24-Epibrassinolide Simultaneously Stimulates Photosynthetic Machinery and Biomass Accumulation in Tomato Plants under Lead Stress: Essential Contributions Connected to the Antioxidant System and Anatomical Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Growth Conditions
2.2. Plants, Containers and Acclimation
2.3. Experimental Design
2.4. 24-Epibrassinolide (EBR) Preparation and Application
2.5. Plant Nutrition and Pb Treatment
2.6. Determination of Pb and Nutrients
2.7. Anatomical Measurements
2.8. Determination of Photosynthetic Pigments
2.9. Measurement of Chlorophyll Fluorescence
2.10. Evaluation of Gas Exchange
2.11. Determination of Antioxidant Enzymes, Superoxide and Soluble Proteins Level
2.12. Superoxide Dismutase Assay
2.13. Catalase Assay
2.14. Ascorbate Peroxidase Assay
2.15. Peroxidase Assay
2.16. Determination of Superoxide Concentration
2.17. Extraction of Nonenzymatic Compounds
2.18. Determination of Hydrogen Peroxide Concentration
2.19. Quantification of Malondialdehyde Concentration
2.20. Determination of Electrolyte Leakage
2.21. Measurements of Biomass
2.22. Data Analysis
3. Results
3.1. Pb Content Was Minimized by EBR in Plants Exposed to Toxicity
3.2. EBR Positively Modulated the Root and Leaf Structures
3.3. Nutrient Contents and Metal Homeostasis Were Up-Regulated by the Steroid
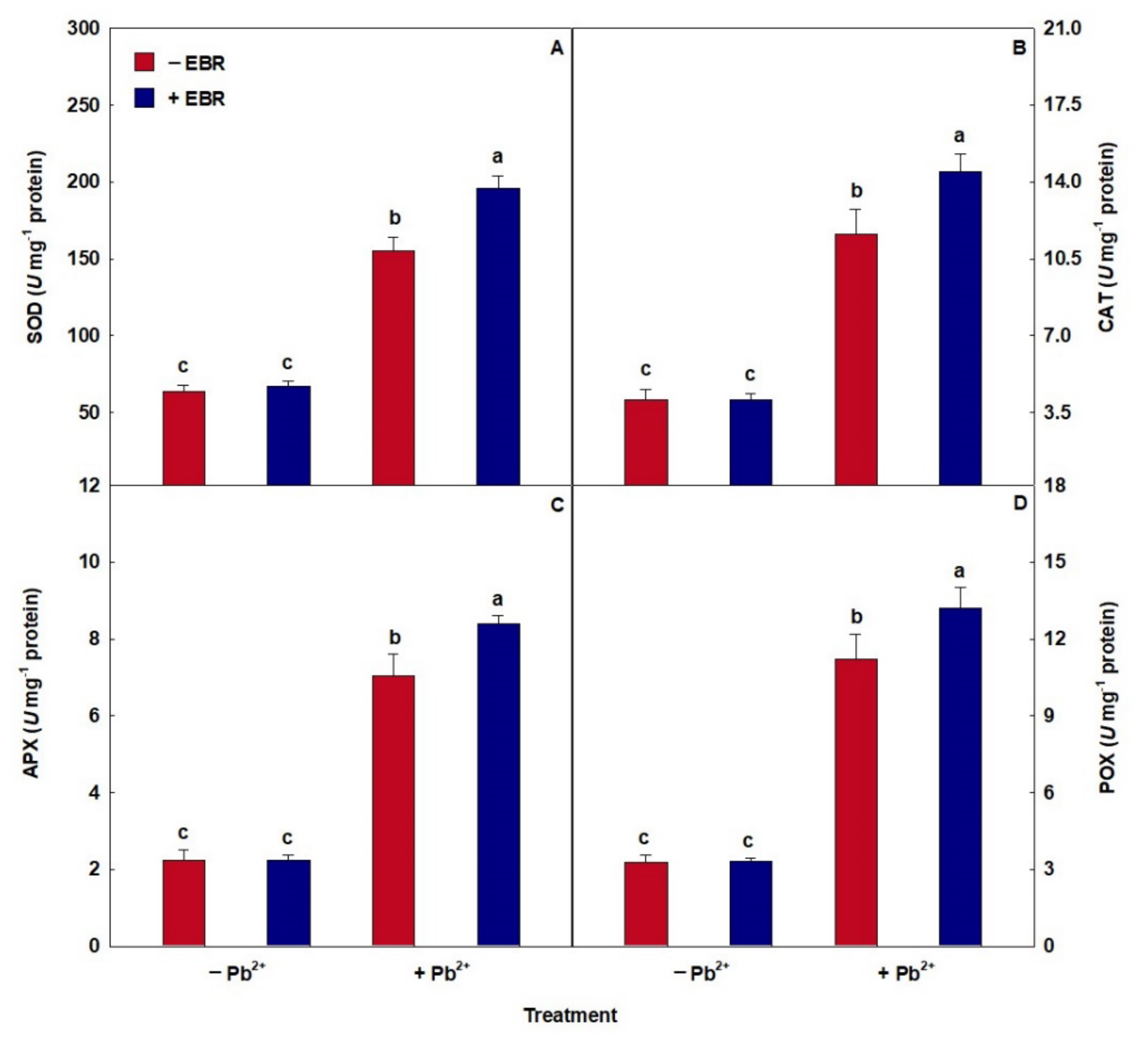
3.4. An Antioxidant System Modulated by EBR
3.5. Steroid Stimulates Oxidative Damage Reduction
3.6. EBR Benefited Maintenance of Membrane Integrity of Chloroplasts and Alleviated the Pb Interference on Light Capture and Gas Exchange
3.7. Limitations on Stomatal Performance Were Attenuated by EBR
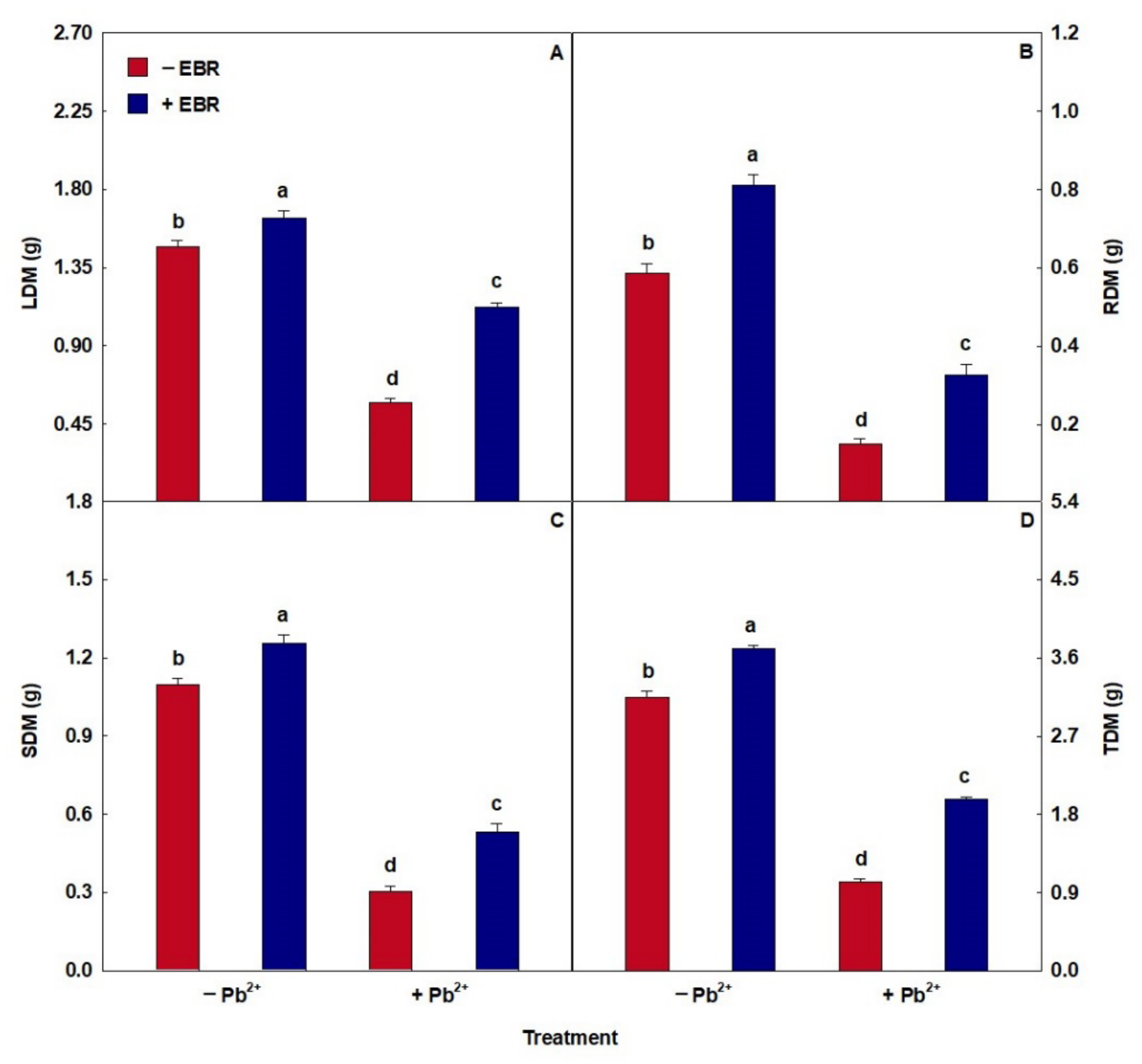
3.8. EBR Promoted Higher Biomass Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar Heavy Metal Uptake, Toxicity and Detoxification in Plants: A Comparison of Foliar and Root Metal Uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological Approaches to Tackle Heavy Metal Pollution: A Survey of Literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1–36. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 1, 25–52. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide Modulates Growth, Nodulation, Antioxidant System, and Osmolyte in Tolerant and Sensitive Varieties of Vigna Radiata under Different Levels of Nickel: A Shotgun Approach. Plant Physiol. Biochem. 2012, 57, 143–153. [Google Scholar] [CrossRef]
- Barros Junior, U.O.; Lima, M.D.R.; Alsahli, A.A.; Lobato, A.K.S. Unraveling the Roles of Brassinosteroids in Alleviating Drought Stress in Young Eucalyptus Urophylla Plants: Implications on Redox Homeostasis and Photosynthetic Apparatus. Physiol. Plant. 2021, 172, 761–784. [Google Scholar] [CrossRef]
- Rodrigues, W.S.; Pereira, Y.C.; Souza, A.L.M.; Batista, B.L.; Lobato, A.K.S. Alleviation of Oxidative Stress Induced by 24-Epibrassinolide in Soybean Plants Exposed to Different Manganese Supplies: UpRegulation of Antioxidant Enzymes and Maintenance of Photosynthetic Pigments. J. Plant Growth Regul. 2020, 39, 1425–1440. [Google Scholar] [CrossRef]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally Designed Mimics of Antioxidant Manganoenzymes: Role of Structural Features in the Quest for Catalysts with Catalase and Superoxide Dismutase Activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- da Silva Lobato, A.K.; Barbosa, M.A.M.; Alsahli, A.A.; Lima, E.J.A.; Da Silva, B.R.S. Exogenous Salicylic Acid Alleviates the Negative Impacts on Production Components, Biomass and Gas Exchange in Tomato Plants under Water Deficit Improving Redox Status and Anatomical Responses. Physiol. Plant. 