A Study of Application and Comparison of Thermal Drying and Freeze Drying of Fresh Edamame Seeds in the Analysis of Seed Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
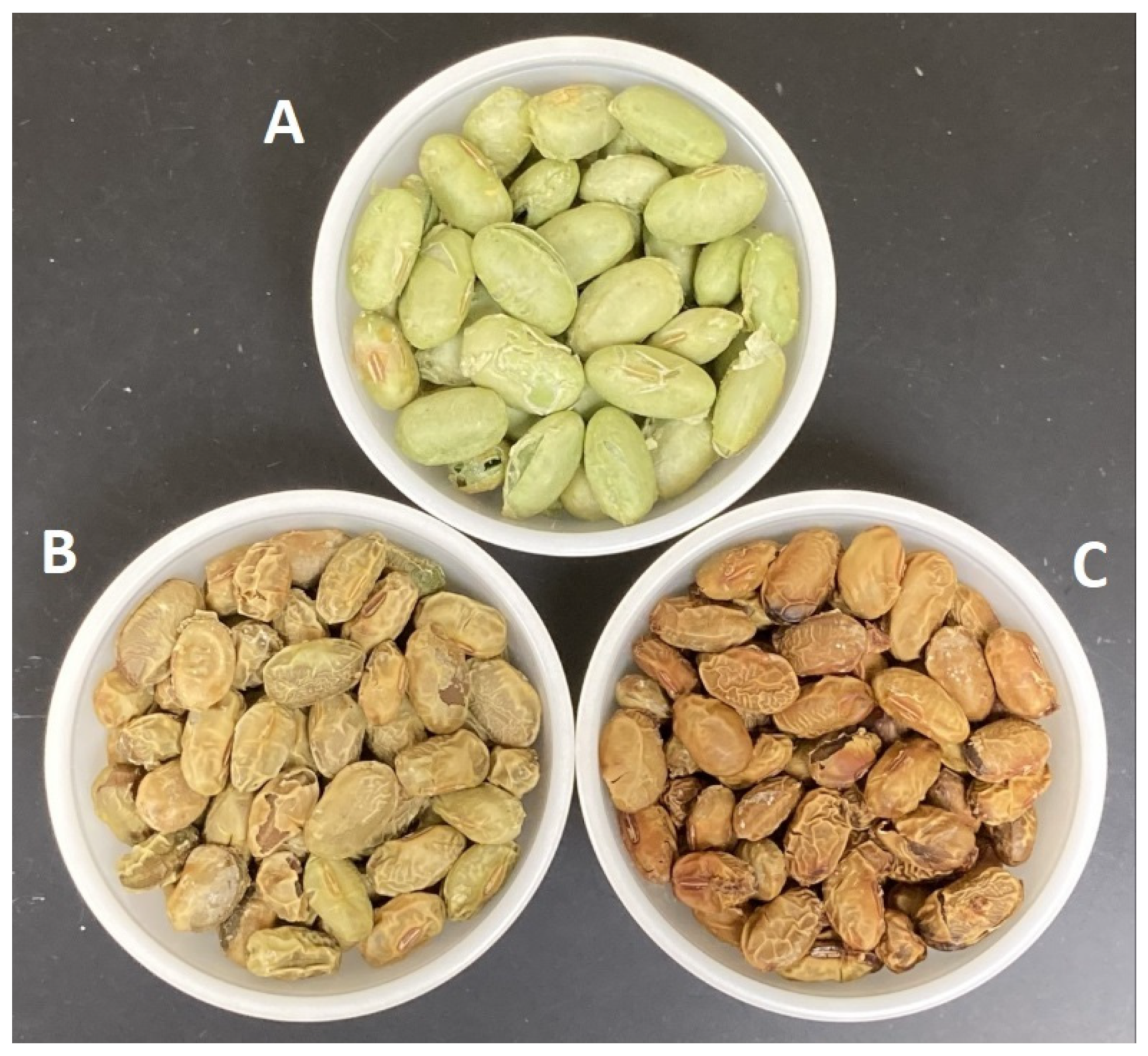
2.2. Edamame Drying
2.3. Seed Composition Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fresh Seed Moisture and Seed Weight
3.2. Comparison of Sample Types
3.3. Comparison of Edamame Drying Methods
3.4. Repeatability and Correlations
3.5. Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.). Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. History of Edamame, Green Vegetable Soybeans, and Vegetable-Type Soybeans (1275–2009): Extensively Annotated Bibliography and Sourcebook; SoyInfo Center: Lafayette, CA, USA, 2009; Available online: https://www.soyinfocenter.com (accessed on 21 March 2022).
- Shanmugasundaram, S.; Yan, M.R. Global expansion of high value vegetable soybean. In Proceedings of the 7th World Soybean Research Conference, Foz do Iguassur, Brazil, 29 February–5 March 2004; pp. 915–920. [Google Scholar]
- Young, D.; Mebrathu, T.; Johnson, J. Acceptability of green soybeans as a vegetable entity. Plant Foods Hum. Nutr. 2000, 55, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.-L.; Rutto, L.K.; Ren, S. Evaluation of soybean lines for edamame yield traits and trait genetic correlation. HortScience 2018, 53, 1732–1736. [Google Scholar] [CrossRef]
- Seid, S. Celebrity Beans Star as New Crop for Emerging Farmers. Sunday Times, 27 April 2014. Available online: https://www.timeslive.co.za/sunday-times/lifestyle/2014-04-27-celebrity-beans-star-as-new-crop-for-emerging-farmers/ (accessed on 11 March 2022).
