Synergistic Effect of Plant-Growth-Promoting Rhizobacteria Improves Strawberry Growth and Flowering with Soil Salinization and Increased Atmospheric CO2 Levels and Temperature Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Treatments
2.2. Rhizobacteria Selection
2.3. Growth Measurements
2.4. Gas Exchange
2.5. Chlorophyll Fluorescence
2.6. Statistical Analysis
3. Results
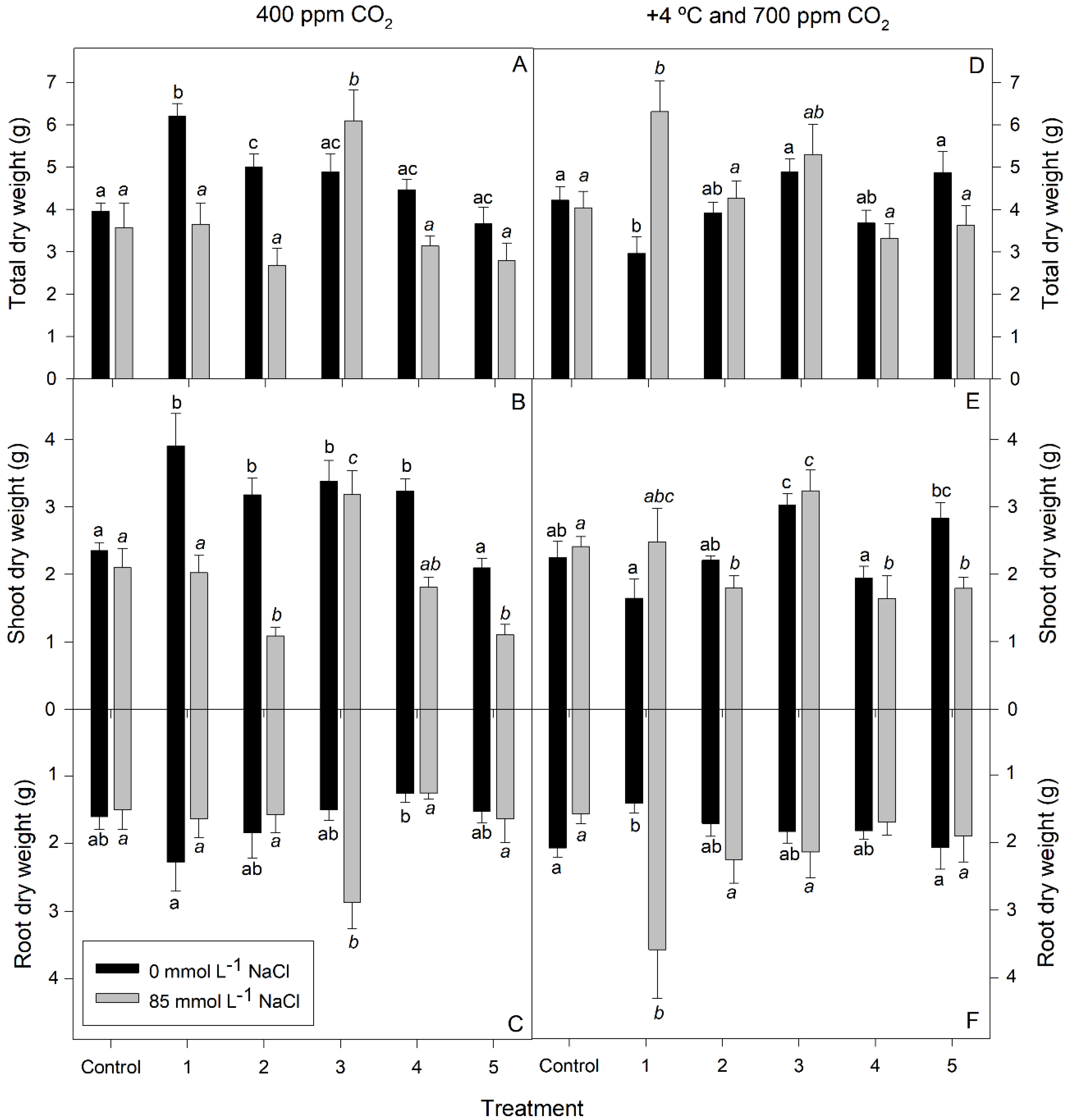
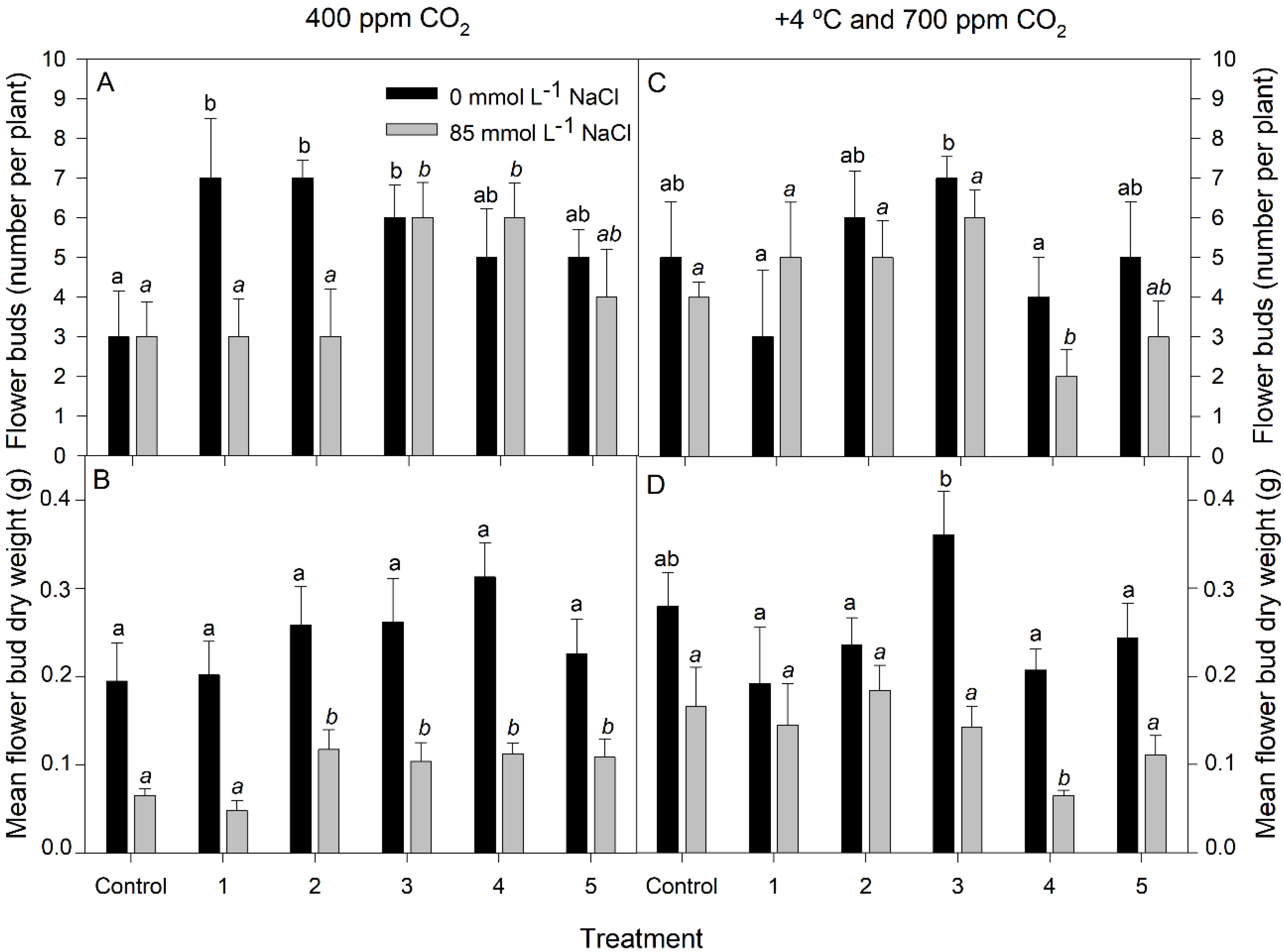
3.1. Growth Measurements
3.2. Gas Exchange
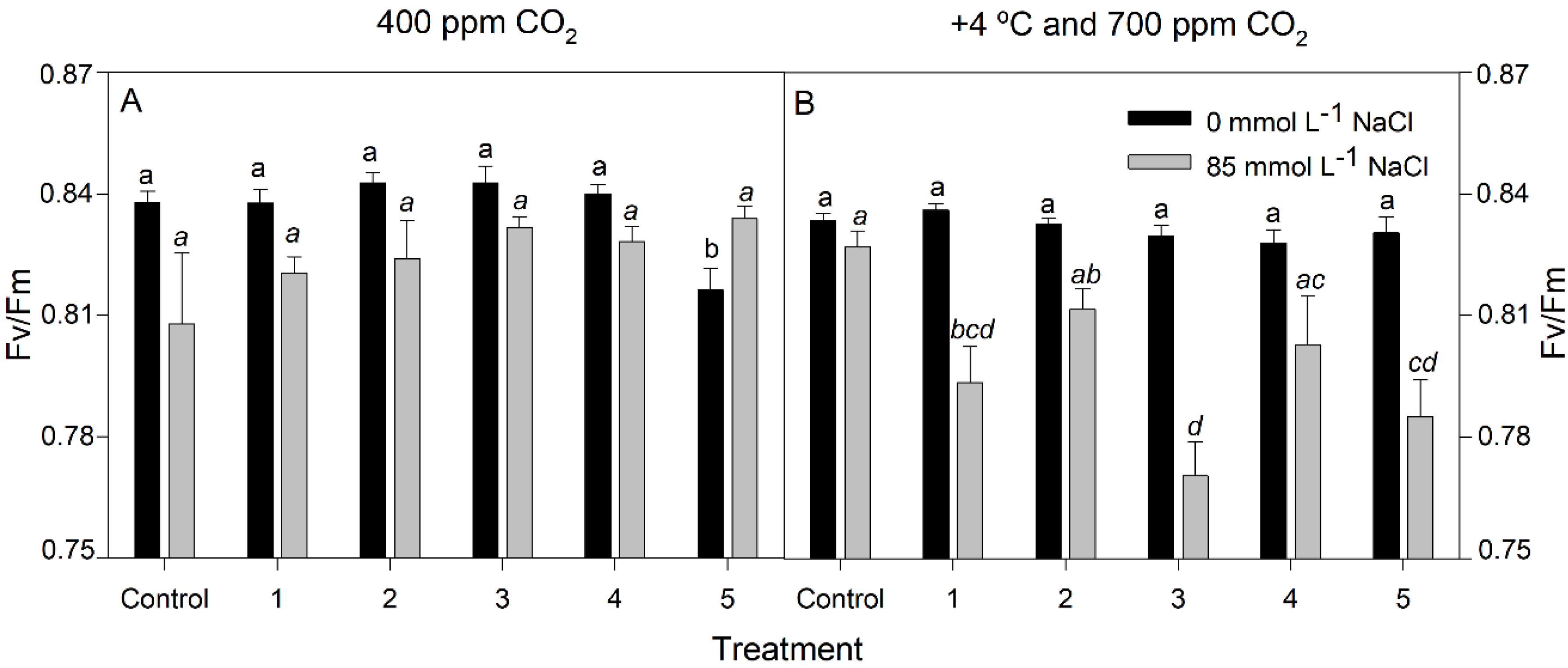
3.3. Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balasooriya, H.N.; Dassanayake, K.B.; Seneweera, S.; Ajlouni, S. Interaction of Elevated Carbon Dioxide and Temperature on Strawberry (Fragaria × ananassa) Growth and Fruit Yield. Int. J. Agric. Biosyst. Eng. 2018, 12, 279–287. [Google Scholar]
- Esitken, A.; Ercisli, S.; Yildiz, H.; Orhan, E. Does climate change have an effect on strawberry yield in colder growing areas? In Workshop on Berry Production in Changing Climate Conditions and Cultivation Systems COST-Action 863; Euroberry Research: From 838; ISHS: Darmstadt, Germany, 2008. [Google Scholar]
- Neri, D.; Baruzzi, G.; Massetani, F.; Faedi, W. Strawberry production in forced and protected culture in europe as a response to climate change. Can. J. Plant. Sci. 2012, 92, 1021–1036. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. In Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Shoba, P.; Ramakrishnan, S.S. Modeling the contributing factors of desertification and evaluating their relationships to the soil degradation process through geomatic techniques. Solid Earth 2016, 7, 341–354. [Google Scholar] [CrossRef]
- Arora, N.; Bhardwaj, R.; Sharma, P.; Arora, H.K. Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol. Plant. 2008, 30, 833–839. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M. Salicylic acid ameliorate the adverse effect of salt stress on strawberry. Sci. Agric. 2009, 66, 180–187. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M.; Pehluvan, M.; Donmez, F. Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria × ananassa). Hortscience 2013, 48, 563–567. [Google Scholar] [CrossRef]