2021, 172, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Hermle, S.; Vollenweider, P.; Gunthardt-Goerg, M.S.; McQuattie, C.J.; Matyssek, R. Leaf Responsiveness of Populus Tremula and Salix Viminalis to Soil Contaminated with Heavy Metals and Acidic Rainwater. Tree Physiol. 2007, 27, 1517–1531. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.-G. Opinion: Prospects for Improving Photosynthesis by Altering Leaf Anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The Formation and Functions of a Fundamental Plant Tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- Glover, B.J.; Airoldi, C.A.; Moyroud, E. Epidermis: Outer Cell Layer of the Plant. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–7. ISBN 9780470015902. [Google Scholar]
- Sorin, C.; Musse, M.; Mariette, F.; Bouchereau, A.; Leport, L. Assessment of Nutrient Remobilization through Structural Changes of Palisade and Spongy Parenchyma in Oilseed Rape Leaves during Senescence. Planta 2015, 241, 333–346. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Wang, R.; Mo, G.; Luo, S.; Luo, X.; He, L.; Gonsamo, A.; Arabian, J.; Zhang, Y.; et al. The Global Distribution of Leaf Chlorophyll Content. Remote Sens. Environ. 2020, 236, 111479. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in Photosynthetic Pigments, Protein, and Carbohydrate Metabolism in a Wild Plant Coronopus didymus L. (Brassicaceae) under Lead Stress. Acta Physiol. Plant. 2017, 39, 176. [Google Scholar] [CrossRef]
- Hussain, I.; Siddique, A.; Ashraf, M.A.; Rasheed, R.; Ibrahim, M.; Iqbal, M.; Akbar, S.; Imran, M. Does Exogenous Application of Ascorbic Acid Modulate Growth, Photosynthetic Pigments and Oxidative Defense in Okra (Abelmoschus esculentus (L.) Moench) under Lead Stress? Acta Physiol. Plant. 2017, 39, 144. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-Lead Interactions: Transport, Toxicity, Tolerance, and Detoxification Mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids Make Plant Life Easier under Abiotic Stresses Mainly by Modulating Major Components of Antioxidant Defense System. Front. Environ. Sci. 2015, 2, 872–876. [Google Scholar] [CrossRef]
- Vidya Vardhini, B. Modifications of Morphological and Anatomical Characteristics of Plants by Application of Brassinosteroids under Various Abiotic Stress Conditions—A Review. Plant Gene 2017, 11, 70–89. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Zulfiqar, B.; Akram, A.; Ashraf, M.Y.; Raheel, M.; Shabbir, R.N.; Hussain, R.A.; Anwar, I.; Aurangzaib, M. Understanding Brassinosteroid-Regulated Mechanisms to Improve Stress Tolerance in Plants: A Critical Review. Environ. Sci. Pollut. Res. 2017, 24, 15959–15975. [Google Scholar] [CrossRef]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Huang, Z.; Jiang, M.; Li, X. Effect of 24-Epibrassinolide on Reactive Oxygen Species and Antioxidative Defense Systems in Tall Fescue Plants under Lead Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109831. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined Effects of Brassinosteroid and Kinetin Mitigates Salinity Stress in Tomato through the Modulation of Antioxidant and Osmolyte Metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Zhang, Y.P.; He, J.; Yang, S.J.; Chen, Y.Y. Exogenous 24-Epibrassinolide Ameliorates High Temperature-Induced Inhibition of Growth and Photosynthesis in Cucumis Melo. Biol. Plant. 2014, 58, 311–318. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid Alleviates Chilling-Induced Oxidative Stress in Pepper by Enhancing Antioxidation Systems and Maintenance of Photosystem II. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Lima, J.V.; Lobato, A.K.S. Brassinosteroids Improve Photosystem II Efficiency, Gas Exchange, Antioxidant Enzymes and Growth of Cowpea Plants Exposed to Water Deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Y.C.; Rodrigues, W.S.; Lima, E.J.A.; Santos, L.R.; Silva, M.H.L.; Lobato, A.K.S. Brassinosteroids Increase Electron Transport and Photosynthesis in Soybean Plants under Water Deficit. Photosynthetica 2019, 57, 181–191. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and Physiological Functions in Plants under Heavy Metal Stress. Front. Plant Sci. 2021; in press. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Agricultural Experiment Station: California, CA, USA, 1950. [Google Scholar]
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide Stimulates Root Protection, and Leaf Antioxidant Enzymes in Lead Stressed Rice Plants: Central Roles to Minimize Pb Content and Oxidative Stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef]
- Maia, C.F.; Silva, B.R.S.; Lobato, A.K.S. Brassinosteroids Positively Modulate Growth: Physiological, Biochemical and Anatomical Evidence Using Two Tomato Genotypes Contrasting to Dwarfism. J. Plant Growth Regul. 2018, 37, 1–14. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective Procedures for the Determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and Rare Earth Elements in Plants and Foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic Staining of Plant Cell Walls by Toluidine Blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Segatto, F.B.; Bisognin, D.A.; Benedetti, M.; Costa, L.C.; Rampelotto, M.V.; Nicoloso, F.T. A Technique for the Anatomical Study of Potato Leaf Epidermis. Ciência Rural 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Castro, E.M.; Pereira, F.J.; Paiva, R. Plant Histology: Structure and Function of Vegetative Organs, 1st ed.; UFLA Publishers: Lavras, Brazil, 2009. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 431–438. ISBN 9780471709084. [Google Scholar]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L. Photosynthesis, Transpiration, and Water Use Efficiency of Caragana Microphylla, C. Intermedia, and C. Korshinskii. Photosynthetica 2004, 42, 65–70. [Google Scholar] [CrossRef]
- Aragão, R.M.; Silva, E.N.; Vieira, C.F.; Silveira, J.A.G. High Supply of NO3−Mitigates Salinity Effects through an Enhancement in the Efficiency of Photosystem II and CO2 Assimilation in Jatropha Curcas Plants. Acta Physiol. Plant. 2012, 34, 2135–2143. [Google Scholar] [CrossRef]
- Badawi, G.H.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kawano, N.; Tanaka, K.; Tanaka, K. Enhanced Tolerance to Salt Stress and Water Deficit by Overexpressing Superoxide Dismutase in Tobacco (Nicotiana tabacum) Chloroplasts. Plant Sci. 2004, 166, 919–928. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of Nitrite Formation from Hydroxylammoniumchloride: A Simple Assay for Superoxide Dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Reactive Oxygen Metabolism in Mycorrhizal and Non-Mycorrhizal Citrus (Poncirus Trifoliata) Seedlings Subjected to Water Stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine Max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic Acid-Induced Thermotolerance in Maize Seedlings Is Mediated by Calcium and Associated with Antioxidant Systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic Internet Publishers: Moorpark, CA, USA, 2006. [Google Scholar]