- Djanta, M.K.A.; Agoyi, E.E.; Agbahougba, S.; Quenum, F.J.-B.; Chadare, F.J.; Assogbadjo, A.E.; Agbangla, C.; Sinsin, B. Vegetable soybean, edamame: Research, production, utilization and analysis of its adoption in Sub-Saharan Africa. J. Hortic. For. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439–450. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Chin, K.L.; Qi, Y. Vegetable soybean: Seed composition and production research. Ital. J. Agron. 2017, 12, 872. [Google Scholar] [CrossRef]
- Binder, K. Edible Soybean Rises in Popularity with U.S. Consumers and Producers. Farm World Newspaper, MidCountry Media, Knightstown, IN, USA, 28 July 2010. Available online: https://www.farmworldonline.com/news/NewsArticle.asp?newsid=10620 (accessed on 10 March 2022).
- Rao, M.S.S.; Bhagsari, A.S.; Mohamed, A.I. Fresh green seed yield and seed nutritional traits of vegetable soybean genotypes. Crop Sci. 2002, 42, 1950–1958. [Google Scholar] [CrossRef]
- Mebrahtu, T.; Devine, T.E. Diallel analysis of sugar composition of 10 vegetable soybean lines. Plant Breed. 2009, 128, 249–252. [Google Scholar] [CrossRef]
- Ogles, C.Z.; Guertal, E.A.; Weaver, D.B. Edamame cultivar evaluation in Central Alabama. Agron. J. 2016, 108, 2371–2378. [Google Scholar] [CrossRef]
- Williams, M.M., II. Phenomorphological characterization of vegetable soybean germplasm lines for commercial production. Crop Sci. 2015, 55, 1274–1279. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Li, D.; Gu, Z. Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max [L.] Merr). Ind. Crops Prod. 2013, 50, 743–749. [Google Scholar] [CrossRef]
- Zandonadi, R.; Stombaugh, T.; Coolong, T.; Pfeiffer, T. Mechanical Harvesting of Edamame. 2010. Available online: https://www.uky.edu/ccd/sites/www.uky.edu.ccd/files/edamame_mechanical_harvest.pdf (accessed on 10 March 2022).
- Font, R.; del Rio-Celestino, M.; de Haro-Bailon, A. The use of near-infrared spectroscopy (NIRS) in the study of seed quality components in plant breeding programs. Ind. Crops Prod. 2006, 24, 307–313. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Katuuramu, D.N.; Xu, Y.; Ren, S.; Rutto, L.K. Analysis and comparison of seed protein, oil, and sugars in edamame dried using two oven-drying methods and mature soybeans. J. Sci. Food Agric. 2020, 100, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists (AACC). Approved Methods of the AACC, Methods 46–30; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Karn, A.; Heim, C.; Flint-Garcia, S.; Bilyeu, K. Development of rigorous fatty acid near-infrared spectroscopy quantitation methods in support of soybean oil improvement. J. Am. Oil Chem. Soc. 2017, 94, 69–76. [Google Scholar] [CrossRef]
- Singh, S.; Patel, S.; Litoria, N.; Gandhi, K.; Faldu, P.; Patel, K.G. Comparative efficiency of conventional and NIR based technique for proximate composition of pigeon pea, soybean and rice cultivars. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 773–782. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, S.; Wu, X.; Xing, C.; Yuan, J. Determination of soybean routine quality parameters using near-infrared spectroscopy. Food Sci. Nutr. 2018, 6, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.G.; Oak, M.D.; Taware, S.P.; Tamhankar, S.A.; Rao, V.S. Nondestructive estimation of fatty acid composition in soybean [Glycine max (L.) Merrill] seeds using near-infrared transmittance spectroscopy. Food Chem. 2010, 120, 1210–1217. [Google Scholar] [CrossRef]
- Pazdernik, D.; Killam, A.S.; Orf, J.H. Analysis of amino and fatty acid composition in soybean seed, using near infrared reflectance spectroscopy. Agron. J. 1997, 89, 679–685. [Google Scholar] [CrossRef]