- Dixit, R.; Agrawal, L.; Srivastava, S.; Chauhan, P.S. Paenibacillus lentimorbus enhanced abiotic stress tolerance through lateral root formation and phytohormone regulation. J. Plant Growth Regul. 2022, 41, 2198–2209. [Google Scholar] [CrossRef]
- Kapadia, C.; Patel, N.; Rana, A.; Vaidya, H.; Afarraj, S.; Ansari, M.J.; Gafur, A.; Poczai, P.; Sayyed, R.Z. Evaluation of plant growth-promoting and salinity ameliorating potential of halophilic bacteria isolated from saine soil. Front. Plant Sci. 2022, 13, 946217. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N. Plant growth promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference in Plant Pathogenic Bacteria, Angers, France, 27 August–2 September 1978; Available online: http://www.bashanfoundation.org/kloepper/kloepperradish.pdf (accessed on 27 July 2022).
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Ann. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Redondo-Gómez, S. Synergic Effect Rhizobacteria—Halophytes as a Rising Strategy to Face a Changing World. In Halophytes and Climatic Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, M., Shabala, S., Fujita, M., Eds.; CABI: Wallingford, UK, 2019; pp. 240–254. [Google Scholar]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Seneviratne, G.; Weerasekara, M.L.M.A.W.; Seneviratne, K.A.C.N.; Zavahir, J.S.; Kecskes, M.L.; Kennedy, I.R. Importance of biofilm formation in plant growth promoting rhizobacterial action. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Das, N.; Basak, L.V.G.; Salam, J.A.; Abigail, E.A. Application of biofilms on remediation of pollutants—An overview. J. Microbiol. Biotechnol. 2012, 2, 783–790. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. Amst. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Gómez, S.; Mesa-Marín, J.; Pérez-Romero, J.A.; López-Jurado, J.; García-López, J.V.; Mariscal, V.; Molina-Heredia, F.P.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Flowers, T.J.; et al. Consortia of plant-growth-promoting rhizobacteria isolated from halophytes improve response of eight crops to soil salinization and climate change conditions. Agronomy 2021, 11, 1609. [Google Scholar] [CrossRef]