- Venables, W.N.; Smith, D.M. R Core Team: An Introduction to R; R Core Team Publishers: USA, 2021; Available online: https://www.studypool.com/documents/8034079/an-introduction-to-r-author-w-n-venables-d-m-smith-the-r-core-team (accessed on 21 August 2022).
- Dalyan, E.; Yüzbaşıoğlu, E.; Akpınar, I. Effect of 24-Epibrassinolide on Antioxidative Defence System Against Lead-Induced Oxidative Stress in The Roots of Brassica juncea L. Seedlings. Russ. J. Plant Physiol. 2018, 65, 570–578. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined Effect of 24-Epibrassinolide and Salicylic Acid Mitigates Lead (Pb) Toxicity by Modulating Various Metabolites in Brassica juncea L. Seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef]
- Rossato, L.V.; Nicoloso, F.T.; Farias, J.G.; Cargnelluti, D.; Tabaldi, L.A.; Antes, F.G.; Dressler, V.L.; Morsch, V.M.; Schetinger, M.R.C. Effects of Lead on the Growth, Lead Accumulation and Physiological Responses of Pluchea Sagittalis. Ecotoxicology 2012, 21, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Talarek-Karwel, M.; Bajguz, A.; Piotrowska-Niczyporuk, A. 24-Epibrassinolide Modulates Primary Metabolites, Antioxidants, and Phytochelatins in Acutodesmus Obliquus Exposed to Lead Stress. J. Appl. Phycol. 2020, 32, 263–276. [Google Scholar] [CrossRef]
- Khan, F.; Hussain, S.; Tanveer, M.; Khan, S.; Hussain, H.A.; Iqbal, B.; Geng, M. Coordinated Effects of Lead Toxicity and Nutrient Deprivation on Growth, Oxidative Status, and Elemental Composition of Primed and Non-Primed Rice Seedlings. Environ. Sci. Pollut. Res. 2018, 25, 21185–21194. [Google Scholar] [CrossRef]
- Gomes, M.P.; de Sá e Melo Marques, T.C.L.L.; de Oliveira Gonçalves Nogueira, M.; de Castro, E.M.; Soares, Â.M. Ecophysiological and Anatomical Changes Due to Uptake and Accumulation of Heavy Metal in Brachiaria Decumbens. Sci. Agric. 2011, 68, 566–573. [Google Scholar] [CrossRef]
- Pereira, Y.C.; da Silva, F.R.; da Silva, B.R.S.; Cruz, F.J.R.; Marques, D.J.; da Silva Lobato, A.K. 24-Epibrassinolide Induces Protection against Waterlogging and Alleviates Impacts on the Root Structures, Photosynthetic Machinery and Biomass in Soybean. Plant Signal. Behav. 2020, 15, 1805885. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhu, S.; Shu, S.; Sun, J.; Guo, S. Regulation of 2,4-Epibrassinolide on Mineral Nutrient Uptake and Ion Distribution in Ca(NO3)2 Stressed Cucumber Plants. J. Plant Physiol. 2015, 188, 29–36. [Google Scholar] [CrossRef]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive Effect of 24-Epibrassinolide and Silicon Alleviates Cadmium Stress via the Modulation of Antioxidant Defense and Glyoxalase Systems and Macronutrient Content in Pisum sativum L. Seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef]
- Santos, L.R.; da Silva, B.R.S.; Pedron, T.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide Improves Root Anatomy and Antioxidant Enzymes in Soybean Plants Subjected to Zinc Stress. J. Soil Sci. Plant Nutr. 2020, 20, 105–124. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Arora, S.; Sharma, A.; Kaur, R.; Bhardwaj, R. Modulation of Antioxidative Defense Expression and Osmolyte Content by Co-Application of 24-Epibrassinolide and Salicylic Acid in Pb Exposed Indian Mustard Plants. Ecotoxicol. Environ. Saf. 2018, 147, 382–393. [Google Scholar] [CrossRef]