- Lee, H.; Cho, B.-K.; Kim, M.S.; Lee, W.-H.; Tewari, J.; Bae, H.; Sohn, S.-I.; Chi, H.-Y. Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sens. Actuators B Chem. 2013, 185, 694–700. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; de Zangirolami, M.S.; Silva, N.O.; Valderrama, P.; Marco, P.H. Rapid non-invasive assessment of quality parameters in ground soybean using near-infrared spectroscopy. Pesq. Agropec. Bras. 2018, 53, 97–104. [Google Scholar] [CrossRef]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, G.-L.; Green, M.; Scott, R.A.; Song, Q.; Hyten, D.L.; Cregan, P.B. Identification and validation of quantitative trait loci for seed yield, oil and protein contents in two recombinant inbred populations of soybean. Mol. Genet. Genom. 2014, 289, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Lu, Y.; Bhusal, S.; Song, Q.; Cregan, P.B.; Yen, Y.; Brown, M.; Jiang, G.-L. Genome-wide scan for seed composition provides insights into soybean quality improvement and the impacts of domestication and breeding. Mol. Plant 2018, 11, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Salmani, Z.; Vijayalakshmi, D.; Sajjan, J.T. Screening of selected vegetable soybean genotypes for nutrient and antinutrient factors. J. Dairy. Foods Home Sci. 2012, 31, 142–145. [Google Scholar]
- Wang, Z.Q.; Senga, E.F.B.; Wang, D.Y. Vegetable soybean (Glycine max (L.) Merrill) from production to processing. Outlook Agri. 2005, 34, 167–172. [Google Scholar] [CrossRef]
- Hu, Q.-G.; Zhang, M.; Mujumdar, A.S.; Du, W.-H.; Sun, J.-C. Effects of different drying methods on the quality changes of granular edamame. Dry. Technol. 2006, 24, 1025–1032. [Google Scholar]
- Jiang, G.-L. Comparison and application of non-destructive NIR evaluations of seed protein and oil content in soybean breeding. Agronomy 2020, 10, 77. [Google Scholar] [CrossRef]
- Sagar, V.R.; Suresh Kumar, P.S. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef]
- Ozkan-Karabacak, A.; Ozean-Sinir, G.; Copur, O.U. Effects of drying methods on the composition of volatile compounds in fruits and vegetables. In Flavour Science: Proceedings of the XV Weurman Flavour Research Symposium, Universitat Graz, Graz, Austria, 18–22 September 2017; Siegmund, B., Leitner, E., Eds.; Verlag der Technischen Universitat Graz: Graz, Austria, 2018; pp. 95–98. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Zepp, M.; Hirneisen, A.; LaBorde, L. Let’s Preserve: Drying Fruits and Vegetables (dehydration). PennState Extension. 2019. Available online: https://extension.psu.edu/lets-preserve-drying-fruits-and-vegetables-dehydration (accessed on 10 March 2022).
- Dehydrator Blog. Dehydrating Time & Temperature Guide: Fruits, Vegetables, Meat, Herbs, Spices & Leather. 2018. Available online: https://dehydratorblog.com/food-dehydrating-time-temperature-guide/ (accessed on 21 March 2022).
- Schmutz, P.; Hoyle, E.H. Drying Foods. Clemson Cooperative Extension. 1999. Available online: https://hgic.clemson.edu/factsheet/drying-foods/ (accessed on 10 April 2022).
- Mercer, D.G. A Basic Guide to Drying Fruits and Vegetables; University of Guelph: Guelph, ON, Canada, 2012; Available online: http://iufost.org/iufostftp/Guide%20to%20Drying-Full.pdf (accessed on 6 August 2022).
- Milligan, L. How to Make Quick and Easy Roasted Edamame. Ideas for the Home. 2018. Available online: https://www.kenarry.com/make-quick-easy-roasted-edamame/ (accessed on 19 August 2022).
- Scheffé, H. The Analysis of Variance; Wiley: New York, NY, USA, 1959. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Chen, P.; Zhang, J.; Florez-Palacios, L.; Zeng, A.; Wang, X.; Bowen, R.A.; Miller, A.; Berry, H. Genetic analysis of sugar composition and its relationship with protein, oil, and fiber in soybean. Crop Sci. 2018, 58, 2413–2421. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. Nutr. 2017, 57, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.A.; Andress, L.E. Preserving Food: Drying Fruits and Vegetables; University of Georgia Cooperative Extension Service: Athens, GA, USA, 2000; Available online: https://nchfps.uga.edu/publications/uga_dry_fruit.pdf (accessed on 21 March 2022).
- Orak, H.H.; Aktas, T.; Yagar, H.; Selen isbilir, S.; Ekinci, N. Effects of hot air and freeze drying methods on antioxidant activity, color and some nutritional characteristics of strawberry tree (Arbutus unedo L.) fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Sablani, S.S. Drying of fruits and vegetables: Retention of nutritional/functional quality. Dry. Technol. 2006, 24, 123–135. [Google Scholar] [CrossRef]
- HWC Magazine. Crunchy Roasted Edamame. Healthy World Cuisine. 2014. Available online: https://www.hwcmagazine.com/recipe/crunchy-roasted-edamame/ (accessed on 19 August 2022).
- Rasmussen, L. Air Fryer Edamame. My Quiet Kitchen. 2021. Available online: https://myquietkitchen.com/air-fryer-edamame/ (accessed on 19 August 2022).