- Satyal, S.S.; Nath, M.; Bhatt, M.D.; Gohil, T.; Bhatt, D. 2021. Molecular mechanisms deciphering cross-talk between quorum sensing genes and major iron regulons in rhizospheric communities. In Microbial Metatranscriptomics Belowground; Nath, M., Bhatt, D., Bhargava, P., Choudhary, D.K., Eds.; Springer: Singapore, 2021; pp. 615–630. [Google Scholar]
- Bhanse, P.; Kumar, M.; Signh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Bernabeu-Meana, M.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Redondo-Gómez, S. Effect of Plant Growth-Promoting Rhizobacteria on Salicornia ramosissima Seed Germination under Salinity, CO2 and Temperature Stress. Agronomy 2019, 9, 655. [Google Scholar] [CrossRef]
- Ariza, M.T.; Soria, C.; Medina-Mínguez, J.J.; Martínez-Ferri, E. Incidence of Misshapen fruits in strawberry plants grown under tunnels is affected by cultivar, planting date, pollination, and low temperatures. Hortic. Sci. 2012, 47, 1569–1573. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils. Front. Microbiol. 2020, 11, 553018. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.L.; Venkatasalam, E.P.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2019, 17, 119–128. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant. Bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased contribution of root exudates to soil carbon input during grassland degradation. Soil Biol. Biochem. 2020, 146, 107817. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apocada, J.; Muñoz-Rueda, A.; Mena-Petite, A. Lettuce production and antioxidant capacity are differentially modified by salt stress and light intensity under ambient and elevated CO2. J. Plant Physiol. 2013, 170, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Marcondes de Andrade, F.; Pereira, T.A.; Pereira Souza, T.; Sales Guimarães, P.H.; Martins, A.D.; Freitas Schwan, R.; Pasqual, M.; Dória, J. Beneficial effects of inoculation of growth-promoting bacteria in strawberry. Microbiol. Res. 2019, 223, 120–128. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, H.; Li, B.; Wang, J.; Jiang, D. Immobilization of Pycnoporus sanguineus laccase on copper tetra-aminophthalocyanine–Fe3O4 nanoparticle composite. Biotechnol. Appl. Biochem. 