- Xia, X.-J.; Wang, Y.-J.; Zhou, Y.-H.; Tao, Y.; Mao, W.-H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.-Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant Steroidal Hormone Epibrassinolide Regulate–Heavy Metal Stress Tolerance in Oryza sativa L. by Modulating Antioxidant Defense Expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huo, S.; Wang, L.; Meng, J.; Zhang, Z.; Xi, Z. Exogenous 24-Epibrassinolide Alleviates Oxidative Damage from Copper Stress in Grape (Vitis vinifera L.) Cuttings. Plant Physiol. Biochem. 2018, 130, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-Epibrassinolide and Salicylic Acid Regulates Pigment Contents, Antioxidative Defense Responses, and Gene Expression in Brassica Juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V.; Sytar, O. Lead Toxicity, Defense Strategies and Associated Indicative Biomarkers in Talinum Triangulare Grown Hydroponically. Chemosphere 2012, 89, 1056–1065. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and Lead Interactive Effects on Oxidative Stress and Antioxidative Responses in Rice Seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Wu, X.; Yao, X.; Chen, J.; Zhu, Z.; Zhang, H.; Zha, D. Brassinosteroids Protect Photosynthesis and Antioxidant System of Eggplant Seedlings from High-Temperature Stress. Acta Physiol. Plant. 2014, 36, 251–261. [Google Scholar] [CrossRef]
- Ali, B.; Mwamba, T.M.; Gill, R.A.; Yang, C.; Ali, S.; Daud, M.K.; Wu, Y.; Zhou, W. Improvement of Element Uptake and Antioxidative Defense in Brassica Napus under Lead Stress by Application of Hydrogen Sulfide. Plant Growth Regul. 2014, 74, 261–273. [Google Scholar] [CrossRef]
- Kohli, S.K.; Bali, S.; Tejpal, R.; Bhalla, V.; Verma, V.; Bhardwaj, R.; Alqarawi, A.A.; Abd_Allah, E.F.; Ahmad, P. In-Situ Localization and Biochemical Analysis of Bio-Molecules Reveals Pb-Stress Amelioration in Brassica juncea L. by Co-Application of 24-Epibrassinolide and Salicylic Acid. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Hou, X.; Han, H.; Cai, L.; Liu, A.; Ma, X.; Zhou, C.; Wang, G.; Meng, F. Pb Stress Effects on Leaf Chlorophyll Fluorescence, Antioxidative Enzyme Activities, and Organic Acid Contents of Pogonatherum Crinitum Seedlings. Flora 2018, 240, 82–88. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Wang, C.; Lu, J. Effects of Pb on the Oxidative Stress and Antioxidant Response in a Pb Bioaccumulator Plant Vallisneria Natans. Ecotoxicol. Environ. Saf. 2012, 78, 28–34. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderón-Urrea, A.; Lyu, J.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome Analysis of Pepper (Capsicum annuum) Revealed a Role of 24-Epibrassinolide in Response to Chilling. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Lead-Induced Toxicity and Interference in Chlorophyll Fluorescence in Talinum Triangulare Grown Hydroponically. Photosynthetica 2015, 53, 66–71. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, Y.J.; Xu, X.L.; Jin, M.; An, L.Z.; Zhang, H. Effect of 24-Epibrassinolide on Drought Stress-Induced Changes in Chorispora Bungeana. Biol. Plant. 2012, 56, 192–196. [Google Scholar] [CrossRef]
- Tadaiesky, L.B.A.; Silva, B.R.S.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids Trigger Tolerance to Iron Toxicity in Rice. Physiol. Plant. 2020, 171, 371–387, Published, ppl.13230. [Google Scholar] [CrossRef]
- Huang, X.H.; Zhu, F.; Yan, W.D.; Chen, X.Y.; Wang, G.J.; Wang, R.J. Effects of Pb and Zn Toxicity on Chlorophyll Fluorescence and Biomass Production of Koelreuteria Paniculata and Zelkova Schneideriana Young Plants. Photosynthetica 2019, 57, 688–697. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Thussagunpanit, J.; Jutamanee, K.; Kaveeta, L.; Chai-arree, W.; Pankean, P.; Homvisasevongsa, S.; Suksamrarn, A. Comparative Effects of Brassinosteroid and Brassinosteroid Mimic on Improving Photosynthesis, Lipid Peroxidation, and Rice Seed Set under Heat Stress. J. Plant Growth Regul. 2015, 34, 320–331. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Confers Tolerance against NaCl Stress in Soybean Plants by up-Regulating Antioxidant System, Ascorbate-Glutathione Cycle, and Glyoxalase System. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef]