| Genotype | Fresh Moisture (%) | Fresh 100-Seed Wt (g) | Dried 100-Seed Wt (g) | Protein | Oil | Fiber | Ash | Sucrose | Stachyose |
|---|---|---|---|---|---|---|---|---|---|
| Asmara | 66.0 ± 0.9 | 48.3 ± 1.3 | 16.4 ± 0.9 | 440.8 ± 13.2 | 207.3 ± 14.1 | 46.9 ± 2.4 | 58.7 ± 0.9 | 61.2 ± 7.5 | 41.1 ± 4.5 |
| Moon Cake | 67.9 ± 4.3 | 38.2 ± 5.2 | 12.3 ± 2.4 | 434.4 ± 13.4 | 205.4 ± 16.9 | 48.8 ± 1.0 | 59.9 ± 1.1 | 62.8 ± 8.5 | 44.4 ± 4.0 |
| N6202-8 | 63.8 ± 2.5 | 46.7 ± 1.7 | 16.9 ± 1.8 | 467.9 ± 21.2 | 200.7 ± 13.6 | 44.0 ± 2.3 | 57.9 ± 0.7 | 52.9 ± 2.2 | 39.3 ± 5.9 |
| NC 346 | 62.9 ± 0.4 | 44.3 ± 5.6 | 16.4 ± 1.9 | 442.5 ± 17.5 | 216.8 ± 13.5 | 46.5 ± 3.6 | 57.9 ± 1.1 | 50.8 ± 17.1 | 52.3 ± 13.9 |
| NC Green | 64.4 ± 2.1 | 61.7 ± 3.8 | 21.9 ± 1.2 | 454.8 ± 12.8 | 207.9 ± 8.3 | 48.6 ± 2.2 | 58.8 ± 2.7 | 45.8 ± 14.5 | 49.1 ± 9.4 |
| NC Raleigh | 64.8 ± 3.4 | 27.4 ± 2.8 | 9.6 ± 0.2 | 393.9 ± 19.7 | 231.9 ± 22.7 | 52.0 ± 1.6 | 57.6 ± 1.8 | 66.5 ± 7.9 | 43.3 ± 7.8 |
| Randolph | 66.5 ± 2.7 | 35.7 ± 6.7 | 12.0 ± 3.0 | 446.9 ± 16.0 | 194.7 ± 16.7 | 48.1 ± 4.2 | 59.6 ± 0.8 | 61.1 ± 14.8 | 48.3 ± 8.6 |
| VS11-0022 | 67.8 ± 1.7 | 62.7 ± 3.1 | 20.1 ± 1.1 | 451.2 ± 10.6 | 203.2 ± 12.5 | 45.1 ± 3.9 | 61.0 ± 0.6 | 49.1 ± 11.5 | 42.4 ± 6.3 |
| VS11-0112 | 66.5 ± 3.1 | 44.6 ± 3.0 | 15.0 ± 1.9 | 434.1 ± 12.9 | 205.4 ± 18.0 | 47.9 ± 1.7 | 59.4 ± 1.5 | 65.7 ± 8.3 | 39.7 ± 3.3 |
| VS11-0137 | 67.2 ± 2.4 | 44.1 ± 2.3 | 14.5 ± 1.8 | 430.8 ± 14.0 | 199.2 ± 18.2 | 45.8 ± 2.2 | 59.3 ± 1.7 | 66.2 ± 12.1 | 39.0 ± 4.8 |
| VS12-0021 | 68.6 ± 2.3 | 76.9 ± 6.7 | 24.2 ± 3.4 | 437.8 ± 11.3 | 205.7 ± 13.9 | 46.5 ± 1.9 | 59.3 ± 1.0 | 63.4 ± 7.5 | 38.7 ± 7.2 |
| VS12-0161 | 68.2 ± 2.0 | 44.1 ± 2.4 | 14.0 ± 1.3 | 439.4 ± 21.7 | 188.4 ± 15.5 | 50.4 ± 1.4 | 60.0 ± 1.5 | 67.5 ± 8.5 | 44.8 ± 3.5 |
| VS15-4007 | 67.6 ± 3.0 | 50.7 ± 4.4 | 16.4 ± 1.1 | 442.2 ± 6.9 | 209.2 ± 9.8 | 48.7 ± 2.9 | 59.3 ± 1.7 | 59.6 ± 18.1 | 41.0 ± 4.7 |
| VS15-4049 | 63.8 ± 3.3 | 26.6 ± 1.8 | 9.7 ± 1.5 | 431.8 ± 15.4 | 212.3 ± 15.4 | 51.2 ± 2.7 | 57.5 ± 1.8 | 60.4 ± 7.9 | 41.9 ± 2.9 |
| VS15-5148 | 64.9 ± 3.3 | 62.0 ± 6.1 | 21.7 ± 1.5 | 453.6 ± 8.2 | 204.5 ± 14.1 | 44.9 ± 2.0 | 59.5 ± 1.3 | 53.5 ± 8.2 | 41.8 ± 7.3 |
| VS15-6005 | 66.4 ± 1.9 | 33.0 ± 3.9 | 11.0 ± 1.0 | 428.1 ± 16.8 | 207.7 ± 6.4 | 50.1 ± 2.6 | 58.8 ± 0.9 | 63.6 ± 8.7 | 42.8 ± 10.8 |
| VS15-6077 | 65.4 ± 1.4 | 42.0 ± 2.5 | 14.5 ± 1.1 | 439.7 ± 15.4 | 199.8 ± 17.5 | 48.0 ± 2.4 | 58.7 ± 1.1 | 61.0 ± 14.3 | 41.8 ± 5.4 |
| VS15-4018 | 64.0 ± 0.2 | 49.5 ± 0.8 | 17.8 ± 0.2 | 462.5 ± 16.1 | 190.8 ± 15.2 | 47.6 ± 1.9 | 59.6 ± 1.1 | 35.4 ± 6.5 | 54.3 ± 15.9 |
| VS15-6021 | 66.9 ± 0.5 | 61.6 ± 1.9 | 20.4 ± 0.8 | 448.1 ± 2.5 | 198.2 ± 14.2 | 45.3 ± 4.0 | 59.7 ± 1.3 | 52.1 ± 9.8 | 44.9 ± 8.3 |