2006, 44, 93–100. [Google Scholar]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic.—Amst. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Pérez_Romero, J.A.; Idaszkin, Y.L.; Duarte, B.; Baeta, A.; Marques, J.C.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Atmospheric CO2 enrichment effect on the Cu-tolerance of the C4 cordgrass Spartina densiflora. J. Plant Physiol. 2018, 220, 155–166. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Davy, A.J.; Fernández-Muñoz, F.; Castellanos, E.M.; Luque, T.; Figueroa, M.E. Growth and photosynthtic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Ann. Bot. 2007, 100, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Figueroa, M.E.; Davy, A.J. Salt stimulation of growth and photosynthesis in a extreme halophyte, Arthrocnemum macrostachyum. Plant Biol. 2010, 12, 79–87. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Lorena Idaszkin, Y.; Barcia-Piedras, J.M.; Duarte, B.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Disentangling the effect of atmospheric CO2 enrichment on the halophyte Salicornia ramosissima J. Woods physiological performance under optimal and suboptimal saline conditions. Plant Physiol. Biochem. 2018, 127, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, N.; Chen, K.; Lenz, F. Responses of strawberry leaf photosynthesis, chlorophyll fluorescence and macronutrient contents to elevated CO2. J. Plant Physiol. 1997, 150, 395–400. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, X.W.; Zhao, M.; Mao, Z.J.; Zhu, S.Y.; Zhang, D.L.; Zhao, Z. Combination of elevated CO2 concentration and elevated temperature and elevated temperature only promote photosynthesis of Quercus mongolica seedlings. Russ. J. Plant Physiol. 2008, 55, 54–58. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Brugnoli, E.; Lauteri, M. Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt- tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol. 1991, 95, 628–635. [Google Scholar] [CrossRef]
- Antolín, M.C.; Sánchez-Díaz, M. Effects of temporary droughts on photosynthesis of alfalfa plants. J. Exp. Bot. 1993, 44, 1341–1349. [Google Scholar] [CrossRef]
