3 ab | 22.5 |
| B. velezensis BZR 517 | 95.7 a | 32.6 a | 32.3 |
| Experimental Options | Development, % | Biological Efficacy against Fusarium Root Rots, %, by Years | Productivity, t/ha | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2012–2013 | 2013–2014 | 2014–2015 | 2012–2013 | 2013–2014 | 2014–2015 | 2012–2013 | 2013–2014 | 2014–2015 | |
| Control | 22.2 b | 36.1 b | 25.3 b | - | - | - | 4.0 b | 6.9 a | 7.7 a |
| Chemical standard | 20.0 b | 26.7 a | 18.8 a | 10.0 | 26.2 | 25.9 | 6.9 c | 7.0 a | 8.3 b |
| Biological standard | – | 29.8 a | 21.3 ab | – | 17.4 | 16.0 | – | 7.4 c | 7.5 a |
| B. velezensis BZR 336 g | 13.9 a | 27.9 a | 21.9 ab | 37.5 | 22.8 | 13.6 | 7.9 a | 7.2 b | 7.9 ab |
| B. velezensis BZR 517 | 12.9 a | 29.9 a | 26.3 b | 45.0 | 17.2 | 0 | 7.9 a | 7.6 d | 7.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaturova, A.M.; Zhevnova, N.A.; Tomashevich, N.S.; Sidorova, T.M.; Homyak, A.I.; Dubyaga, V.M.; Nadykta, V.D.; Zharikov, A.P.; Kostyukevich, Y.I.; Tupertsev, B.S. Evaluation of Bacillus velezensis Biocontrol Potential against Fusarium Fungi on Winter Wheat. Agronomy 2022, 12, 1956. https://doi.org/10.3390/agronomy12081956
Asaturova AM, Zhevnova NA, Tomashevich NS, Sidorova TM, Homyak AI, Dubyaga VM, Nadykta VD, Zharikov AP, Kostyukevich YI, Tupertsev BS. Evaluation of Bacillus velezensis Biocontrol Potential against Fusarium Fungi on Winter Wheat. Agronomy. 2022; 12(8):1956. https://doi.org/10.3390/agronomy12081956
Chicago/Turabian StyleAsaturova, Anzhela Mikhailovna, Natalya Andreevna Zhevnova, Natalia Sergeevna Tomashevich, Tatiana Mikhailovna Sidorova, Anna Igorevna Homyak, Valentina Mikhailovna Dubyaga, Vladimir Dmitrievich Nadykta, Artem Pavlovich Zharikov, Yuri Irodionovich Kostyukevich, and Boris Sergeevich Tupertsev. 2022. "Evaluation of Bacillus velezensis Biocontrol Potential against Fusarium Fungi on Winter Wheat" Agronomy 12, no. 8: 1956. https://doi.org/10.3390/agronomy12081956