| VS15-6023 | 66.4 ± 1.1 | 50.0 ± 1.3 | 16.8 ± 0.2 | 432.7 ± 13.5 | 198.7 ± 11.4 | 48.0 ± 2.9 | 59.4 ± 1.1 | 61.2 ± 6.0 | 49.2 ± 5.2 |
| Genotype | Raffinose | Total Sugar | Palmitic acid | Stearic acid | Oleic acid | Linoleic acid | Linolenic acid | ADF | NDF |
| Asmara | 15.0 ± 1.4 | 117.3 ± 5.5 | 11.7 ± 0.5 | 5.2 ± 0.6 | 29.0 ± 1.5 | 40.5 ± 4.3 | 7.4 ± 2.6 | 15.1 ± 2.4 | 15.5 ± 0.8 |
| Moon Cake | 14.8 ± 1.5 | 122.0 ± 12.9 | 11.6 ± 1.1 | 5.0 ± 0.4 | 27.2 ± 2.6 | 43.6 ± 4.5 | 7.7 ± 2.0 | 14.9 ± 2.0 | 15.9 ± 0.9 |
| N6202-8 | 13.5 ± 1.9 | 105.7 ± 6.3 | 11.2 ± 0.7 | 5.0 ± 0.6 | 29.7 ± 1.8 | 42.3 ± 5.0 | 7.3 ± 3.7 | 14.8 ± 2.0 | 15.3 ± 0.7 |
| NC 346 | 12.8 ± 3.6 | 115.9 ± 17.6 | 11.5 ± 1.0 | 4.5 ± 0.6 | 27.1 ± 2.4 | 43.7 ± 4.6 | 9.2 ± 4.4 | 15.2 ± 1.8 | 15.8 ± 1.0 |
| NC Green | 12.6 ± 3.3 | 107.5 ± 9.6 | 11.3 ± 0.9 | 4.9 ± 0.8 | 27.6 ± 1.7 | 42.9 ± 5.4 | 8.8 ± 3.7 | 15.8 ± 2.4 | 15.7 ± 1.0 |
| NC Raleigh | 15.3 ± 1.1 | 125.1 ± 13.1 | 11.6 ± 0.6 | 4.8 ± 0.4 | 26.8 ± 3.2 | 45.5 ± 3.8 | 6.8 ± 1.9 | 15.2 ± 0.8 | 16.8 ± 0.6 |
| Randolph | 14.5 ± 2.3 | 123.9 ± 18.8 | 11.8 ± 0.6 | 4.9 ± 0.4 | 29.2 ± 2.0 | 40.7 ± 5.4 | 8.6 ± 4.2 | 14.2 ± 1.5 | 15.4 ± 1.1 |
| VS11-0022 | 15.0 ± 1.7 | 106.5 ± 10.8 | 11.3 ± 0.8 | 5.4 ± 0.3 | 33.4 ± 2.6 | 37.3 ± 4.0 | 6.9 ± 2.5 | 14.7 ± 1.3 | 15.9 ± 1.0 |
| VS11-0112 | 15.4 ± 1.7 | 120.8 ± 10.7 | 11.4 ± 0.6 | 5.0 ± 0.4 | 30.2 ± 3.9 | 40.3 ± 8.2 | 7.6 ± 3.0 | 13.8 ± 1.1 | 15.5 ± 1.0 |
| VS11-0137 | 15.5 ± 1.6 | 120.7 ± 13.2 | 11.5 ± 0.6 | 5.0 ± 0.4 | 29.8 ± 1.9 | 41.5 ± 3.9 | 7.2 ± 2.4 | 14.1 ± 1.4 | 15.5 ± 1.0 |
| VS12-0021 | 14.8 ± 0.5 | 116.8 ± 13.2 | 11.1 ± 0.5 | 5.6 ± 0.6 | 28.7 ± 2.1 | 41.0 ± 5.3 | 8.5 ± 3.2 | 15.1 ± 1.0 | 16.4 ± 0.9 |
| VS12-0161 | 15.5 ± 1.6 | 127.8 ± 11.1 | 11.6 ± 0.5 | 5.2 ± 0.6 | 28.4 ± 3.6 | 40.8 ± 5.4 | 8.1 ± 3.9 | 14.1 ± 0.8 | 15.3 ± 0.5 |
| VS15-4007 | 15.7 ± 2.1 | 116.3 ± 22.9 | 11.8 ± 0.9 | 5.1 ± 0.6 | 27.0 ± 3.1 | 42.5 ± 5.7 | 7.9 ± 3.0 | 14.6 ± 1.7 | 15.8 ± 1.1 |
| VS15-4049 | 14.9 ± 0.6 | 117.2 ± 10.2 | 11.5 ± 0.3 | 4.9 ± 0.4 | 23.6 ± 2.2 | 48.2 ± 3.5 | 7.7 ± 2.4 | 14.8 ± 1.0 | 16.1 ± 0.7 |
| VS15-5148 | 13.9 ± 2.1 | 109.2 ± 8.0 | 11.1 ± 0.6 | 5.0 ± 0.5 | 33.3 ± 2.9 | 36.7 ± 7.2 | 8.2 ± 3.9 | 14.3 ± 1.7 | 15.4 ± 0.6 |
| VS15-6005 | 14.8 ± 1.2 | 121.3 ± 9.3 | 11.7 ± 0.8 | 4.8 ± 0.4 | 26.5 ± 2.0 | 43.4 ± 4.6 | 8.7 ± 2.9 | 14.7 ± 0.8 | 16.1 ± 0.3 |
| VS15-6077 | 14.1 ± 2.4 | 116.8 ± 15.5 | 11.3 ± 0.6 | 4.9 ± 0.5 | 29.4 ± 1.9 | 42.5 ± 3.0 | 7.3 ± 3.3 | 14.6 ± 1.7 | 15.7 ± 1.3 |
| VS15-4018 | 11.3 ± 2.1 | 101.0 ± 8.1 | 11.2 ± 0.3 | 4.8 ± 0.7 | 30.5 ± 0.6 | 38.4 ± 4.6 | 10.8 ± 5.2 | 15.7 ± 1.4 | 16.0 ± 0.4 |
| VS15-6021 | 13.6 ± 0.8 | 110.6 ± 16.1 | 11.2 ± 0.2 | 4.9 ± 0.7 | 35.2 ± 1.9 | 35.1 ± 1.7 | 8.4 ± 3.6 | 13.7 ± 0.9 | 15.1 ± 0.4 |