- Santos, L.R.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids Mitigate Cadmium Toxicity in Cowpea Plants. Photosynthetica 2018, 56, 591–605. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M.; Tabassam, Q.; Hussain, M.; Firdous, H. Lead (Pb)-Induced Regulation of Growth, Photosynthesis, and Mineral Nutrition in Maize (Zea mays L.) Plants at Early Growth Stages. Biol. Trace Elem. Res. 2011, 144, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nas, F.S. The Effect of Lead on Plants in Terms of Growing and Biochemical Parameters: A Review. MOJ Ecol. Environ. Sci. 2018, 3. [Google Scholar] [CrossRef]
- Cunha, L.F.S.; Oliveira, V.P.; Nascimento, A.W.S.; Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; Silva Lobato, A.K. Leaf Application of 24-epibrassinolide Mitigates Cadmium Toxicity in Young Eucalyptus Urophylla Plants by Modulating Leaf Anatomy and Gas Exchange. Physiol. Plant. 2020. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.; Ma, J.; Yang, L.; Wei, Y. The Effects of Lead Stress on Photosynthetic Function and Chloroplast Ultrastructure of Robinia Pseudoacacia Seedlings. Environ. Sci. Pollut. Res. 2017, 24, 10718–10726. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Oniosun, B.; Akpoilih, O.A.; Adeseko, A.; Arowo, D.O. Anatomical Changes, Osmolytes Accumulation and Distribution in the Native Plants Growing on Pb-Contaminated Sites. Environ. Geochem. Health 2021, 43, 1537–1549. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Vanhoutte, I.; Boudolf, V.; Betti, C.; Dhondt, S.; Coppens, F.; Mylle, E.; Maes, S.; González-García, M.-P.; Caño-Delgado, A.I.; et al. Brassinosteroid Production and Signaling Differentially Control Cell Division and Expansion in the Leaf. New Phytol. 2013, 197, 490–502. [Google Scholar] [CrossRef]
- dos Santos, L.R.; de Sousa Paula, L.; Pereira, Y.C.; da Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; da Silva Lobato, A.K. Brassinosteroids-Mediated Amelioration of Iron Deficiency in Soybean Plants: Beneficial Effects on the Nutritional Status, Photosynthetic Pigments and Chlorophyll Fluorescence. J. Plant Growth Regul. 2021, 40, 1803–1823. [Google Scholar] [CrossRef]
- Oliveira, V.P.; Lima, M.D.R.; Silva, B.R.S.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids Confer Tolerance to Salt Stress in Eucalyptus Urophylla Plants Enhancing Homeostasis, Antioxidant Metabolism and Leaf Anatomy. J. Plant Growth Regul. 2019, 38, 557–573. [Google Scholar] [CrossRef]
- Pereira, M.P.; de Almeida Rodrigues, L.C.; Corrêa, F.F.; de Castro, E.M.; Ribeiro, V.E.; Pereira, F.J. Cadmium Tolerance in Schinus Molle Trees Is Modulated by Enhanced Leaf Anatomy and Photosynthesis. Trees 2016, 30, 807–814. [Google Scholar] [CrossRef]