- Ohad, I.; Kyle, D.J.; Arntzen, C.J. Membrane protein damage and repair: Removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J. Cell Biol. 1984, 99, 481–485. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Wharmby, C.; Castillo, J.M.; Mateos-Naranjo, E.; Luque, C.J.; de Cires, A.; Luque, T.; Davy, A.J.; Figueroa, M.E. Growth and photosynthetic responses to salinity in an extreme halophyte, Sarcocornia fruticosa. Physiol. Plant. 2006, 128, 116–124. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Romano-Rodríguez, E.; Mesa-Marín, J.; Sola-Elías, C.; Mateos-Naranjo, E. Consortia of plant-growth-promoting rhizobacteria isolated from halophytes improve the response of Swiss chard to soil salinization. Agronomy 2022, 12, 468. [Google Scholar] [CrossRef]
- Thiagarajan, A.; Lada, R.; Joy, P. Compensatory effects of elevated CO2 concentration on the inhibitory effects of high temperature and irradiance on photosynthetic gas exchange in carrots. Photosynthetica 2007, 45, 355–362. [Google Scholar] [CrossRef]
- Baker, J.; Allen Jr., L. Contrasting crop species responses to CO2 and temperature: Rice, soybean and citrus. Vegetatio 1993, 104, 239–260. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Umrao, V.; Singh, M. Effect of GA3 and IAA on growth and flowering of carnation. Hortflora Res. Spectr. 2012, 1, 69–72. [Google Scholar]
- Kurokura, T.; Hiraide, S.; Shimamura, Y.; Yamane, K. PGPR improves yield of strawberry species under less-fertilized conditions. Environ. Control Biol. 2017, 55, 121–128. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Achard, P.; Baghour, M.; Chapple, A.; Harberd, N.P. The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc. Natl. Acad. Sci. USA 2007, 104, 6484–6489. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Lei, M.; Fu, Y.; Zhao, J.; Ao, M.; Xu, L. Integrated DNA methylome and transcriptome analysis reveals the ethylene-induced flowering pathway genes in pineapple. Sci. Rep. 2017, 7, 17167. [Google Scholar] [CrossRef]
- Sun, P.; Mantri, N.; Lou, H.; Hu, Y.; Sun, D.; Zhu, Y.; Dong, T.; Lu, H. Effects of elevated CO2 and temperature on yield and fruit quality of strawberry (Fragaria × ananassa Duch.) at two levels of nitrogen application. PLoS ONE 2012, 7, e41000. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Biofertilizers | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||||||