| VS15-6023 | 13.8 ± 1.7 | 124.1 ± 9.2 | 11.6 ± 0.2 | 4.9 ± 0.8 | 27.9 ± 3.4 | 40.8 ± 1.0 | 9.6 ± 3.6 | 14.6 ± 1.1 | 15.5 ± 0.3 |
| Trait | Freeze Drying | Low-Heat Drying | High-Heat Drying | |||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| Fresh 100-seed weight (g) | 46.5 ± 13.2 | 24.4–87.5 | 47.3 ± 13.1 | 24.2–84.3 | 45.4 ± 13.1 | 23.7–85.2 |
| Fresh moisture (%) | 65.7 ± 2.9 | 59.6–71.2 | 66.0 ± 2.7 | 60.3–71.7 | 66.2 ± 2.8 | 58.3–72.0 |
| Dried 100-seed weight (g) | 15.9 ± 4.4 | 8.3–31.6 | 16.0 ± 4.3 | 8.2–29.2 | 15.8 ± 4.5 | 7.8–32.8 |
| Dried moisture (%) | 10.3 ± 0.5 | 8.4–11.6 | 10.6 ± 0.8 | 9.1–12.4 | 9.9 ± 0.9 | 8.1–11.9 |
| Trait a | Mean | Difference | Repeatability (%) | |||
|---|---|---|---|---|---|---|
| Ground Samples | Whole Seed Samples | Value | % of Ground Samples | Ground Samples | Whole Samples | |
| Protein | 440.0 ± 19.6 | 441.8 ± 19.5 | 1.8 * | 0.41 | 98.90 | 98.35 |
| Oil | 201.8 ± 16.5 | 199.8 ± 16.6 | −2.0 * | −1.00 | 97.45 | 96.45 |
| Fiber | 49.3 ± 4.1 | 45.9 ± 6.5 | −3.4 * | −6.85 | 91.79 | 89.77 |
| Ash | 58.3 ± 2.4 | 58.9 ± 8.3 | 0.6 NS | 1.04 | 77.99 | 84.35 |
| Sucrose | 53.8 ± 16.3 | 53.6 ± 16.3 | −0.1 NS | −0.26 | 87.79 | 83.30 |
| Stachyose | 45.9 ± 8.9 | 54.9 ± 10.8 | 9.0 * | 19.58 | 91.26 | 84.79 |
| Raffinose | 14.6 ± 2.2 | 15.7 ± 2.0 | 1.1 * | 7.36 | 91.95 | 47.60 |
| Total Sugar | 114.3 ± 15.4 | 124.2 ± 15.9 | 9.9 * | 8.69 | 83.68 | 68.39 |
| Palmitic acid | 11.3 ± 1.1 | 11.6 ± 0.8 | 0.3 * | 3.08 | 63.28 | 67.60 |
| Stearic acid | 4.9 ± 0.6 | 4.9 ± 0.4 | 0.0 NS | 0.28 | 58.52 | 84.26 |
| Oleic acid | 29.5 ± 6.3 | 29.3 ± 6.3 | −0.2 NS | −0.56 | 82.98 | 95.67 |
| Linoleic acid | 42.2 ± 8.0 | 41.7 ± 8.2 | −0.5 NS | −1.16 | 79.41 | 94.06 |
| Linolenic acid | 8.1 ± 3.5 | 7.9 ± 2.5 | −0.2 NS | −1.97 | 55.76 | 84.32 |
| ADF | 14.4 ± 1.8 | 15.0 ± 1.9 | 0.6 * | 4.18 | 79.47 | 73.45 |
| NDF | 15.6 ± 1.3 | 15.9 ± 1.9 | 0.3 * | 1.96 | 86.06 | 83.73 |
| Trait a | Ground Samples | Whole Seed Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| MSY b | MSM | MSG | MSMG | MSY | MSM | MSG | MSMG | |
| Protein | 10435.00 ** | 44.60 | 2315.83 ** | 25.45 | 5183.18 ** | 1176.93 ** | 2715.85 ** | 44.82 |
| Oil | 9724.18 ** | 1185.00 ** | 940.31 ** | 24.00 | 536.27 ** | 14232.00 ** | 998.40 ** | 35.45 |
| Fiber | 359.91 ** | 168.68 ** | 46.58 ** | 3.82 | 1430.33 ** | 584.76 ** | 65.86 ** | 6.74 |
| Ash | 5.15 | 185.44 ** | 5.74 | 1.26 | 4712.69 ** | 441.16 ** | 7.61 ** | 1.19 |
| Sucrose | 1236.46 ** | 7408.47 ** | 486.24 ** | 59.38 | 835.36 ** | 19797.00 ** | 294.82 ** | 49.23 |
| Stachyose | 517.80 ** | 327.68 ** | 222.93 ** | 19.48 | 5409.63 ** | 2020.87 ** | 71.65 ** | 10.90 |
| Raffinose | 190.92 ** | 3.63 | 8.15 ** | 0.66 | 9.85 ** | 73.30 ** | 4.99 | 2.61 |