- Ribeiro, A.T.; de Oliveira, V.P.; de Oliveira Barros Junior, U.; da Silva, B.R.S.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide Mitigates Nickel Toxicity in Young Eucalyptus Urophylla S.T. Blake Plants: Nutritional, Physiological, Biochemical, Anatomical and Morphological Responses. Ann. For. Sci. 2020, 77. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy Metal-Induced Oxidative Damage, Defense Reactions, and Detoxification Mechanisms in Plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Ahmed, M.; Mir, B.A.; Yusuf, M.; Khan, T.A. 24-Epibrassinolide Mitigates the Adverse Effects of Manganese Induced Toxicity through Improved Antioxidant System and Photosynthetic Attributes in Brassica Juncea. Environ. Sci. Pollut. Res. 2015, 22, 11349–11359. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide Alleviates the Injurious Effects of Cr(VI) Toxicity in Tomato Plants: Insights into Growth, Physio-Biochemical Attributes, Antioxidant Activity and Regulation of Ascorbate–Glutathione and Glyoxalase Cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]



| Pb | EBR | Pb in Root (µg g DM−1) | Pb in Stem (µg g DM−1) | Pb in Leaf (µg g DM−1) |
|---|---|---|---|---|
| − | − | 0.20 ± 0.02 c | 0.00 ± 0.00 d | 0.00 ± 0.00 c |
| − | + | 0.51 ± 0.04 c | 0.07 ± 0.00 c | 0.07 ± 0.01 c |
| + | − | 115.58 ± 8.33 a | 0.91 ± 0.07 a | 1.25 ± 0.10 a |
| + | + | 51.64 ± 3.93 b | 0.32 ± 0.02 b | 0.69 ± 0.04 b |
| Pb | EBR | RET (µm) | RDT (µm) | RCT (µm) | VCD (µm) | RMD (µm) |
|---|---|---|---|---|---|---|
| − | − | 21.1 ± 1.5 a | 18.8 ± 1.3 a | 208 ± 08 a | 155 ± 10 a | 37.7 ± 2.9 a |
| − | + | 20.9 ± 1.4 a | 19.7 ± 1.2 a | 209 ± 10 a | 156 ± 10 a | 39.1 ± 2.2 a |
| + | − | 15.5 ± 1.4 c | 13.3 ± 0.9 c | 166 ± 11 b | 142 ± 11 a | 25.4 ± 1.9 c |
| + | + | 19.1 ± 1.5 b | 16.4 ± 0.9 b | 169 ± 11 b | 148 ± 11 a | 30.4 ± 2.3 b |
| Pb | EBR | ETAd (µm) | ETAb (µm) | PPT (µm) | SPT (µm) | Ratio PPT/SPT |
| − | − | 19.02 ± 0.60 a | 12.82 ± 1.04 a | 67.21 ± 1.21 a | 73.42 ± 1.57 a | 0.91 ± 0.02 a |
| − | + | 19.99 ± 0.78 a | 13.27 ± 1.01 a | 67.37 ± 1.08 a | 73.78 ± 1.39 a | 0.91 ± 0.03 a |
| + | − | 16.01 ± 0.89 c | 8.08 ± 0.58 c | 59.36 ± 1.49 c | 63.86 ± 1.76 c | 0.93 ± 0.04 a |
| + | + | 17.65 ± 0.71 b | 9.80 ± 0.67 b | 64.73 ± 1.17 b | 70.28 ± 1.45 b | 0.92 ± 0.02 a |
| Pb | EBR | Mg (mg g DM−1) | K (mg g DM−1) | Ca (mg g DM−1) | Cu (µg g DM−1) | Zn (µg g DM−1) | Mn (µg g DM−1) |
|---|---|---|---|---|---|---|---|
| Contents in root | |||||||
| − | − | 8.62 ± 0.26 a | 27.72 ± 0.44 b | 3.55 ± 0.15 a | 8.37 ± 0.33 a | 25.90 ± 0.32 b | 315.11 ± 7.82 a |
| − | + | 8.85 ± 0.43 a | 29.99 ± 0.56 a | 3.68 ± 0.19 a | 8.55 ± 0.26 a | 26.65 ± 0.26 a | 314.78 ± 9.27 a |
| + | − | 5.80 ± 0.29 c | 20.09 ± 0.94 d | 2.48 ± 0.15 c | 5.44 ± 0.24 c | 19.89 ± 0.53 d | 272.14 ± 8.21 c |
| + | + | 7.54 ± 0.19 b | 23.86 ± 0.81 c | 3.20 ± 0.11 b | 7.66 ± 0.20 b | 23.04 ± 0.75 c | 290.44 ± 9.54 b |
| Contents in stem | |||||||
| − | − | 5.66 ± 0.21 a | 124.47 ± 0.71 a | 7.42 ± 0.18 a | 4.51 ± 0.18 a | 21.02 ± 0.33 b | 32.00 ± 0.66 a |
| − | + | 5.74 ± 0.25 a | 124.87 ± 0.58 a | 7.68 ± 0.31 a | 4.77 ± 0.18 a | 22.19 ± 0.57 a | 31.70 ± 0.48 a |
| + | − | 4.95 ± 0.14 c | 118.59 ± 0.58 c | 7.05 ± 0.52 a | 2.99 ± 0.20 c | 17.74 ± 0.46 d | 29.48 ± 0.79 b |
| + | + | 5.26 ± 0.15 b | 122.07 ± 0.75 b | 7.29 ± 0.15 a | 4.02 ± 0.16 b | 19.92 ± 0.54 c | 30.69 ± 0.53 b |
| Contents in leaf | |||||||
| − | − | 7.57 ± 0.27 a | 29.36 ± 0.61 a | 17.54 ± 0.30 a | 7.22 ± 0.20 a | 16.51 ± 0.23 b | 76.73 ± 0.99 a |
| − | + | 7.44 ± 0.22 a | 30.13 ± 0.69 a | 17.84 ± 0.52 a | 7.40 ± 0.30 a | 17.30 ± 0.26 a | 75.90 ± 0.99 a |
| + | − | 5.77 ± 0.37 c | 23.76 ± 0.92 c | 14.96 ± 0.77 c | 4.59 ± 0.36 c | 12.87 ± 0.28 d | 64.02 ± 1.07 c |
| + | + | 6.58 ± 0.28 b | 28.19 ± 0.52 b | 16.68 ± 0.53 b | 6.46 ± 0.30 b | 15.13 ± 0.33 c | 73.36 ± 1.11 b |
| Pb | EBR | Chl a (mg g−1 FM) | Chl b (mg g−1 FM) | Total Chl (mg g−1 FM) | Car (mg g−1 FM) | Ratio Chl a/Chl b | Ratio Total Chl/Car |
|---|---|---|---|---|---|---|---|