| PGPR Properties | SDT3 | SDT13 | SDT14 | RA1 | RA15 | RA18 | SMT383 | SMT483 | SMT513 | HPJ2 | HPJ15 | HPJ50 | SRT1 | SRT8 | SRT15 |
| N fixation | × | × | × | × | × | × | × | × | × | × | |||||
| P solubilization | × | × | × | × | × | × | × | × | |||||||
| Siderophores production | × | × | × | × | × | × | × | × | × | × | × | ||||
| IAA production | × | × | × | × | × | × | × | × | × | × | × | ||||
| Biofilm production | × | × | × | × | × | × | |||||||||
| ACC deaminase | × | × | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redondo-Gómez, S.; García-López, J.V.; Mesa-Marín, J.; Pajuelo, E.; Rodriguez-Llorente, I.D.; Mateos-Naranjo, E. Synergistic Effect of Plant-Growth-Promoting Rhizobacteria Improves Strawberry Growth and Flowering with Soil Salinization and Increased Atmospheric CO2 Levels and Temperature Conditions. Agronomy 2022, 12, 2082. https://doi.org/10.3390/agronomy12092082
Redondo-Gómez S, García-López JV, Mesa-Marín J, Pajuelo E, Rodriguez-Llorente ID, Mateos-Naranjo E. Synergistic Effect of Plant-Growth-Promoting Rhizobacteria Improves Strawberry Growth and Flowering with Soil Salinization and Increased Atmospheric CO2 Levels and Temperature Conditions. Agronomy. 2022; 12(9):2082. https://doi.org/10.3390/agronomy12092082
Chicago/Turabian StyleRedondo-Gómez, Susana, Jesús V. García-López, Jennifer Mesa-Marín, Eloísa Pajuelo, Ignacio D. Rodriguez-Llorente, and Enrique Mateos-Naranjo. 2022. "Synergistic Effect of Plant-Growth-Promoting Rhizobacteria Improves Strawberry Growth and Flowering with Soil Salinization and Increased Atmospheric CO2 Levels and Temperature Conditions" Agronomy 12, no. 9: 2082. https://doi.org/10.3390/agronomy12092082
APA StyleRedondo-Gómez, S., García-López, J. V., Mesa-Marín, J., Pajuelo, E., Rodriguez-Llorente, I. D., & Mateos-Naranjo, E. (2022). Synergistic Effect of Plant-Growth-Promoting Rhizobacteria Improves Strawberry Growth and Flowering with Soil Salinization and Increased Atmospheric CO2 Levels and Temperature Conditions. Agronomy, 12(9), 2082. https://doi.org/10.3390/agronomy12092082