| Total Sugar | 674.13 * | 5440.94 ** | 328.61 * | 53.62 | 2653.27 ** | 12203.00 ** | 283.97 ** | 89.78 |
| Palmitic acid | 34.93 ** | 33.70 ** | 0.53 | 0.20 | 20.41 ** | 21.26 ** | 0.48 ** | 0.16 |
| Stearic acid | 16.14 ** | 5.63 ** | 0.23 | 0.09 | 1.97 ** | 1.38 ** | 0.31 ** | 0.05 |
| Oleic acid | 901.29 ** | 62.88 * | 115.23 ** | 19.61 | 958.69 ** | 759.32 ** | 108.74 ** | 4.71 |
| Linoleic acid | 2398.41 ** | 31.94 | 114.25 ** | 23.52 | 2677.52 ** | 1244.39 ** | 111.32 ** | 6.62 |
| Linolenic acid | 771.53 ** | 24.16 ** | 3.52 * | 1.56 | 345.17 ** | 29.95 ** | 4.78 ** | 0.75 |
| ADF | 118.05 ** | 32.58 ** | 3.51 ** | 0.72 | 32.22 ** | 253.38 ** | 2.68 ** | 0.71 |
| NDF | 12.38 ** | 54.89 ** | 2.48 ** | 0.35 | 3.94 ** | 289.54 ** | 3.75 ** | 0.61 |
| Trait a | Freeze Drying | Low-Heat Drying | High-Heat Drying |
|---|---|---|---|
| Protein | 0.960 ** | 0.972 ** | 0.953 ** |
| Oil | 0.968 ** | 0.945 ** | 0.886 ** |
| Fiber | 0.776 ** | 0.758 ** | 0.817 ** |
| Ash | 0.598 ** | 0.675 ** | −0.368 |
| Sucrose | 0.924 ** | 0.753 ** | 0.873 ** |
| Stachyose | 0.445 * | 0.555 * | 0.558 * |
| Raffinose | 0.523 * | 0.624 ** | 0.359 |
| Total Sugar | 0.668 ** | 0.672 ** | 0.416 |
| Palmitic acid | 0.582 ** | 0.795 ** | 0.545 * |
| Stearic acid | 0.820 ** | 0.826 ** | 0.030 |
| Oleic acid | 0.841 ** | 0.961 ** | 0.819 ** |
| Linoleic acid | 0.873 ** | 0.957 ** | 0.752 ** |
| Linolenic acid | 0.789 ** | 0.562 ** | −0.136 |
| ADF | 0.557 * | 0.554 * | 0.609 ** |
| NDF | 0.761 ** | 0.668 ** | 0.699 ** |
| Trait c | Freeze Drying | Low-Heat Drying | High-Heat Drying | Relative Difference (%) d | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Low-Heat | High-Heat | |
| Protein | 439.0 ± 18.9 | 380.0–475.1 | 440.4 ± 19.8 | 373.4–485.0 | 440.6 ± 20.4 | 369.7–491.2 | 0.31 | 0.35 |
| Oil | 197.3 ± 19.0 | 160.5–250.7 | 204.7 ± 15.9 | 173.4–256.4 | 203.5 ± 13.3 | 181.2–259.3 | 3.78 a | 3.16 a |
| Fiber | 49.3 ± 4.9 | 37.3–60.3 | 47.8 ± 3.1 | 40.5–54.7 | 50.8 ± 3.6 | 41.2–58.3 | 3.18 b | 2.98 a |
| Ash | 56.5 ± 1.6 | 51.8–59.8 | 59.1 ± 1.5 | 55.1–62.2 | 59.4 ± 2.8 | 54.8–68.2 | 4.54 a | 5.04 a |
| Sucrose | 60.6 ± 14.1 | 33.7–81.5 | 58.3 ± 12.2 | 29.3–84.0 | 42.5 ± 18.9 | 8.0–83.2 | 3.68 | 29.84 a |
| Stachyose | 46.2 ± 9.1 | 26.8–65.9 | 43.8 ± 7.8 | 32.1–64.9 | 47.8 ± 9.4 | 33.4–71.5 | 5.13 | 3.64 |
| Raffinose | 14.8 ± 2.5 | 9.7–19.8 | 14.4 ± 2.0 | 9.0–18.0 | 14.5 ± 2.0 | 9.8–19.0 | 2.81 | 2.17 |
| Total Sugar | 121.5 ± 9.8 | 102.7–142.8 | 116.5 ± 13.2 | 91.9–149.5 | 104.8 ± 17.2 | 76.4–143.8 | 4.13 a | 13.75 b |
| Palmitic acid | 11.8 ± 0.5 | 10.4–13.0 | 11.5 ± 0.6 | 10.3–13.1 | 10.5 ± 1.5 | 7.9–13.0 | 3.25 a | 10.95 b |
| Stearic acid | 5.2 ± 0.6 | 4.0–6.0 | 5.0 ± 0.5 | 4.0–6.1 | 4.6 ± 0.7 | 3.3–5.9 | 3.48 a | 10.47 b |