| − | − | 4.84 ± 0.14 b | 2.05 ± 0.12 b | 6.89 ± 0.17 b | 0.67 ± 0.04 b | 2.37 ± 0.16 b | 10.27 ± 0.80 a |
| − | + | 5.16 ± 0.16 a | 2.38 ± 0.14 a | 7.54 ± 0.12 a | 0.78 ± 0.05 a | 2.18 ± 0.18 b | 9.70 ± 0.67 a |
| + | − | 3.60 ± 0.19 d | 1.29 ± 0.11 d | 4.89 ± 0.22 d | 0.45 ± 0.03 d | 2.79 ± 0.21 a | 10.80 ± 0.84 a |
| + | + | 4.35 ± 0.17 c | 1.61 ± 0.09 c | 5.96 ± 0.21 c | 0.58 ± 0.04 c | 2.70 ± 0.14 a | 10.29 ± 0.91 a |
| Pb | EBR | ΦPSII | qP | NPQ | ETR (µmol m−2 s−1) | EXC (µmol m−2 s−1) | ETR/PN |
| − | − | 0.271 ± 0.006 b | 0.378 ± 0.008 b | 0.72 ± 0.02 c | 39.86 ± 0.87 b | 0.667 ± 0.008 c | 2.75 ± 0.04 c |
| − | + | 0.312 ± 0.005 a | 0.417 ± 0.009 a | 0.57 ± 0.03 d | 45.81 ± 0.75 a | 0.621 ± 0.008 d | 2.73 ± 0.05 c |
| + | − | 0.200 ± 0.003 d | 0.324 ± 0.007 d | 1.06 ± 0.05 a | 29.41 ± 0.47 d | 0.740 ± 0.005 a | 2.98 ± 0.04 a |
| + | + | 0.248 ± 0.006 c | 0.361 ± 0.007 c | 0.82 ± 0.03 b | 36.39 ± 0.93 c | 0.690 ± 0.009 b | 2.86 ± 0.06 b |
| Pb | EBR | PN (µmol m−2 s−1) | E (mmol m−2 s−1) | gs (mol m−2 s−1) | Ci (µmol mol−1) | WUE (µmol mmol−1) | PN/Ci (µmol m−2 s−1 Pa−1) |
| − | − | 14.48 ± 0.43 b | 2.88 ± 0.05 a | 0.306 ± 0.021 a | 275 ± 4 b | 5.03 ± 0.23 b | 0.053 ± 0.002 b |
| − | + | 16.78 ± 0.41 a | 2.94 ± 0.09 a | 0.330 ± 0.022 a | 262 ± 4 c | 5.71 ± 0.18 a | 0.064 ± 0.003 a |
| + | − | 9.87 ± 0.21 d | 2.72 ± 0.07 b | 0.222 ± 0.019 c | 293 ± 6 a | 3.63 ± 0.16 d | 0.034 ± 0.001 d |
| + | + | 12.72 ± 0.19 c | 2.78 ± 0.04 b | 0.262 ± 0.020 b | 280 ± 5 b | 4.58 ± 0.20 c | 0.045 ± 0.002 c |
| Pb | EBR | SD (Stomata Per mm2) | PDS (µm) | EDS (µm) | SF | SI (%) |
|---|---|---|---|---|---|---|
| Adaxial face | ||||||
| − | − | 14.37 ± 0.50 b | 12.09 ± 0.38 b | 21.08 ± 0.58 c | 0.58 ± 0.05 a | 11.96 ± 0.61 a |
| − | + | 16.30 ± 0.98 a | 11.73 ± 0.44 b | 19.86 ± 0.55 d | 0.59 ± 0.04 a | 12.73 ± 0.89 a |
| + | − | 11.72 ± 0.77 d | 13.46 ± 0.31 a | 24.16 ± 0.81 a | 0.56 ± 0.04 a | 9.39 ± 0.53 c |
| + | + | 13.27 ± 0.59 c | 12.86 ± 0.34 a | 22.50 ± 0.74 b | 0.57 ± 0.04 a | 10.60 ± 0.64 b |
| Abaxial face | ||||||
| − | − | 32.21 ± 1.17 b | 10.43 ± 0.35 b | 19.75 ± 0.52 c | 0.53 ± 0.04 a | 21.71 ± 0.98 b |
| − | + | 36.09 ± 1.26 a | 10.14 ± 0.30 b | 18.65 ± 0.49 d | 0.54 ± 0.04 a | 25.34 ± 1.07 a |
| + | − | 19.37 ± 1.02 d | 11.51 ± 0.51 a | 22.98 ± 0.61 a | 0.50 ± 0.03 a | 17.28 ± 0.62 d |
| + | + | 22.94 ± 1.05 c | 11.25 ± 0.43 a | 21.57 ± 0.74 b | 0.52 ± 0.03 a | 18.80 ± 0.70 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Bajguz, A.; Lobato, A.K.d.S. 24-Epibrassinolide Simultaneously Stimulates Photosynthetic Machinery and Biomass Accumulation in Tomato Plants under Lead Stress: Essential Contributions Connected to the Antioxidant System and Anatomical Structures. Agronomy 2022, 12, 1985. https://doi.org/10.3390/agronomy12091985
Maia CF, da Silva BRS, Batista BL, Bajguz A, Lobato AKdS. 24-Epibrassinolide Simultaneously Stimulates Photosynthetic Machinery and Biomass Accumulation in Tomato Plants under Lead Stress: Essential Contributions Connected to the Antioxidant System and Anatomical Structures. Agronomy. 2022; 12(9):1985. https://doi.org/10.3390/agronomy12091985
Chicago/Turabian StyleMaia, Camille Ferreira, Breno Ricardo Serrão da Silva, Bruno Lemos Batista, Andrzej Bajguz, and Allan Klynger da Silva Lobato. 2022. "24-Epibrassinolide Simultaneously Stimulates Photosynthetic Machinery and Biomass Accumulation in Tomato Plants under Lead Stress: Essential Contributions Connected to the Antioxidant System and Anatomical Structures" Agronomy 12, no. 9: 1985. https://doi.org/10.3390/agronomy12091985