| Oleic acid | 29.0 ± 3.6 | 19.9–37.0 | 28.9 ± 3.4 | 21.3–37.2 | 30.4 ± 9.8 | 13.1–49.4 | 0.19 | 4.95 a |
| Linoleic acid | 42.7 ± 5.3 | 32.4–53.6 | 41.5 ± 5.1 | 28.9–53.3 | 42.3 ± 11.7 | 19.4–63.1 | 2.86 | 1.04 |
| Linolenic acid | 7.6 ± 3.1 | 2.9–12.9 | 8.1 ± 3.0 | 3.3–13.9 | 8.6 ± 4.2 | 2.2–16.2 | 6.95 a | 14.35 b |
| ADF | 13.7 ± 1.4 | 10.0–15.9 | 14.7 ± 1.4 | 12.1–18.0 | 14.9 ± 2.3 | 9.6–18.1 | 7.36 a | 8.80 a |
| NDF | 14.7 ± 0.8 | 12.6–16.7 | 15.7 ± 0.8 | 13.9–17.6 | 16.4 ± 1.6 | 12.7–18.8 | 6.98 a | 11.40 b |
| Average | 3.91 | 7.90 | ||||||
| Trait a | Repeatability (%) | Correlation with Freeze Drying | |||
|---|---|---|---|---|---|
| Freeze Drying | Low-Heat Drying | High-Heat Drying | Low-Heat Drying | High-Heat Drying | |
| Protein | 93.91 | 92.04 | 91.19 | 0.975 ** | 0.961 ** |
| Oil | 91.84 | 86.84 | 90.47 | 0.917 ** | 0.946 ** |
| Fiber | 56.50 | 69.65 | 75.35 | 0.705 ** | 0.790 ** |
| Ash | 52.80 | 56.27 | 6.33 | 0.660 ** | 0.373 |
| Sucrose | 86.34 | 74.82 | 57.94 | 0.841 ** | 0.591 ** |
| Stachyose | 39.13 | 39.22 | 44.51 | 0.741 ** | 0.680 ** |
| Raffinose | 75.12 | 70.45 | 53.95 | 0.933 ** | 0.793 ** |
| Total Sugar | 62.00 | 72.23 | −0.32 | 0.851 ** | 0.508 * |
| Palmitic acid | 83.80 | 44.98 | 54.31 | 0.683 ** | 0.330 |
| Stearic acid | 66.95 | 70.49 | 1.46 | 0.712 ** | 0.072 |
| Oleic acid | 84.38 | 87.34 | 69.70 | 0.914 ** | 0.714 ** |
| Linoleic acid | 82.64 | 85.89 | 66.46 | 0.916 ** | 0.659 ** |
| Linolenic acid | 42.99 | 49.66 | 5.07 | 0.731 ** | 0.436 |
| ADF | 71.46 | 27.43 | 64.74 | 0.653 ** | 0.635 ** |
| NDF | 73.65 | 62.62 | 64.14 | 0.622 ** | 0.869 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.-L.; Townsend, W.; Sismour, E.; Xu, Y. A Study of Application and Comparison of Thermal Drying and Freeze Drying of Fresh Edamame Seeds in the Analysis of Seed Composition. Agronomy 2022, 12, 1993. https://doi.org/10.3390/agronomy12091993
Jiang G-L, Townsend W, Sismour E, Xu Y. A Study of Application and Comparison of Thermal Drying and Freeze Drying of Fresh Edamame Seeds in the Analysis of Seed Composition. Agronomy. 2022; 12(9):1993. https://doi.org/10.3390/agronomy12091993
Chicago/Turabian StyleJiang, Guo-Liang, William Townsend, Edward Sismour, and Yixiang Xu. 2022. "A Study of Application and Comparison of Thermal Drying and Freeze Drying of Fresh Edamame Seeds in the Analysis of Seed Composition" Agronomy 12, no. 9: 1993. https://doi.org/10.3390/agronomy12091993
APA StyleJiang, G.-L., Townsend, W., Sismour, E., & Xu, Y. (2022). A Study of Application and Comparison of Thermal Drying and Freeze Drying of Fresh Edamame Seeds in the Analysis of Seed Composition. Agronomy, 12(9), 1993. https://doi.org/10.3390/agronomy12091993





